Summary
Killer immunoglobulin-like receptor (KIR)-ligand mismatched natural killer (NK) cells play a key role in achieving durable remission after haplo-identical transplantation for acute myeloid leukaemia. We investigated the feasibility of transfusing haplo-identical, T-cell depleted, KIR-ligand mismatched NK cells, after conditioning therapy with melphalan and fludarabine, to patients with advanced multiple myeloma (MM) followed by delayed rescue with autologous stem cells. No graft-versus-host disease or failure of autologous stem cells to engraft was observed. There was significant variation in the number of allo-reactive NK cells transfused. However, all NK products containing allo-reactive NK cells killed the NK cell target K562, the MM cell line U266, and recipient MM cells when available. Post NK cell infusion there was a rise in endogenous interleukin-15 accompanied by increasing donor chimaerism. Donor chimaerism was eventually lost, which correlated with the emergence of potent host anti-donor responses indicating that the immunosuppressive properties of the conditioning regimen require further optimization. Further, blocking of inhibitory KIR-ligands with anti-human leucocyte antigen antibody substantially enhanced killing of MM cells thus highlighting the potential for modulating NK/MM cell interaction. Encouragingly, 50% of patients achieved (near) complete remission. These data set the stage for future studies of KIR-ligand mismatched NK cell therapy in the autologous setting.
Keywords: haplo-identical, natural killer cell, immunotherapy, multiple myeloma
High-dose chemotherapy supported by autologous (auto) peripheral blood stem cell transplantation (auto-PBSCT) can substantially prolong disease-free survival in multiple myeloma (MM) (Attal et al, 2003; Child et al, 2003; Barlogie et al, 2006a). Although the majority of patients eventually relapse, we have recently reported a plateau of continuous complete remissions (CR) apparent at 10 years (Barlogie et al, 2006b). Novel agents alone or in combination, including bortezomib, thalidomide and lenalidomide, can induce responses including CRs in newly diagnosed and relapsed MM. However, the durability of these responses is yet to be appreciated (Rajkumar et al, 2002, 2005; Richardson et al, 2005). Gene expression profiling can identify patients who are likely to fare poorly both with auto-PBSCT and novel drugs based on a risk score derived from 70 key genes (Zhan et al, 2006; Shaughnessy et al, 2007). Although CR is easily achieved in high-risk myeloma, disease control is often of short duration due to rapid regrowth of chemotherapy-refractory MM cells. Thus, new therapeutic strategies are urgently needed for such patients.
The graft-versus-myeloma (GvM) reactions have been attributed to donor T-lymphocytes, but emerging evidence suggests that natural killer (NK) cells also have anti-MM activity (Tricot et al, 1996a; Lokhorst et al, 2000; Salama et al, 2000; Zeiser et al, 2004). In vitro studies demonstrated that allogeneic (allo) and auto-NK cells have the ability to kill CD138-purified primary MM cells (Szmania et al, 2006). In contrast, CD34+ haematopoietic stem cells, as well as peripheral blood mononuclear cells (PBMNC), were completely resistant to NK cell killing under similar conditions (Frohn et al, 2002).
In humans, NK cells are regulated by clonally distributed killer immunoglobulin-like receptors (KIRs) that recognize allotypic determinants displayed by different human leucocyte antigen (HLA) class I alleles. Inhibitory KIRs are generally dominant and prevent NK cells from killing autologous cells. NK cells expressing KIR2DL1 are inhibited by HLA-C group 2, which have Asn77-Lys80 on the α1 helix of HLA-C. KIR2DL2 and KIR2DL3 recognize Ser77-Asn80 on HLA-C group 1 alleles, whilst KIR3DL1 has specificity for HLA-Bw4. The frequency of the HLA class I KIR ligands C-group1, C-group2 and HLA-Bw4 amongst Caucasians vary and are approximately 80%, 65% and 55% respectively (Single et al, 2007). As a consequence, transplantation across histocompatibility barriers may trigger donor NK cell allo-reactivity if the recipient lacks KIR ligands present in the donor, that can activate inhibitory NK receptors and thereby prevent killing (‘missing self’) (Farag et al, 2002; Ruggeri et al, 2002; Parham & McQueen, 2003). Ruggeri et al (2002) first reported that NK cells from KIR-ligand mismatched donors exert a potent anti-leukaemic effect and prevent relapse after haplo-identical transplantation for acute myeloid leukaemia (AML).
Haplo-identical transplantation is not an option for the vast majority of MM patients. We therefore designed a treatment protocol for patients with advanced MM that aimed to harness the beneficial effects of allogeneic NK cells without subjecting patients to a full haplo-identical transplant. We evaluated whether NK cell infusions from haplo-identical KIR-ligand mismatched donors in the setting of a delayed auto-PBSCT may confer additional anti-myeloma effects.
Patients and methods
Patients and donors
Ten patients with relapsed MM after single (n = 4) or tandem PBSCT (n = 6) were enroled. The characteristics of these patients are listed in Table I. Informed consent was obtained from patients and their haplo-identical donors according to the Declaration of Helsinki and the study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. The clinical protocol was conducted under the Investigational New Drug Application BB-IND 11347. Patients and donors were typed by serological techniques for HLA-A and -B. HLA-C alleles were assigned by high resolution molecular typing by polymerase chain reaction (PCR) amplification with sequence-specific primers following the manufacturer’s instructions (Pel-Freez\Dynal Biotech, Brown Deer, WI, USA). Donor selection criteria were strictly based on the ligand/ligand model as previously described (Aversa et al, 1998, 2005; Ruggeri et al, 2002). Additional KIR genotyping and phenotyping of the donor were performed, but played no role in the selection of the donor, since these data became available only later. Standard criteria were used to assess response and relapse of the treated patients (Blade et al, 1998).
Table I.
