Abstract
Circulating cortisol and psychosocial stress may contribute to the pathogenesis of obesity and metabolic syndrome. To evaluate these relationships, we performed a cross-sectional study of 369 overweight and obese subjects and 60 healthy volunteers and reviewed the previous literature.
Overweight and obese subjects had at least two other features of Cushing’s syndrome. They underwent measurements representing cortisol dynamics (24h urine cortisol excretion (UFC), bedtime salivary cortisol, 1 mg dexamethasone suppression test) and metabolic parameters (BMI, blood pressure (BP); fasting serum triglycerides, HDL, insulin, and glucose). Subjects also completed the Perceived Stress Scale (PSS). UFC, salivary cortisol and weight from 60 healthy volunteers were analyzed.
No subject had Cushing’s syndrome. UFC and dexamethasone responses were not associated with BMI or weight. However, salivary cortisol showed a trend to increase as BMI increased (P< 0.0001), and correlated with waist circumference (WC) in men (rs=0.28, P=0.02) and systolic BP in women (rs=0.24, P =0.0008). Post-dexamethasone cortisol levels were weak to moderately correlated with fasting insulin (rs=− 0.31, P=0.01) and HOMA-IR (rs=−0.31, P=0.01) in men and systolic (rs=0.18, P= 0.02) and diastolic BP (rs=0.20, P=0.009) in women. PSS results were higher in obese subjects than controls, but were not associated with cortisol or metabolic parameters. As expected, WC correlated with fasting insulin, HOMA-IR and systolic BP (adjusted for BMI and gender; P < 0.01). Literature showed inconsistent relationships between cortisol and metabolic parameters.
Taken together, these data do not support a strong relationship between systemic cortisol or stress and obesity or metabolic syndrome.
Keywords: cortisol, metabolic syndrome, insulin resistance, obesity
Introduction
The current “epidemic” of overweight and obesity in the United States is associated with a high prevalence of co-morbidities characterized as metabolic syndrome (MS), including hypertension, diabetes, and dyslipidemia. This may be why obesity confers a higher risk of death from cardiovascular disease, diabetes, and some cancers (1). Thus, insight into factors that cause and/or maintain obesity and the metabolic syndrome have important therapeutic implications.
Like obesity, Cushing’s syndrome is associated with components of the metabolic syndrome as well as increased mortality from cardiovascular disease (2). Hypercortisolism is the pathophysiologic underpinning of Cushing’s syndrome. The similarity of co-morbidities raises the question of whether cortisol plays a role in the development and maintenance of obesity and the metabolic syndrome.
Some previous reports suggested a causal relationship between cortisol and obesity or cortisol and metabolic syndrome. In 87 obese women, 24 h urine cortisol excretion correlated significantly with abdominal diameter and abdominal obesity (3). Also, cortisol responses to ACTH and corticotropin-releasing factor were increased in 16 obese women with abdominal compared to peripheral fat distribution (4). Two studies identified a significant relationship between 0900h fasting plasma cortisol and components of the metabolic syndrome (5, 6). In addition to direct relationships between cortisol, obesity and metabolic syndrome, evidence also suggests that perceived stress may exert adverse metabolic effects through cortisol (7, 8). These putative relationships have reached the lay press (9), which has deemed cortisol “the real culprit” for obesity, and underlie sales of weight loss products that claim to block cortisol action (10).
To further examine the role of cortisol in obesity and metabolic syndrome, we investigated associations between various measures of systemic hypercortisolism and weight, metabolic syndrome, and psychosocial stress in an obese population. To extend this analysis to lower BMI ranges we added data from healthy volunteers to evaluate relationships between urine and salivary cortisol and weight. We reviewed the available literature on this topic.
Methods
The NICHD Institutional Review Board approved both studies and all subjects provided written informed consent.
Overweight and Obese Subjects
The primary outcome measure of the study (NCT00361777), the diagnostic accuracy of screening tests for Cushing’s syndrome, has been published (11). This report is a prospectively planned secondary analysis.
From October 2003 to April 2008, obese and overweight individuals presenting for weight loss treatment at The George Washington University Weight Management Program were invited to participate in this study. Inclusion criteria were age 18 to 75 years, weight gain and the presence of at least two other features of Cushing’s syndrome from the following list: impaired short term memory, lethargy, osteopenia or recent fracture, recent onset or difficult to control hypertension, plethora, hirsutism, eccymoses, weakness, edema, female balding, decreased libido, irritability, decreased concentration, changes in appetite, menstrual changes, headache, glucose intolerance, recurrent infections, striae wider than 1 cm and purple in color, abnormal fat distribution, thin skin, and acne (11). Exclusion criteria included weight > 350 lbs (159 kg); serum creatinine > 2.6 mg/dl; pregnancy; serious medical conditions that might alter pituitary-adrenal function; and recent or anticipated use of medications affecting glucocorticoid physiology, including glucocorticoids, black licorice, chewing tobacco, phenytoin, barbiturates, loperamide, and opiates.
