Abstract
Circulating adiponectin reflects the degree of energy homeostasis and insulin sensitivity of adult individuals. Low abundance of the high molecular mass multimers (HMW), the most active forms mediating the insulin-sensitizing effects of adiponectin, is indicative of impaired metabolic status. The increase in fetal adiponectin HMW compared with adults is a distinctive features of human neonates. In order to further understand the functional properties of adiponectin during fetal life, we have evaluated the associations of adiponectin with insulin sensitivity, body composition and gender. Umbilical cord adiponectin, adiponectin complexes and metabolic parameters were measured at term by elective Cesarean section. The associations between adiponectin, measures of body composition and insulin sensitivity were evaluated in relation to fetal gender in 121 singleton neonates. Higher total adiponectin concentrations in females compared with male fetuses (34.3±9.5 vs 24.9±8.6, p<0.001) were associated with a 3.2-fold greater abundance in circulating HMW complexes (0.20±0.03 vs 0.08±0.03, p<0.001, n=9). Adiponectin was positively correlated with neonatal fat mass (r= 0.27, p< 0.04) and percent body fat in female fetuses (r= 0.28, p<0.03) and with lean mass in males (r= 0.28, p<0.03). There was no significant correlation between cord adiponectin and fasting insulin concentrations or fetal insulin sensitivity as estimated by HOMA-IR. The gender dimorphism for plasma adiponectin concentration and complex distribution first appears in utero. In sharp contrast to the inverse correlation found in adults, the positive relationship between adiponectin and body fat is a specific feature of the fetus.
Keywords: Adiponectin complexes, insulin sensitivity, body composition, adiposity, human, fetus
INTRODUCTION
Adiponectin is a circulating hormone produced exclusively by adipose tissue in adult humans and rodents (1). It is secreted under trimeric subunits that can be separated by applying stringent reducing and denaturing conditions in vitro. Once released in the systemic circulation adiponectin aggregates into multimeric complexes of various molecular mass consisting of 3 to 18 monomers. The so-called low molecular weigh (LMW) hexamers and high molecular weight (HMW) 12–18 oligomers are the main circulating forms which may account for up to 80 percent of total adiponectin (2, 3). Each oligomeric isoform elicits distinct biological action(s) in the target tissues. The HMW oligomers are the major active form mediating the peripheral insulin-sensitizing effects, whereas the central actions are attributed primarily to the LMW and trimeric oligomers (4). A decrease in adiponectin concentrations is a hallmark of the metabolic transition between insulin sensitive and insulin resistant states. The insulin resistance of obesity, type 2 and gestational diabetes is associated with lower concentrations of total plasma adiponectin (5–7). Similarly, decreased adiponectin levels are observed in late pregnancy reflecting the maternal insulin resistance which is greatest during the third trimester (8). The lower abundance of the HMW complexes in several situations of insulin resistance has pointed to HMW as better markers of insulin resistance than total plasma adiponectin (9–11).
Changes in adiponectin concentrations in response to metabolic effectors are regulated at the molecular level. The production of adiponectin by the adipocytes is controlled at the level of gene expression through specific promoter regions (12,13). Additionally, complex post-translational modifications involving extensive hydroxylation and glycosylation reactions regulate the assembly and release of oligomers in the systemic circulation (14). However, the contribution of adipose tissue mass to the regulation of adiponectin production following changes in BMI, weight gain or body composition is not well understood. The inverse relationship between adiponectin concentrations and adipose tissue mass is paradoxical for an adipocyte derived hormone. Indeed, the negative correlation between adiponectin and adiposity is in sharp contrast to other adipocytokines such as leptin whose circulating concentrations are positively correlated with adipose tissue mass (15). The hormonal regulation of adiponectin secretion and circulating concentrations is mediated through insulin and testosterone action (16, 17). Testosterone has the potential for contributing to the gender difference in adiponectin with lower levels appearing in boys during the progression of puberty (18, 19).
A developmental regulation of adiponectin has also been suggested based on the higher adiponectin concentrations in early developmental stages. The concentration of adiponectin is 4-fold higher in umbilical cord plasma at birth and 2-fold higher in 2-month old neonates compared with adults (20–22). The adiponectin producing tissues have not been well characterized in newborns however, the expression of adiponectin in fetal white adipose tissue and vascular cells likely contributes to the high circulating concentrations at birth (20, 23). To gain further insight into the mechanisms of adiponectin action, we have characterized the relationships of adiponectin with regard to insulin sensitivity and adiposity in human fetuses and their association with gender.