Patient characteristics and clinical outcome.
| Subject | Age (years) | Sex | Status at enrolment | Number of prior autotransplants | Metaphase cytogenetics | Best response on protocol | Outcome on protocol | Salvage therapy | Current status | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | Refractory relapse | 2 | Abnormal | n-CR | Relapse day 77 | Yes | Dead day 201 | PD |
| 2 | 52 | F | Relapse | 1 | Abnormal | CR | Relapse day 133 | Yes | Dead day 615 | Pneumonia post further PBSCT for PD |
| 3 | 62 | M | Refractory relapse | 1 | Abnormal | n-CR | Relapse day 31 | Yes | CR & alive day 1111 | N/A |
| 4 | 56 | M | Refractory relapse | 2 | Normal | PD | PD | Yes | Dead day 825 | PD |
| 5 | 67 | M | Relapse | 2 | Abnormal | CR | Relapse day 532 | Yes | n-CR & alive day 850 | N/A |
| 6 | 48 | M | Relapse | 2 | Normal | PD | PD | Yes | Dead day 206 | PD |
| 7 | 49 | M | Relapse | 2 | Abnormal | PR | Relapse day 75 | Yes | Dead day 285 | PD |
| 8 | 50 | M | Refractory relapse | 1 | Abnormal | MR | Relapse day 58 | Yes | Dead day 202 | PD |
| 9 | 60 | M | Relapse | 1 | Normal | SD | SD | No | SD & alive day 533 | N/A |
| 10 | 44 | M | Relapse | 2 | Abnormal | n-CR | Relapse day 259 | Yes | n-CR & alive day 541 | N/A |
F, female; M, male; CR, complete remission; n-CR, near complete remission; PR, partial response; MR, minimal response; SD, stable disease; PD, progressive disease; N/A, not applicable; PBSCT, peripheral blood stem cell transplantation.
Study design
The protocol schema is depicted in Fig 1. Mononuclear cells (MNC) were collected from each KIR-ligand mismatched haplo-identical family donor by steady state 24-l aphaeresis on two occasions. The aphaeresis products were enriched for NK cells by T-cell depletion using the CliniMACS CD3 depletion system (Miltenyi, Auburn, CA, USA) under good manufacturing practice conditions. The CD3-depleted products of the first four patients were incubated overnight at 1 × 106 cells/ml in AIM-V medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2% human serum albumin (American Red Cross, Washington, DC, USA) and 300 IU/ml of interleukin-2 (IL-2) (Chiron, Emeryville, CA, USA) to allow review of the product safety data prior to infusion of the NK cell enriched product. The aphaeresis products of patients 5–10 were exposed to IL-2 (300 IU/ml) during incubation with anti-CD3 beads and transfused on the same day once in-house endotoxin testing had been established. NK cell enriched aphaeresis products were transfused on day 0, i.e. the day after the high-dose melphalan (MEL), and day +2. Infusion targets were more than 1.0 × 106 /kg CD56+ CD3− NK cells and less than 1.0 × 105 /kg CD3+ CD56− T cells. All cell products contained more than 95% viable cells prior to infusion. No graft-versus-host disease (GvHD) prophylaxis was used. Three million units of IL-2 were administered subcutaneously from day +1 to +11 to support NK cell survival in vivo. Patients received a delayed, unmanipulated auto-PBSCT on day +14, i.e. 15 d after infusion of high-dose MEL, with 3–8 × 106 /kg CD34+ cells. All transplant recipients received infectious disease prophylaxis as per standard practice (Barlogie et al, 2006a). Engraftment of autologous CD34+ cells and GvHD were assessed by consensus criteria (Przepiorka et al, 1995). Patients were monitored for NK cell-related infusion reactions, side effects of IL-2 administration and occurrence of infections in the context of a delayed autologous stem cell infusion.
Fig 1.

Protocol schema ‘haplo-identical NK cell therapy combined with delayed autografting’. Conditioning therapy comprised fludarabine (Flu, 25 mg/m2 on day −5 to day −2), dexamethasone (Dex, 40 mg/d on days −5 to −2), and melphalan (MEL, 140 mg/m2 IV on day −1). Flu and Dex were given to deplete patient lymphocytes in order to prevent rejection of allogeneic NK cells. MEL 140 mg/m2 was used for tumour reduction. NK cells from a haplo-identical KIR-ligand mismatched family donor were transfused on days 0 and +2. IL-2 was given daily to support the survival and expansion of NK cells in vivo. Autologous PBSCT was delayed to provide a window for donor NK cell product to kill residual myeloma cells.
KIR genotyping and phenotyping
KIR genotyping was performed as previously described (Hsu et al, 2002). Immunophenotyping of aphaeresis products and NK enriched products for infusion were analysed by flow cytometry (Leung et al, 2004). Fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll-alpha protein (PerCP), and allophycocyanin (APC)-conjugated control immunoglobulins or specific antibodies (abs) directed at CD56, CD3, CD14, CD15, CD19, CD33, CD158a (KIR2DL1), CD158b (KIR2DL2/L3), and NKB1 (KIR3DL1) (BD Phar-Mingen, San Diego, CA, USA) were used to evaluate NK and other cells of treated patients. Absolute NK cell numbers/kg of recipient body weight were calculated as (total MNC/ml × volume of pre-infusion NK final product × percentage of CD56 positive cells)/kg (recipient body weight). Alloreactive NK cells were quantitated by determining the number of cells positive for KIR receptor(s) for which the corresponding HLA-ligand was absent in the recipient. Absolute CD3+ numbers/kg of recipient body weight were expressed as (MNC/ml × volume of pre-infusion NK product × percentage of CD3 positive cells)/kg (recipient body weight). The percentage of KIR− cells was determined by staining purified (>97%) CD3− CD56+ NK cells with CD158a (KIR2DL1), CD158b (KIR2DL2/L3) and NKB1 (KIR3DL1) abs conjugated to FITC, PE or APC. Analysis was performed with all abs in the same tube.
Cytotoxicity, chimaerism assay, enzyme-linked immunosorbent assay and flow cytometric bead array
The ability of the haplo-identical NK cells to kill the NK sensitive cell line K562, recipient MM cells (when available) or the MM cell line U266 [HLA-C*0304 (group 1), HLAC* 0702 (group 1), Bw6], and patient phytohaemagglutinin-induced (PHA)-blasts was evaluated in standard 4-h 51Cr release assays. Anti-human HLA-ABC monoclonal antibody (mAb) (clone W6/32; Serotec, Raleigh, NC, USA) (10 μg/ml) was used in HLA class I-blocking experiments. Persistence of donor cells was assessed by real-time PCR-based chimaerism assay, analysing polymorphisms of HLA-DRB1 or short tandem repeat (STR) as reported (Reed et al, 2002; Suskind et al, 2004). The IL-15 levels in the serum were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). The serum levels of interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-10, IL-6, and IL-4 were determined using a flow cytometric bead array (CBA) (human Th1/Th2 cytokine kit II; BD Biosciences, San Diego, CA, USA).