At the first visit, staff reviewed the medical history and performed a physical examination including measurements of weight, height, and waist circumference, systolic and diastolic blood pressure (SBP, DBP). Fasting blood sugar, insulin, triglyceride, and HDL levels were measured. Subjects without known diabetes underwent an oral glucose tolerance test.
Each subject underwent at least 2 screening tests for Cushing’s syndrome: 1) 1 mg overnight dexamethasone suppression test (DST) with measurement of serum dexamethasone (radioimmunoassay [RIA] October 2003 – January 2005; high pressure liquid chromatography and isotope dilution mass spectrometry January 2005 onwards; R2 = 0.972; Esoterix Laboratories [ETX], Calabasas Hills, CA) and cortisol (ETX, RIA October 2003–July 2004; liquid chromatography tandem mass spectrometry [LC-MS/MS] July 2004 onwards; R2 = 0.985); the reported lower limit of detection in both assays was 1.0 ug/dl [27.6 nmol/l]); 2) measurements of 24-hour urine creatinine and cortisol excretion (UFC) (tandem mass spectrometry, [LC-MS/MS], Mayo Laboratories, [Mayo] Rochester, MN); or 3) measurement of bedtime salivary cortisol (LC-MS/MS and/or RIA), as described previously (11). Biochemical testing and the Perceived Stress Scale (PSS) questionnaire were completed before beginning the weight loss program, usually within two weeks after the first visit (12).
Healthy Volunteers
To evaluate cortisol relationships over a larger BMI range, we included data from a separate study (NCT00156767) performed at the NIH Clinical Center. In that study, healthy volunteers provided urine and saliva collections for measurement of daily cortisol excretion (chemiluminescence immunometric assay, Nichols Advantage one site, NIH, earlier in the study; and LC-MS/MS, Mayo later in the study) and bedtime salivary cortisol levels (LC-MS/MS, Mayo). Inclusion and exclusion criteria and subject demographics have been described previously (13). These subjects did not complete the PSS or the 1 mg DST.
Literature Review
To evaluate extant data regarding the relationship(s) of basal urine or salivary cortisol levels and dexamethasone suppression testing, and measures of obesity and/or metabolic syndrome in adults, the following text words were searched in MEDLINE: cortisol, hypothalamic-pituitary-adrenal axis, metabolic syndrome, obesity, weight, BMI, urine free cortisol, salivary cortisol, and 1mg dexamethasone suppression test. Articles reporting on measures of 17-keto- or 17-hydroxy steroids were excluded, thus, setting the time frame for this search to articles published after 1980. Only English language reports were reviewed. We did not analyze results of cortisol metabolites or dynamic testing responses of the adrenal axis.
Analysis
Simple descriptive statistics and frequency distributions were used to characterize subject clinical presentation and demographics of the subjects. Data are reported as mean ± SD for demographics and comparison to normative data, but otherwise are median and inter-quartile range (IQR; 25th percentile, 75th percentile). Subjects who completed only one screening test were excluded from analysis (n=6). Women known to be taking estrogenic preparations (n=50) and those without data regarding estrogen use (n=41) were excluded from analyses of post-dexamethasone cortisol levels.
Subjects were considered to have a diagnosis of diabetes if they were taking a glucose-lowering medication, had a fasting blood glucose ≥126 mg/dl [7.0 mmol/l] or a 2-h post glucose ≥ 200 mg/dl [11.1 mmol/l]). Those with impaired fasting glucose (fasting blood glucose ≥ 100 mg/dl [5.6 mmol/l]; n=65) or impaired glucose tolerance (blood glucose of 140–199 mg/dl [7.8–11.0 mmol/l]; n=24) (14) were excluded from analyses involving diabetes. Insulin resistance was evaluated by HOMA-IR. Subjects were considered to have hypertension if they were taking a medication to lower blood pressure or if SBP was ≥140 mmHg and/or DBP was ≥ 90 mmHg.
Subjects were classified as having metabolic syndrome (MS) if they had 3 of the 5 risk factors listed in the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP III) report (15). In a separate analysis, subjects were classified as having metabolic syndrome (MSRx) if they met the aforementioned criteria and/or received medical treatment for diabetes, hypertension, or elevated triglycerides (Table 1).
Table 1.