METHODS AND PROCEDURES
Subjects
Volunteers gave signed informed consent in accordance with the Case Western Reserve University and MetroHealth Medical Center guidelines for the protection of human subjects. A total of 121 non-laboring women (100 with uncomplicated pregnancy and 21 with gestational diabetes) without evidence of clinical infection were recruited at the time of elective Cesarean section delivery after overnight fast. Gestational diabetes was diagnosed at 20–24 weeks gestation based on a positive 100 g oral glucose tolerance test according to the criteria defined by Carpenter and Coustan (24). Umbilical venous blood was obtained upon delivery of the placenta by puncture of the double clamped cord. Plasma was separated by centrifugation and kept frozen at −20°C for adiponectin, glucose, insulin and leptin assays.
Metabolic and anthropometric measurements
Gestational age was determined based on record of the last menstrual period and verified by ultrasound prior to week 20 of gestation. Placenta weight was recorded on a calibration scale after trimming of umbilical cord and fetal membranes. Neonatal length and weight were determined using measuring board and calibrated scale to the nearest of 0.1 cm and 10 g respectively. Neonatal body composition measurements were evaluated using skinfolds within 24 hours from delivery by one examiner experienced in technique (25). Two subjects were not included in final analysis based on their % neonatal body fat <2. The insulin resistance indexes were calculated according to the homeostasis model assessment (HOMA): HOMA = fasting plasma insulin (μU/ml) x fasting blood glucose (mmol/liter)/22.5.
Adiponectin electrophoretic measurements
Oligomeric adiponectin complexes in umbilical plasma were analyzed by western blot in five females and four males from the cohort of subjects matched for maternal age, gestational age and ethnicity. Four μl of a 1/10 plasma dilution (approx 12 μg protein) from male and female fetuses were electrophoresed under non-reducing and non-denaturing conditions and transferred to nitrocellulose membrane. Membranes were blocked overnight then incubated for 2 hours using a rabbit polyclonal anti-adiponectin antibody (Chemicon), and goat anti-rabbit IgG HRP-conjugated as secondary antibody. Immuno-complexes were visualized by chemiluminescence (Amersham, Piscataway, NJ). Abundance of high molecular weight adiponectin multimers were determined by densitometry with a gel-doc system (Biorad, Hercules, CA) and expressed as percent total adiponectin multimers. For determination of total adiponectin, all plasma samples were run in duplicate in a single assay using commercial ELISA kits with intra-assays coefficients of variation of 7.4 %.
Other plasma assays
Insulin was measured using radioimmunoassay (Linco, St Charles, MO). Glucose was assessed by the glucose oxidase method using a glucose analyser (Yellow Springs Instrument, Yellow Springs, OH). Leptin was measured using ELISA kits (Linco) with intra-assays coefficients of variation (CV) of 2.6–6.2%.
Data analysis
Values are presented as means ± SD. Differences between dependent variables were examined with one-way repeated measures analysis of variance (ANOVA). Statistical significant mean differences were identified with a Fisher’s PLSD post-hoc test. The relationships between fetal adiponectin metabolic and body composition parameters were estimated on bivariate correlation analysis. The correlations between adiponectin and variables of interest were evaluated by analysis of covariance and simultaneously adjusted for potential confounders (gestational age, maternal age, maternal pre-gravid BMI and ethnicity) using partial correlation. The data were analyzed using the Statview III statistical package (Abacus Concepts, Berkeley, CA) and statistics (Tallahassee, FL). P values were computed using the Fisher’s Z statistics. The level for statistical significance was set at 0.05.
RESULTS
Adiponectin association with gender
Demographic and metabolic variables of the neonates are presented in Table 1. The inclusion of pregnant women with and without gestational diabetes allowed us to analyze neonates with a wide range in neonatal adiposity at birth (4.4 to 22.8 % total body fat). The wide range of adipose tissue mass was reflected by the wide distribution of umbilical leptin concentrations from 3.4 to 96.6 ng/ml. There was a gender difference in birth length, birth weight and placental weight which were all higher in males. In contrast, the amount of adipose tissue mass was similar in male and female neonates at delivery (Table 1) as reported previously by our group. Total adiponectin measured in umbilical cord plasma at birth was higher in female as compared with male fetuses (34.3 ± 9.5 vs 29.4± 9.0 μg/ml, p< 0.004). The gender difference in total plasma adiponectin was associated with a 3.2-fold greater abundance (0.20±0.03 vs 0.08±0.03) of circulating HMW multimeric complexes (Figure 1) with no significant difference in LMW and trimers (p=0.1). There was no difference between plasma adiponectin in offspring of women with GDM and normal glucose tolerance either as a group or split by gender (29.4±9.0 vs 28.5±3.9 in males and 34.3±9.5 vs 35±11.9 μg/ml in females).