Mixed lymphocyte cultures (MLR)
PBMNC were collected from patients between day −5 and day +14. Patient cells were used as responders against irradiated donor PBMNC obtained from the aphaeresis product to measure the host response to transfused donor NK cell product in MLR. Irradiated donor stimulators (5 × 104 cells/well) were incubated with limiting diluting numbers of recipient PBMNC in triplicate wells for 72 h followed by addition of 925 kBq 3H-thymidine/well. Results were expressed as mean stimulation index.
Results
Donor/recipient matching
The missing KIR ligand was HLA-C group 2 in seven, HLABw4 in two and HLA-C group 2/HLA-Bw4 in one recipient respectively. All 10 patients and eight of 10 donors expressed at least one HLA-C group 1 allele, whilst two of 10 patients and 10 of 10 donors expressed at least one HLA-C group 2 allele. The fact that only two of the 10 enroled patients expressed a HLA-C group 2 allele is somewhat surprising, but consistent with population studies indicating that the HLA-C group 1 allele is more frequent in European populations (Single et al, 2007).
Clinical outcome
All 10 patients had relapsed MM after single or double auto-PBSCT and had received further salvage therapies prior to enrolment on protocol. An overview of their clinical outcome is presented in Table I. All patients engrafted in a timely fashion after infusion of autologous CD34+ cells. The median times to absolute neutrophil count >0.5 × 109/l and platelet count >50 × 109/l after auto-PBSCT were 12.5 (range: 10–20) and 14.5 d (range: 10–22) respectively. None of the subjects developed GvHD. Five patients achieved a CR or near CR (n-CR) (response rate 50%), whilst one patient each had a partial response (PR) or minimal response (MR). One patient had stable disease (SD), whilst two had progressive disease (PD) after auto-PBSCT. Five patients sustained an early relapse at a median of 75 d (range: 31–133) post-NK cell infusions. Two patients had late relapses at 8.6 and 17.7 months, respectively, and one had SD at 17.7 months. Four of these relapsed patients are currently alive at 1.4, 1.5, 2.3 and 3.0 years post-NK cell therapy with three having received further salvage therapies. We compared the outcome of the present study cohort with 20 patients (2:1 pair-mate analysis) matched for age, sex and cytogenetic abnormalities who received a MEL 200 salvage transplant. The rate of (n)CR was 50% for patients on the present study receiving NK cell therapy and a MEL 140 transplant versus a 40% rate of (n)CR for those receiving a MEL 200 transplant without NK cell therapy (P = 0.32).
Toxicity
A transient, but severe infusion-related acute lung injury event responding to steroids was observed in the first patient treated. This was attributed to a red cell lysis step (Silliman et al, 1998), which was subsequently eliminated from the manufacturing process. In the first four patients the NK cell product was incubated overnight in IL-2 300 IU/ml to allow for endotoxin testing to meet lot release criteria. These patients all had rigors, chills and hypotension requiring vasopressor support. We noted that the NK cell products after overnight incubation contained significant amounts of the pro-inflammatory cytokines IFN-γ and TNF-α (Fig S1), which may have contributed to the hypotensive episodes observed. After validation of in-house endotoxin testing the next six patients were transfused with the NK cell products immediately upon completion of manufacture and only incubated with IL-2 during cell processing. These products contained significantly less pro-inflammatory cytokines. Product infusion was uneventful and without hypotensive episodes. Administration of IL-2 post NK cell infusion was also better tolerated in these patients. Patients 5–10 received 89% of planned IL-2 doses in full, whilst patients 1–4 received 64% of all IL-2 doses. Serial measurement of cytokine levels after NK cell infusion showed peak levels of IFN-γ, IL-10 and IL-6 on 8–9 d post NK cell infusion (Fig S2). There was no elevation in levels of TNF-α and IL-4.
The extent of prior therapy to which this patient cohort had been exposed was reflected by their low CD4+ counts (median: 0.106 × 109/l; range: 0.019–0.522 × 109 /l) prior to conditioning. Only two patients had a CD4+ count >0.140 × 109 /l. All patients were therefore closely monitored for infections, particularly as the delayed auto-PBSCT prolonged the duration of neutropenia. Patient 6 (CD4+ count 0.038 × 109 /l) developed fungal pneumonia during neutropenia despite liposomal amphotericine prophylaxis. This patient also developed disseminated Herpes simplex virus (HSV) infection and influenza virus pneumonia. Patient 8 (CD4+ count 0.038 × 109 /l) developed fungal sinusitis during neutropenia. Both patients recovered fully.
Donor NK cells killed KIR-ligand mismatched primary MM cells and the cell line U266
The composition of the NK cell products is shown in Table II. There was considerable donor-to-donor variation in the absolute number of NK cells collected (median: 12.5 × 106/kg; range: 2.7–92.0), as well as the percentage of allo-reactive NK cells (median: 15.0; range: 0.0–55.4). This resulted in a wide range of the absolute number of allo-reactive NK cells infused (median: 1.7 × 106/kg; range: 0.0–25.5). There was considerably less spread in NK cells not expressing any KIR-receptor (median: 40%; range: 21–65%). The median number of CD3+ T cells infused was low at 5.5 × 104 /kg (range: 0.0–8.0).
Table II.
Composition of NK cell products.
| Transplant pairs | Donor’s KIR genotyping | Donor’s uninhibited receptor | Cell dose × 106 cells/kg)
|
Missing donor KIR ligand in recipient | |||
|---|---|---|---|---|---|---|---|
| MNC | NK cells | Alloreactive NK cells (%) | CD3 | ||||
| 1 | 2DL1, 2DL3, 3DL1 | 3DL1 | 36.8 | 2.7 | 0.3 (11.1) | 0.02 | HLA-Bw4 |
| 2 | 2DL1, 2DL2/3, 3DL1 | 2DL1 | 100.1 | 11.4 | 1.5 (13.1) | 0.05 | HLA-C2 |
| 3 | 2DL1, 2DL3, 3DL1 | 2DL1 | 39.5 | 11.7 | 1.0 (8.5) | 0.06 | HLA-C2 |
| 4 | 2DL1, 2DL3, 3DL1 | 3DL1 | 95.5 | 10.1 | 0.3 (3.0) | 0.06 | HLA-Bw4 |
| 5 | 2DL1, 2DL3, 3DL1 | 2DL1 | 273.2 | 92.0 | 25.5 (28) | 0.08 | HLA-C2 |
| 6 | 2DL1, 2DL2, 3DL1 | 2DL1 | 95.1 | 13.3 | 2.2 (16.9) | 0.08 | HLA-C2 |
| 7 | 2DL1, 2DL2, 3DL1 | 2DL1 | 102.0 | 20.1 | 0.0 (0) | 0.07 | HLA-C2 |
| 8 | 2DL1, 2DL3, 3DL1 | 2DL1 | 150.2 | 37.2 | 20.5 (55.4) | 0.01 | HLA-C2 |
| 9 | 2DL1, 2DL3, 3DL1 | 2DL1 | 173.3 | 26.7 | 4.7 (17.6) | 0.04 | HLA-C2 |
| 10 | 2DL1, 2DL2/3, 3DL1 | 2DL1, 3DL1 | 53.4 | 9.8 | 1.8 (18.3) | 0.00 | HLA-C2 and HLA-Bw4 |
Combined numbers for the two aphaeresis products of each donor are depicted.
NK, natural killer; KIR, killer immunoglobulin-like receptor; MNC, mononuclear cells; D, domains; L, long cytoplasmic tail; HLA, human leucocyte
Standard cytolytic assays were performed with NK cell products against the MM cell line U266, recipient primary MM (when available) to evaluate anti-MM effects mediated by KIR-ligand mismatched NK cells in vitro. Patient PHA-blasts, representing recipient normal tissues, were used as negative control. K562 cells were used as the positive control. Donor NK cells killed K562 and U266 at low E:T ratios (<10:1) but not recipient PHA-blasts (Fig 2). In five cases primary MM cells from the recipients were available for assay. There was no killing of the recipient MM cells by the cells from Donor 7 who lacked any allo-reactive NK cells, based on immunopheno-typing of NK receptors, although the existence of such a population was predicted by HLA-typing. The MM cells of the other four recipients were killed albeit less avidly than the K562 cells and the MM cell lines (median 28%, range 24–37%) (Fig 2). These in vitro data do not predict for in vivo activity, although all patients were given IL-2 to enhance the survival and activity of the transfused donor NK cells. As post-infusion NK cells were not available for analysis we evaluated the cytolytic ability of resting donor NK cells. Resting donor NK cells killed the cell lines K562 and U266, although their activity was somewhat attenuated compared to the IL-2 activated NK cell products (Fig S3).
Fig 2.
All donor NK products killed KIR-ligand mismatched MM cells. Donor NK cells lysed MM cell targets lacking inhibitory KIR-ligands including patient MM cells (when available), with the exception of donor 7, who did not have allo-reactive NK cells. K562, patient MM cells, U266 MM cell line (homozygous C group 1 and HLA-Bw4 negative), and patient PHA blasts were employed as targets in a standard 4-h 51Cr release assay, at E/T ratios of <10:1. Patient primary MM cells were available for patients 2, 3, 6, 7 and 8. Specific lysis percentage was calculated as (test release − spontaneous release)/(maximal release − spontaneous release) × 100. All experiments were performed in triplicate wells and the mean ± SD were presented. One of three independent experiments was shown. All experiments were performed with the final NK cell product, which had been incubated overnight (products 1–4) or during cell processing with 300 IU/ml of IL-2 (products 5–10).
We subsequently evaluated if the interaction between MM cells and NK cells could be modulated to enhance NK cell alloreactivity directed towards primary MM cells. Therefore, recipient MM cells were pre-treated with the HLA class I ab, W6/32, which blocks both the inhibitory ligands HLA-C and HLA-Bw4. Primary MM cells of patients 2, 6, 7 and 8 were tested. Both donor and recipient expressed HLA-C group 1. Furthermore, KIR genotyping and phenotyping showed that donor cells expressed KIR2DL2 and/or 2DL3. We postulated that blocking of HLA class I should therefore release KIR2DL2/3 expressing NK cells from inhibition, thereby increasing the percentage lysis of recipient MM cells. HLA class I blocked recipient MM cells were indeed killed more effectively compared to isotype treated controls (Fig 3). The level of killing achieved was similar to that of K562 and U266 (data not shown), thus demonstrating the potential for increasing NK cell-mediated cytotoxicity by modulating MM/NK cell interactions.
Fig 3.
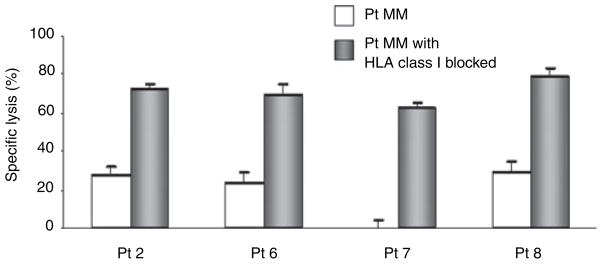
Blocking of HLA class I molecules increases NK cell-mediated lysis of primary MM cells. Anti-human HLA-ABC monoclonal antibody, clone W6/32, (10 μg/ml) was used in the blocking experiments. Control and blocked cells were employed as targets in the cytotoxicity assay, at E/T ratios of <10:1. Specific lysis percentage was expressed as (test release − spontaneous release)/(maximal release − spontaneous release) × 100. All experiments were performed in triplicate wells and the mean ± SD was present. One of three independent experiments was shown. Effectors comprised the corresponding haplo-identical donor’s final NK cell product after IL-2 incubation. Pt, patient.