Table 1. Neonatal anthropometric and metabolic parameters at delivery.
n=60 females (including 11 from women with GDM) and 61 males (including 10 from women with GDM). Means ± SD.
| males | females | p-value | |
|---|---|---|---|
| Maternal age (yr) | 29.1 ± 5.6 | 26.5 ± 5.7 | 0.01 |
| Gestational Age (wk) | 38.7 ± 0.7 | 38.8 ± 0.6 | 0.26 |
| Placental Weight (g) | 691 ± 185 | 635 ± 134 | 0.01 |
| Weight (g) | 3406 ± 532 | 3199 ± 445 | 0.002 |
| Length (cm) | 49.6 ± 2.1 | 48.6 ± 2.0 | 0.0004 |
| PI (g/cm3) | 2.78 ± 0.27 | 2.79 ± 0.28 | 0.88 |
| Fat Mass (g) | 446 ± 194 | 407 ± 162 | 0.11 |
| Lean Mass (g) | 2951 ± 355 | 2783 ± 308 | 0.0002 |
| Body Fat (%) | 12.6 ± 3.8 | 12.4 ± 3.3 | 0.65 |
| Leptin (ng/ml) | 11.0 ± 9.8 | 17.3 ± 18.4 | 0.005 |
| Insulin (μU/ml) | 8.6 ± 5.5 | 10.3 ± 10.6 | 0.19 |
| HOMA-IR index | 1.3 ± 0.8 | 1.6 ± 1.5 | 0.09 |
GDM, gestational diabetes mellitus; HOMA-IR, homeostasis model assessment of insulin resistance; PI, pondral index
Figure 1. distribution of adiponectin oligomer complexes in umbilical circulation.
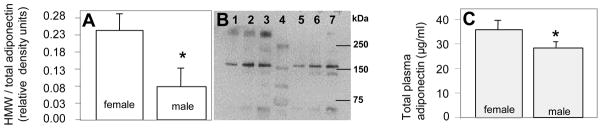
A: Distribution of HMW and LMW adiponectin complexes quantified by densitometry. B: western blot under non-reducing and non-denaturing SDS-PAGE conditions showing high molecular weight oligomers (HMW) migrating at approximately 340 kDa, low molecular weight hexamers (LMW) migrating at 160 kDa and trimers migrating at 67 kDa. Lanes 1–3: umbilical plasma (1, 2, and 4 ul per well) from one representative female fetus, lanes 4: molecular mass markers, lanes 4–6: umbilical plasma (1, 2 and 4 μl per well) from one representative male fetus. Data are means ± SE with n = 5 females and 4 males from the cohort of subjects described in Table 1 matched for maternal age, gestational age and ethnicity. C: Total adiponectin in umbilical plasma at delivery with n= 61 males and 60 females. *p<0.001 between males and females.
Adiponectin association with body composition
There was a positive correlation between umbilical cord adiponectin and fetal adiposity at birth estimated by percent body fat (r=0.19, p < 0.03) but not between adiponectin and lean body mass (r=0.10, p=0.26) (Figure 2). When analyzed by gender the association between adiponectin and percent body fat was significant only in females (r= 0.28, p<0.03) whereas the association between adiponectin and lean mass was significant only in males (r= 0.31, p<0.02) (Table 2).
Figure 2. gender specific relationship between fetal adiponectin and body composition.