Donor cells persisted transiently post NK product infusion
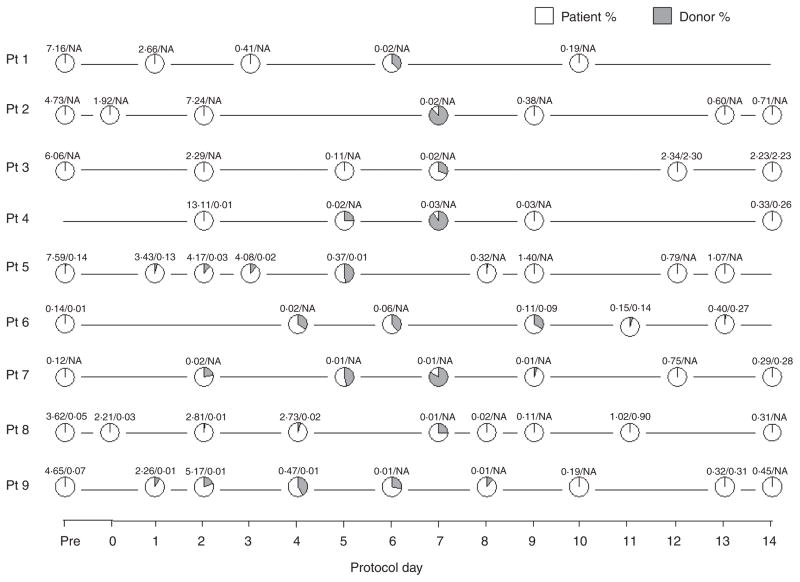
All patients had donor cells detectable in the peripheral blood, which reached its maximum around day 7 (median 41%, range 26–90%), but chimaerism reverted to fully recipient by day 14, indicating disappearance of donor cells (Fig 4). All but one patient had increasing percentages of donor chimaerism in the first 7 d post-NK cell infusions. This suggests that there may have been some expansion of NK cells in vivo, particularly in view of the relatively low number of donor cells transfused. However, we did not formally demonstrate in vivo expansion of NK cells.
Fig 4.
Persistence of donor cells in vivo in patients post NK cell infusion. Persistence of donor cells was assessed by real-time PCR-based chimaerism assay analysing polymorphisms of HLA-DRB1 or short tandem repeat. The total WBC and lymphocyte count (×109/dl) are indicated above each data point. Pt, patient; NA, not available.
In all patients the white blood cell count reached a nadir around day 5–7 after the first NK cell infusion. Lymphocyte recovery started approximately 8 d after NK infusion, which coincided with the disappearance of donor chimaerism. The principal cells circulating between days 8–14 were CD3+/8+ (50–80%) and CD3+/4+ T cells (20–50%). All patients lost donor cell chimaerism between days 8 and 14, which coincided with progressively increasing host T-cell concentrations (data not shown). This raised the question whether there was active immunological rejection of transfused donor NK cells. MLRs in the recipient versus donor rejection with responder cells collected early after NK cell infusion were low. Increasing alloreactivity was observed and potent anti-donor responses were present 8–14 d after NK cell infusion (Fig 5), suggesting that expanding residual recipient T cells had become sensitized to donor allo-antigen. These responses may have been enhanced by IL-2 administration.
Fig 5.
Patient T cells responded to donor cells post NK cell infusion. Mean ± SD of stimulation index (SI) is shown. The data shown are representative of an average of four recipient-donor MLRs analysed. The composition of the responder cells at each day is shown for each time point to demonstrate that the increase in SI reflected an increase in actual T-cell responsiveness over time rather than a change in CD3+ T-cell composition. SI = CPM in cultures with donor NK cell product/CPM in cultures with autologous PHA blasts.
IL-15 levels increase post NK infusion
We next studied whether IL-15 played a role in the transient persistence of donor cells because it is well established that IL-15 is important to NK cell survival and expansion in humans and in mice (Cooper et al, 2002; Prlic et al, 2003; Miller et al, 2005). There was an increase in serum IL-15 concentration in the MM patients post-NK product infusion, which peaked 3–7 d after NK cell infusion and returned to normal after 2 weeks (Fig 6). Elevated levels of IL-15 were not present in healthy donors or in the NK cell products. There was only a modest elevation of IL-15 in control patients treated with standard MEL 200 mg/m2 auto-PBSCT without NK infusions (Fig 6), indicating that MEL conditioning therapy or infusion of autologous cells did not produce a marked surge in IL-15 serum levels. From the present data it cannot be excluded that administration of Fludarabine (Flu) may have contributed to the observed rises in IL-15.
Fig 6.
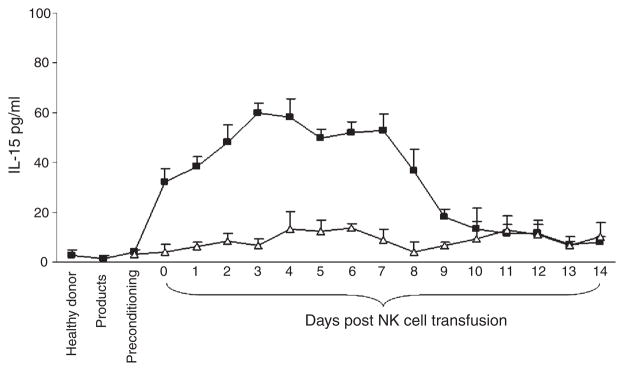
NK cell growth factor IL-15 was detected in vivo post NK cell infusion. Serum levels of IL-15 were measured by ELISA. Black squares represent the mean values ± SD of nine recipient-donor pairs studied. Open triangles represent the mean values ± SD of two patients treated with autologous PBSCT after standard melphalan 200 mg/m2 conditioning therapy.
Discussion
Several investigators have reported that KIR ligand mismatching predicts for donor/recipient allo-reactivity, which reduces leukaemia relapse post-allogeneic PBSCT (Ruggeri et al, 1999, 2002, 2007; Giebel et al, 2003; Morishima et al, 2003; Leung et al, 2004, 2005; Dawson & Spencer, 2005; Elmaagacli et al, 2005; Hsu et al, 2005, 2006; Kroger et al, 2005; Cooley et al, 2007). Others have shown no influence of KIR ligand status, which is probably explained by heterogeneity in transplant procedures, underlying disease and grafts which are either T-replete or vary in extent of T-cell depletion (Davies et al, 2002; Lowe et al, 2003; Bornhauser et al, 2004; Farag et al, 2006; Kroger et al, 2006). The present study evaluated the efficacy of haplo-identical KIR-ligand mismatched NK cell infusion in the setting of an auto-PBSCT for relapsed/refractory MM. The premise was to create a 2-week window for donor-derived NK cells to mediate an anti-MM effect. This was accomplished by preparing patients with MEL, Flu and dexamethasone, followed by a delayed rescue with auto-PBSCT. MEL was incorporated into the conditioning regimen rather than cyclophosphamide (Cy), because of its superior anti-MM effect, while its immunosuppressive effect was surmised to be comparable to that of Cy.
It is difficult to ascertain the relative contribution of adoptively transferred NK cells and the preparative regimen, MEL (140 mg/m2) to the clinical outcome in a cohort of heavily pre-treated patients. Five patients relapsed early, between 31 and 133 d after NK cell infusion, and two patients had PD. Two patients relapsed at 259 and 532 d and are alive after further salvage therapy. One patient is alive with stable disease. However, it should be pointed out that all patients had relapsed after single or tandem auto-PBSCT with a MEL 200 mg/m2-based or similar intensity conditioning regimen. We are encouraged by the high CR and n-CR rate (50%). Using a pair-mate analysis we observed (n)CR rates, which were at least equivalent to similar patients receiving a salvage auto-PBSCT with MEL 200 mg/m2 (Tricot et al, 1996b).
The haplo-identical NK cell infusions were safe and did not impair engraftment or cause GvHD. The method of preparation of NK cells was of importance with regards to minimizing side effects. Overnight incubation of the product with IL-2 added substantially to toxicity, including hypotension, whilst incubation with IL-2 during cell processing abrogated these effects. NK cell products incubated with IL-2 during cell processing, instead of overnight, contained significantly lower cytokine levels. Incubation with IL-2 during cell processing also allowed for delivery of a substantial larger number of s.c. IL-2 doses to the patients, which were intended to enhance survival of the NK cells.
All donors were predicted to have NK cell populations exhibiting KIR receptors, which were not inhibited by recipient HLA (‘missing self’). NK cell product analysis showed that the number of allo-reactive NK cells was substantially lower compared to total NK cell content and a large variability between donors was observed. Approximately 40% of NK cells were KIR−. KIR− populations probably represent NKG2A+/KIR− NK cells or developmentally immature NKG2A−/KIR− cells (Cooley et al, 2007; Ruggeri et al, 2007). Further, other investigators have shown that the KIR genotype does not directly correlate with KIR phenotype due to allelic polymorphisms and epigenetic silencing (Gardiner et al, 2001; Santourlidis et al, 2002; Shilling et al, 2002; Chan et al, 2003; Goodridge et al, 2003; Kikuchi-Maki et al, 2003; Pando et al, 2003; Leung et al, 2005). This produced a broad range in the percentage of NK cells expressing genomically encoded KIR receptors. In the present study, one KIR3DL1 positive donor (Donor 7) lacked any allo-reactive NK cells highlighting the need for KIR phenotyping. KIR phenotyping is not only useful for donor selection, but also allows for estimating the size of the allo-reactive NK cell population, which has been correlated with the degree of cytotoxicity exhibited (Pende et al, 2005). All NK cell products killed effectively K562 and the MM cell line U266, whilst recipient PHA-blasts, a proxy target representing normal cells, were not killed. Primary MM cells were lysed in all instances with the exception of the donor who did not have allo-reactive NK cells. As all the NK cell products had been activated in vitro with IL-2 during processing we also demonstrated that resting donor NK cells, not activated with IL-2, were cytolytic. However, it was not certain whether the cytotoxicity observed towards myeloma targets in vitro was retained in vivo after infusion of the NK cell product.
Chimaerism data indicated that donor cells persisted transiently and mostly disappeared by day 9–14. We observed an early surge of recipient T cells, which responded to donor cells in MLR, suggesting that there may have been active rejection mediated by persistent recipient T cells, implying that the preparative regimen may have been insufficiently immunosuppressive. It is unclear whether the drop in IL-15 serum levels or the discontinuation of exogenous IL-2 by day +11 might have contributed to the disappearance of the NK cells, although that seems less likely. Miller et al (2005) reported that the combination of low dose Cy and methylprednisone or Flu alone were insufficient to prevent immunologic rejection of transfused haplo-identical NK cells. In vivo persistence and clinical responses were only observed after preparing patients with high dose Cy and Flu (Miller et al, 2005). A similar lymphocyte-depleting chemotherapy also appeared to be crucial for the repopulation of melanoma patients with autologous ex-vivo expanded tumour infiltrating lymphocytes (Rosenberg & Dudley, 2004; Dudley et al, 2005). Preparation of patients with Cy/Flu may therefore be preferable to MEL/Flu, despite the superior anti-MM activity of MEL. It may also abrogate the need for auto-PBSCT.
IL-15 is the primary survival and growth factor for NK cells during NK lymphopoiesis (Carson et al, 1997; Huntington et al, 2007). We observed an increase in IL-15 levels post-NK cell infusion similar to that reported by Miller et al (2005) after high dose Cy/Flu. Interestingly, IL-15 was not elevated immediately after conditioning with MEL/Flu nor was it present in the NK cell product. Furthermore, we did not observe a significant increase in IL-15 post standard auto-PBSCT with MEL 200 mg/m2 indicating that high dose cytotoxic therapy or auto-PBSCT does not in itself induce endogenous production of IL-15. Taken together, these findings suggest that the transfused NK cell products elicited endogenous IL-15 secretion by an as yet undefined mechanism.
The present study points the way for future protocols to enhance the efficacy of this approach. First, preparative therapy with Cy/Flu may provide more potent immunosuppression and allow for longer persistence and expansion of NK cells. Second, the number of allo-reactive NK cells was too low to eradicate all MM cells, taking into account that MM patients, even when in remission, may harbor tumour burdens as large as 109 MM cells (Tricot et al, 1998). In this context, it is important to point out that patients receiving haplo-identical transplants for AML have considerably better outcome when transplanted in remission rather than frank relapse, suggesting that allo-reactive NK cells cannot overcome very large tumour burdens (Ruggeri et al, 2007). Several investigators have reported methods to expand NK cells in vitro, which may help to overcome a potentially large tumour mass (Shi et al, 2005; Berg et al, 2007; Fujisaki et al, 2007; Xing et al, 2007; Alici et al, 2008). Third, we demonstrated that blocking of HLA class I can restore potent MM cell killing to levels comparable to the NK cell sensitive target K562 by recruiting non-alloreactive NK cell populations. We have recently demonstrated that bortezomib can downregulate HLA class I and potentiate NK cell-mediated killing of MM cells (Shi et al, 2008). Blocking of KIR receptors with anti-KIR ab is a promising alternative (Wagtmann et al, 2007). Lastly, recent studies in our laboratory have demonstrated that CS1 ab enhances antibody-dependent lysis of MM cells both in vitro and in mice (Szmania et al, 2006; Tai et al, 2007). Insights into the control of NK cell function may usher in a new era of NK cell therapy and may offer the prospect of developing effective NK cell therapy for myeloma and other malignancies.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institutes of Health (PO1 grant CA55819).