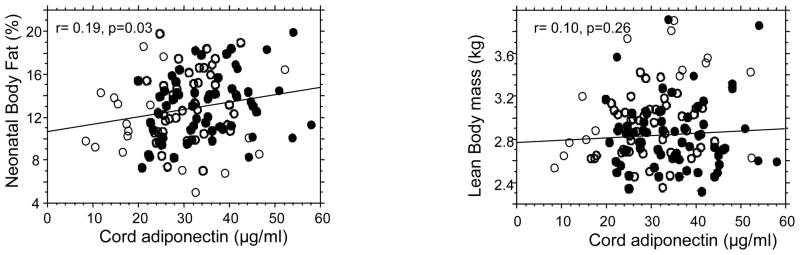
Regression analyses show a significant positive correlation between umbilical adiponectin and percent total body fat in females (r=0.19, p=0.03) but not between adiponectin and lean body mass (r=0.10, p=0.25). Black circles: females, open circles, males.
Table 2. Sex discordant associations between umbilical cord plasma adiponectin concentrations and neonatal body composition at birth.
p and r values are provided without adjustment and after simultaneous adjustment for maternal age, maternal ethnicity, gestational age and pre-gravid BMI.
| Adiponectin in girls | Adiponectin in girls | Adiponectin in boys | Adiponectin in boys | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | adjusted | r | p | adjusted | |||
| r | p | r | p | |||||
| HOMA-IR index | 0.09 | 0.50 | 0.10 | 0.45 | −0.02 | 0.90 | −0.05 | 0.71 |
| Insulin | 0.15 | 0.25 | 0.15 | 0.27 | −0.05 | 0.72 | −0.04 | 0.75 |
| Leptin | 0.20 | 0.14 | 0.20 | 0.13 | 0.11 | 0.37 | 0.16 | 0.23 |
| Neonatal lean mass | 0.08 | 0.55 | 0.15 | 0.35 | 0.28 | 0.03 | 0.31 | 0.02 |
| Neonatal fat mass | 0.24 | 0.07 | 0.27 | 0.04 | 0.19 | 0.14 | 0.24 | 0.07 |
| Percent body fat | 0.28 | 0.03 | 0.28 | 0.03 | 0.13 | 0.31 | 0.21 | 0.21 |
| Birth weight | 0.13 | 0.33 | 0.20 | 0.14 | 0.26 | 0.03 | 0.29 | 0.02 |
HOMA-IR, homeostasis model assessment of insulin resistance.
Adiponectin association with insulin sensitivity
Neonatal insulin sensitivity at birth was estimated by calculating homeostasis model assessment of insulin resistance (HOMA-IR) indices in offspring of mothers fasted overnight prior to delivery. The mean value for HOMA–IR indices and insulin concentrations were higher in male fetuses suggesting that male fetuses are slightly less insulin sensitive than females (Table 1). However, there was no significant correlation between cord adiponectin and HOMA-IR or between adiponectin and fasting insulin concentrations (Figure 3, Table 2).
Figure 3. adiponectin is not correlated with insulin sensitivity of the fetus.
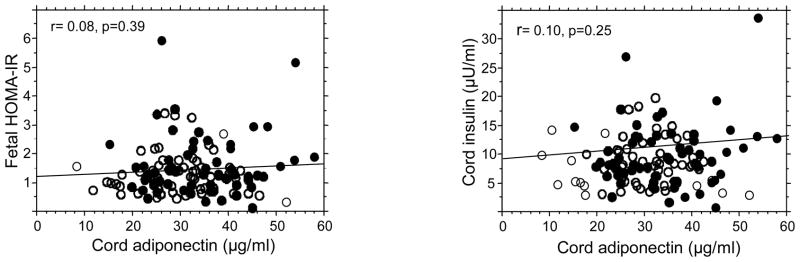
Regression analyses show an absence of significant correlation between umbilical cord plasma adiponectin concentrations, insulin sensitivity of the fetus estimated by HOMA-IR and fetal insulin concentrations. Black circles: females, open circles, males.
DISCUSSION
Gender dimorphism
Sex differences in total adiponectin with higher circulating concentrations in females as compared with males have been identified in adults, adolescents and children (18,26,27). The sexual dimorphism appears primarily related to the higher levels of the HMW complexes with no significant differences in lower molecular weight multimers (2, 28). We report that the gender difference in adiponectin already exists at birth with greater umbilical plasma concentrations as well as a greater proportion of HMW complexes in female neonates as compared with males. Our findings indicate that the abundance of adiponectin is regulated at early stages of human development, through mechanisms which reflect the gender specificity of the intrauterine environment. The HMW and LMW systemic complexes do not interchange in the circulation and thus directly reflect adiponectin secretion (29). Assuming that HMW abundance reflects the level of secretion by fetal tissues rather than a difference in clearance rates, the lower HMW in males may reflect regulated adiponectin production in vascular cells and adipocytes (20). Testosterone selectively reduces the HMW form by inhibiting its secretion from adipocytes (28, 30). Hence, the higher testosterone concentration in male as compared with female fetuses may contribute to the decreased HMW abundance we have observed in umbilical adiponectin (31). Androgens may also indirectly regulate adiponectin distribution through a decrease in adipocyte number and size in male fetuses (32, 33) providing that the pathways for hormonal regulation of adipose tissue growth are functional before birth. A potential reason why previous studies may have failed to document a sex difference in fetal/neonatal adiponectin concentrations is unclear (21,34,35). We regard the technique-inherent variability with large inter and intra-assay coefficients of variation of most commercial adiponectin assay kits as a potential source of bias.