Footnotes
Authorship
Conceptualized work and supervised the research: FvR, JS, GT; wrote paper: JS, GT, FvR; performed research: JS, SS, NR, TKG, PAM, AM, BD, KCH, LBL, MF, JDS; enroled and treated patients on research protocols: BB, GT, FvR.
Additional supporting information may be found in the online version of this article.
Fig S1. NK cell products incubated overnight with IL-2 (products 1–4) contained significantly more IFN-γ, TNF-α and IL-6 when compared to NK cell products incubated with IL-2 only during cell processing (products 5–10). Four products were analyzed from patients 1–4 and seven products were analyzed from patients 5–10. Mean ± SD of products tested are shown. Cytokine levels were measured by flow cytometric bead array.
Fig S2. Serum cytokine levels after NK cell infusions show maximum elevation of IFN-γ, IL-10 and IL-6. There was no elevation in levels of TNF-α and IL-4. Each point represents the mean ± SD of serum samples from nine treated patients. Cytokine levels were tested by flow cytometric bead array.
Fig S3. Comparison of the cytolytic activity of resting donor NK cells and the IL-2 activated NK cell product using a standard 4-h 51Cr release assay. Resting NK cells were obtained from donor’s leucaphaeresis product. Cytotoxicity of resting NK cells and the final, IL-2 activated, NK cell product was tested against NK-cell sensitive cell line K562 and U266. Each data point represents percentage killing of the mean ± SD form two donors.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren HG, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–3162. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. Single versus double autologous stem-cell transplantation for multiple myeloma. New England Journal of Medicine. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. New England Journal of Medicine. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, Aloisi T, Saab JP, Santucci A, Perruccio K, Martelli MP, Mecucci C, Reisner Y, Martelli MF. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. Journal of Clinical Oncology. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, Fassas A, Zangari M, Hollmig K, Pineda-Roman M, Lee C, Talamo G, Thertulien R, Kiwan E, Krishna S, Fox M, Crowley J. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. New England Journal of Medicine. 2006a;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, Shaughnessy JD, Jagannath S, Bolejack V, Gurley J, Hoering A, Vesole D, Desikan R, Siegel D, Mehta J, Singhal S, Munshi NC, Dhodapkar M, Jenkins B, Attal M, Harousseau JL, Crowley J. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. British Journal of Haematology. 2006b;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- Berg M, Lundqvist A, Fan Y, McCoy J, Yokoyama H, Childs R. In vitro-expanded NK cells have increased TRAIL and NKG2D expression and enhanced TRAIL-mediated tumor cytotoxicity compared to non-expanded NK cells. Blood. 2007;110:2744a. [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British Journal of Haematology. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103:2860–2861. doi: 10.1182/blood-2003-11-3893. author reply 2862. [DOI] [PubMed] [Google Scholar]
- Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. The Journal of Clinical Investigation. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HW, Kurago ZB, Stewart CA, Wilson MJ, Martin MP, Mace BE, Carrington M, Trowsdale J, Lutz CT. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. The Journal of Experimental Medicine. 2003;197:245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. New England Journal of Medicine. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, McQueen KL, Guethlein LA, Parham P, Miller JS. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, Velardi A, Blazar BR. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Spencer A. Successful use of haploidentical stem-cell transplantation with KIR mismatch as initial therapy for poor-risk myelodysplastic syndrome. Journal of Clinical Oncology. 2005;23:4473–4474. doi: 10.1200/JCO.2005.01.3581. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of Clinical Oncology. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli AH, Ottinger H, Koldehoff M, Peceny R, Steckel NK, Trenschel R, Biersack H, Grosse-Wilde H, Beelen DW. Reduced risk for molecular disease in patients with chronic myeloid leukemia after transplantation from a KIR-mismatched donor. Transplantation. 2005;79:1741–1747. doi: 10.1097/01.tp.0000164500.16052.3c. [DOI] [PubMed] [Google Scholar]
- Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, Boudreau C, Nelson G, Oudshoorn M, van Rood J, Velardi A, Maiers M, Setterholm M, Confer D, Posch PE, Anasetti C, Kamani N, Miller JS, Weisdorf D, Davies SM. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biology of Blood and Marrow Transplantation. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Frohn C, Hoppner M, Schlenke P, Kirchner H, Koritke P, Luhm J. Anti-myeloma activity of natural killer lymphocytes. British Journal of Haematology. 2002;119:660–664. doi: 10.1046/j.1365-2141.2002.03879.x. [DOI] [PubMed] [Google Scholar]
- Fujisaki H, Kakuda H, Lockey T, Eldridge P, Leung W, Campana D. Expanded natural killer cells for cellular therapy of acute myeloid leukemia. Blood. 2007;110:2743a. [Google Scholar]
- Gardiner CM, Guethlein LA, Shilling HG, Pando M, Carr WH, Rajalingam R, Vilches C, Parham P. Different NK cell surface phenotypes defined by the DX9 antibody are due to KIR3DL1 gene polymorphism. Journal of Immunology. 2001;166:2992–3001. doi: 10.4049/jimmunol.166.5.2992. [DOI] [PubMed] [Google Scholar]
- Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, Maccario R, Bonetti F, Wojnar J, Martinetti M, Frassoni F, Giorgiani G, Bacigalupo A, Holowiecki J. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Witt CS, Christiansen FT, Warren HS. KIR2DL4 (CD158d) genotype influences expression and function in NK cells. Journal of Immunology. 2003;171:1768–1774. doi: 10.4049/jimmunol.171.4.1768. [DOI] [PubMed] [Google Scholar]
- Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. Journal of Immunology. 2002;169:5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O’Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KC, Gooley T, Malkki M, Pinto-Agnello C, Dupont B, Bignon JD, Bornhauser M, Christiansen F, Gratwohl A, Morishima Y, Oudshoorn M, Ringden O, van Rood JJ, Petersdorf E. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biology of Blood and Marrow Transplantation. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, Motoyama N, Huang DC, Nutt SL, Tarlinton DM, Strasser A. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nature Immunology. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Maki A, Yusa S, Catina TL, Campbell KS. KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. Journal of Immunology. 2003;171:3415–3425. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A, Nagler A, Binder T, Eiermann T, Madrigal A, Schwerdtfeger R, Kiehl M, Sayer HG, Beyer J, Bornhauser M, Ayuk F, Zander AR, Marks DI. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. British Journal of Haematology. 2005;129:631–643. doi: 10.1111/j.1365-2141.2005.05513.x. [DOI] [PubMed] [Google Scholar]