Adiponectin and adiposity
The intuitive principle that the circulating concentration of a molecule secreted by adipose tissue varies accordingly to changes in adipose mass, which applies to most adipokines, is considerably more complex for adiponectin. The well established inverse correlation between adiponectin and body mass index in adults (36) has been questioned in young children and adolescents (19,27). In neonates, there is a positive association between umbilical adiponectin and birth weight (34,35,37,38) but the relationship with fat mass is unclear (39,40). In our cohort of 121 neonates, cord adiponectin at delivery was positively correlated with percent body fat in girls but not in boys reflecting a sexual dimorphism during in utero development. The weaker correlation between adiponectin and adiposity in fetal compared with adults (6,8) may reflect the multiple sites for adiponectin expression in the fetus (20). Our data provide experimental support to the hypothesis by Ong et al that the association between adiponectin and body size progressively switches from positive at birth to negative in adults (27). It further highlights that the association is based on adiposity rather than simply reflecting body weight. In adults, adiponectin secretion displays a depot difference with higher secretion by central/visceral as compared with subcutaneous fat (16,41). We thus speculate that the gender specificity that we have observed between adiponectin and adiposity reflects early specificities in adipose tissue development with greater visceral than subcutaneous fat mass in female fetuses. Additionally, our data suggest that the postnatal increase in adipose tissue mass contributes to production of inhibitors of adiponectin which either are not produced or are not functional in utero. The identification of such factors may provide valuable tools for further understanding adiponectin regulation.
Adiponectin and insulin sensitivity
Fetal insulin sensitivity was estimated by calculating HOMA-IR indices in our study cohort at birth. Insulin sensitivity was slightly lower in males compared with female fetuses, As previously reported the HOMA-IR indices were several orders of magnitude lower than in adults, suggesting a state of higher insulin sensitivity in utero (20). The absence of significant correlation between cord adiponectin concentration and HOMA-IR indices or umbilical insulin concentrations is in sharp contrast to the inverse relationship reported in adults (6,8,42). In agreement with estimates of insulin sensitivity in newborns, our data support the concept that early developmental stages are characterized by higher insulin sensitivity particularly of peripheral tissues (43). Taken together, these findings suggest that the combination of high insulin sensitivity and high adiponectin levels may have functional consequences in creating a favorable environment for rapid tissue growth during fetal life.
In conclusion, we report that the regulation of adiponectin in prenatal life displays gender characteristics. The sexual dimorphism between females and males suggests that adiponectin secretion by fetal tissues may be sensitive to the action of androgens as part of early metabolic programming. Our findings set the basis for further understanding the function of adiponectin during human development.
Acknowledgments
This work was supported by research support from Diabetes Association of Greater Cleveland to SHM, American Diabetes Association, NIH RO1-HD22965 to PMC/SHM and UL1 RR 024989 (CTSA). The authors are grateful to Pat Mencin for help with subject recruitment and body composition measurements at delivery and to Joan Lippus for technical assistance with the plasma assays.