- Kroger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, Ayuk F, Dahlke J, Eiermann T, Zander A. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82:1024–1030. doi: 10.1097/01.tp.0000235859.24513.43. [DOI] [PubMed] [Google Scholar]
- Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. Journal of Immunology. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- Leung W, Iyengar R, Triplett B, Turner V, Behm FG, Holladay MS, Houston J, Handgretinger R. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. Journal of Immunology. 2005;174:6540–6545. doi: 10.4049/jimmunol.174.10.6540. [DOI] [PubMed] [Google Scholar]
- Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, Donk NW, Verdonck LF. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. Journal of Clinical Oncology. 2000;18:3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- Lowe EJ, Turner V, Handgretinger R, Horwitz EM, Benaim E, Hale GA, Woodard P, Leung W. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. British Journal of Haematology. 2003;123:323–326. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Yabe T, Inoko H. Clinical significance of killer Ig-like receptor (KIR) on acute GvHD, rejection and leukemia relapse in patients transplanted non-T cell depleted marrow from unrelated donors; role of inhibitory KIR epitope matching and activating KIR genotype. Blood. 2003;102:526a. [Google Scholar]
- Pando MJ, Gardiner CM, Gleimer M, McQueen KL, Parham P. The protein made from a common allele of KIR3DL1 (3DL1*004) is poorly expressed at cell surfaces due to substitution at positions 86 in Ig domain 0 and 182 in Ig domain 1. Journal of Immunology. 2003;171:6640–6649. doi: 10.4049/jimmunol.171.12.6640. [DOI] [PubMed] [Google Scholar]
- Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nature Reviews Immunology. 2003;3:108–122. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. The Journal of Experimental Medicine. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR, Geyer S, Iturria N, Fonseca R, Lust JA, Kyle RA, Witzig TE. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. Journal of Clinical Oncology. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Gertz MA. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W, Kong DZ, Lee TH, Cowan MJ, Busch MP, Baxter-Lowe LA. Non-invasive determination of the paternal HLA haplotype of a fetus using kinetic PCR to detect fetal microchimerism in maternal plasma. Bone Marrow Transplantation. 2002;29:527–529. doi: 10.1038/sj.bmt.1703411. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, Topini F, Bianchi E, Aversa F, Martelli MF, Velardi A. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, Nevill T, Marcellus D, Parker P, Johnson M, Kirk A, Porter D, Giralt S, Levine JE, Drobyski W, Barrett AJ, Horowitz M, Collins RH. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplantation. 2000;26:1179–1184. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, Uhrberg M. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. Journal of Immunology. 2002;169:4253–4261. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Shi J, Tricot G, Szmania S, Camet C, Rosen N, Cottler-Fox M, Barlogie B, Campana D, van Rhee F. Stimulation with K562 cells transfected with 4-1BBL and IL-15 expands and activates natural killer (NK) cells with specific cytotoxicity for multiple myeloma (MM) Blood. 2005;106:3392a. [Google Scholar]
- Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, Storrie B, Mulder A, Shaughnessy JD, Jr, Barlogie B, van Rhee F. Bortezomib down-regulates the cell surface expression of HLA-class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling HG, Guethlein LA, Cheng NW, Gardiner CM, Rodriguez R, Tyan D, Parham P. Allelic polymorphism synergizes with variable gene content to individualize human KIR genotype. Journal of Immunology. 2002;168:2307–2315. doi: 10.4049/jimmunol.168.5.2307. [DOI] [PubMed] [Google Scholar]
- Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. The Journal of Clinical Investigation. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. Global diversity and evidence for coevolution of KIR and HLA. Nature Genetics. 2007;39:1114–1119. doi: 10.1038/ng2077. [DOI] [PubMed] [Google Scholar]
- Suskind DL, Rosenthal P, Heyman MB, Kong D, Magrane G, Baxter-Lowe LA, Muench MO. Maternal microchimerism in the livers of patients with biliary atresia. BMC Gastroenterology. 2004;4:14. doi: 10.1186/1471-230X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmania S, Balasa B, Malaviarachchi P, Zhan F, Huang Y, Draksharapu A, Vexler V, Shaughnessy J, Barlogie B, Tricot G, Afar D, van Rhee F. CS1 is expressed on myeloma cells from early stage, late stage, and drug-treated multiple myeloma patients, and is selectively targeted by the huluc63 antibody. Blood. 2006;108:660a. [Google Scholar]
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weler E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2007 doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996a;87:1196–1198. [PubMed] [Google Scholar]
- Tricot G, Jagannath S, Vesole DH, Bracy D, Desikan KR, Siegel D, Barlogie B. Hematopoietic stem cell transplants for multiple myeloma. Leukemia & Lymphoma. 1996b;22:25–36. doi: 10.3109/10428199609051725. [DOI] [PubMed] [Google Scholar]
- Tricot G, Gazitt Y, Leemhuis T, Jagannath S, Desikan KR, Siegel D, Fassas A, Tindle S, Nelson J, Juttner C, Tsukamoto A, Hallagan J, Atkinson K, Reading C, Hoffman R, Barlogie B. Collection, tumor contamination, and engraftment kinetics of highly purified hematopoietic progenitor cells to support high dose therapy in multiple myeloma. Blood. 1998;91:4489–4495. [PubMed] [Google Scholar]
- Wagtmann N, Andre P, Zahn S, Spee P, Anfossi N, Gauthier L, Blaser B, Caligiuri M, Capanni M, Ruggeri L, Velardi A, Romagne F. Anti-KIR (1-7F9): a fully human monoclonal antibody (mAb) that blocks KIR2DL1, -2 and -3, promoting natural killer (NK) cell-mediated lysis of tumor cells in vitro and in vivo. Blood. 2007;110:582a. [Google Scholar]
- Xing D, Fang W, Decker W, Li S, Robinson S, Yang H, Steiner D, Thomas M, Champlin R, McMannis J, Shpall E, Zweidler-Mckay P. Ex vivo expansion of cord blood NK cell have in vivo efficacy against leukemia. Blood. 2007;110:2741a. [Google Scholar]
- Zeiser R, Bertz H, Spyridonidis A, Houet L, Finke J. Donor lymphocyte infusions for multiple myeloma: clinical results and novel perspectives. Bone Marrow Transplantation. 2004;34:923–928. doi: 10.1038/sj.bmt.1704670. [DOI] [PubMed] [Google Scholar]
- Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD., Jr The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.