Footnotes
Disclosure information
B. S., L. L., P. L., C. P., HM. S. have nothing to declare.
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 3.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 4.Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 5.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 7.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-de Mouzon S. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–13562. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 10.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 11.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 12.Barth N, Langmann T, Schölmerich J, Schmitz G, Schäffler A. Identification of regulatory elements in the human adipose most abundant gene transcript-1 (apM-1) promoter: role of SP1/SP3 and TNF-alpha as regulatory pathways. Diabetologia. 2002;45:1425–1433. doi: 10.1007/s00125-002-0895-5. [DOI] [PubMed] [Google Scholar]
- 13.Qiao L, Schaack J, Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006;147:865–874. doi: 10.1210/en.2005-1030. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 15.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;11:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 16.Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, Rosato FE, Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 18.Woo JG, Dolan LM, Daniels SR, Goodman E, Martin LJ. Adolescent sex differences in adiponectin are conditional on pubertal development and adiposity. Obes Res. 2005;13:2095–2101. doi: 10.1038/oby.2005.260. [DOI] [PubMed] [Google Scholar]
- 19.Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 20.Pinar H, Basu S, Hotmire K, Laffineuse L, Presley L, Carpenter M, Catalano PM, Hauguel-de Mouzon S. High molecular weight multimer complexes and vascular expression contribute to high adiponectin in the fetus. J Clin Endocrinol Metab. 2008;93(7):2885–2890. doi: 10.1210/jc.2008-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 22.Ibáñez L, Sebastiani G, Díaz M, Lopez-Bermejo A, Gómez-Roig MD, de Zegher F. Gender Specificity of Body Adiposity and Circulating Adiponectin, Visfatin, Insulin and IGF-I at Term Birth: Relation to Prenatal Growth. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-0526. In press. [DOI] [PubMed] [Google Scholar]
- 23.Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, Bondioni S, Beck-Peccoz P, Spada A. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005;90:2397–2402. doi: 10.1210/jc.2004-1553. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982:144768–14773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 25.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173:1176–1181. doi: 10.1016/0002-9378(95)91348-3. [DOI] [PubMed] [Google Scholar]
- 26.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003:46459–46469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 27.Ong KK, Frystyk J, Flyvbjerg A, Petry CJ, Ness A, Dunger DB. Sex-discordant associations with adiponectin levels and lipid profiles in children. Diabetes. 2006;55:1337–1341. doi: 10.2337/db05-1272. [DOI] [PubMed] [Google Scholar]
- 28.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 29.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 31.Forest MG, De Peretti E, Bertrand J. Hypothalamic-pituitary-gonadal relationships in man from birth to puberty. Clin Endocrinol (Oxf) 1976;5:551–569. doi: 10.1111/j.1365-2265.1976.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson LA, McTernan PG, Barnett AH, Kumar S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab. 2001;86:5045–5051. doi: 10.1210/jcem.86.10.7955. [DOI] [PubMed] [Google Scholar]
- 34.Kamoda T, Saitoh H, Saito M, Sugiura M, Matsui A. Serum adiponectin concentrations in newborn infants in early postnatal life. Pediatr Res. 2004;56:690–693. doi: 10.1203/01.PDR.0000142711.24999.8A. [DOI] [PubMed] [Google Scholar]
- 35.Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Hyperadiponectinemia in newborns: relationship with leptin levels and birth weight. Obes Res. 2004;4:521–524. doi: 10.1038/oby.2004.59. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 37.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am J Obstet Gynecol. 2005;193:1238–1242. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 38.Kadowaki K, Waguri M, Nakanishi I, Miyashita Y, Nakayama M, Suehara N, Funahashi T, Shimomura I, Fujita T. Adiponectin concentration in umbilical cord serum is positively associated with the weight ratio of fetus to placenta. J Clin Endocrinol Metab. 2006;91:5090–5094. doi: 10.1210/jc.2005-2846. [DOI] [PubMed] [Google Scholar]
- 39.Inami I, Okada T, Fujita H, Makimoto M, Hosono S, Minato M, Takahashi S, Harada K, Yamamoto T. Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatr Res. 2007;61:604–606. doi: 10.1203/pdr.0b013e3180459f8a. [DOI] [PubMed] [Google Scholar]
- 40.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, Ho SC, Chu CH. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf) 2004;61:88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 41.Perrini S, Laviola L, Cignarelli A, Melchiorre M, De Stefano F, Caccioppoli C, Natalicchio A, Orlando MR, Garruti G, De Fazio M, Catalano G, Memeo V, Giorgino R, Giorgino F. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–164. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 42.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 43.Farrag HM, Nawrath LM, Healey JE, Dorcus EJ, Rapoza RE, Oh W, Cowett RM. Persistent glucose production and greater peripheral sensitivity to insulin in the neonate vs. the adult. Am J Physiol. 1997;272:E86–E93. doi: 10.1152/ajpendo.1997.272.1.E86. [DOI] [PubMed] [Google Scholar]