Modified ATP III criteria for metabolic syndrome
| Risk Factor | Threshold criterion |
|---|---|
|
| |
| Waist circumference, Women | > 35 inches (89 cm) |
| Waist circumference, Men | > 40 inches (102 cm) |
| Triglycerides | ≥ 150 mg/dl (1.7 mmol/l) OR < 150 mg/dl + MED* |
| HDL cholesterol, Women | < 50 mg/dl (1.29 mmol/l) |
| HDL cholesterol, Men | < 40 mg/dl (1.03 mmol/l) |
| Systolic Blood Pressure | ≥ 130 (mmHg) OR < 130 + MED* |
| Diastolic Blood Pressure | ≥ 85 (mmHg) OR < 85 + MED* |
| Fasting Glucose | ≥ 100 mg/dl (5.6 mmol/l) OR < 100 + MED* |
MED: triglyceride, blood pressure, or glucose lowering medications
Cortisol parameters were considered abnormal if they exceeded the laboratory’s established normal range or published guidelines: UFC > 45 ug/24 hour (124 nmol/24 hour); salivary cortisol > 170 ng/dl (4.7 nmol/l) (RIA) or > 100 ng/dl (2.8 nmol/l) (LC-MS/MS); or post-DST cortisol ≥ 1.8 ug/dl (50 nmol/l) (16).
Comparisons were made between cortisol abnormalities, MS, MSRx, diabetes, hypertension, and perceived stress. Categorical data and prevalence were compared using Fisher’s exact tests, and continuous data were compared by non-parametric (Wilcoxon rank sum) tests. Correlation analyses utilized Pearson product-moment or Spearman rank correlation coefficients, as appropriate. Multiple regression analyses adjusted for BMI and sex as necessary. The Abelson-Tukey ANOVA test was used to evaluate trend.
Study subjects’ PSS scores were compared to published normative data using t-tests. The PSS score ranges from 0 to 40 points (13). Higher scores indicate increased stress. Normative data are available by gender and age. Mean PSS score for women is 13.7± 6.6 and for males is 12.1±5.9. Mean PSS scores for ages 18–29 y is 14.2±6.2; 30–44 y, 13.0±6.2; 45–54 y, 12.6±6.1; 55–64 y, 11.9±6.9, and 65 years and older, 12.0±6.3.
BMI, weight, salivary cortisol and 24 h UFC results of healthy volunteers were combined with those of the overweight and obese group for analyses involving cortisol and weight parameters. Analyses involving metabolic syndrome and/or its individual features did not include this group.
In healthy volunteers, UFC results measured by the chemiluminescence immunometric assay at the NIH were converted to values representative of the LC-MS/MS assay using the equation: 0.218 x NIH value + 6.105, as determined by the Department of Laboratory Medicine, NIH. LC-MS/MS values, actual or converted, were used for analyses.
Missing data were due to laboratory error, inadequate specimen, subjects not providing specimens, or unavailable vital signs. Data were analyzed using SAS software (v. 9.2, SAS Institute, Inc, Cary, NC).
Results
Demographics: Overweight and Obese Subjects
Three hundred sixty-nine subjects (72.4% women; 82.7% Caucasian) completed at least two screening tests (UFC, n=348; salivary cortisol by RIA, n=232; salivary cortisol by LC-MS/MS, n=269; DST, n=270) and were eligible for analysis. Men and women had similar age (50 ± 12 vs 48 ± 12 y), but not weight (128 ± 29 vs 102 ± 20 kg), BMI (41 ± 9 vs 38 ± 7 kg/M2) or waist circumference (125 ± 16 vs 105 ± 15 cm) (all P<0.01). The BMI distribution was: 25–29.9 kg/M2, 11%; 30–34.9 kg/M2, 26%; 35–39.9 kg/M2, 25%; 40–83 kg/M2, 38%. The frequency distribution of MSRx, MS, diabetes, and hypertension were: 186/348 (53.4%), 159/345 (46.1%), 72/362 (19.9%), and 170/357 (47.6%), respectively.
The most common clinical features reported by subjects were: lethargy, irritability, cognitive impairment and hypertension. The least common clinical features reported were: change in appetite, weakness, skin problems, recurrent infections and osteopenia (11).
Demographics: Healthy Volunteers
Sixty healthy volunteers were enrolled into the study (50% women; 68% Caucasian) from three age groups (< 40, 40–55, > 55 years, evenly divided). Fifty-six subjects provided 24 h UFC samples and 57 provided salivary samples. Men and women had similar ages (46 ± 15 vs 45 ± 15 years) and BMI (27 ± 5 vs 25 ± 5 kg/M2) but not weight (87 ± 11 vs 67 ± 14 kg). The BMI distribution in this group was: 18.6–24.9 kg/M2,, 33%; 25–29.9 kg/M2, 50%; 30–34.9 kg/M2, 15%; 35–35.3 kg/M2, 2%; > 35.4 kg/M2, 0%.
Abnormal cortisol test results
63 subjects in the overweight and obese group had one abnormal result and 21 had two or more abnormalities. After further evaluation, none was diagnosed with Cushing’s syndrome (11). Two subjects in the healthy volunteer group had an abnormal salivary cortisol level (402 ng/dl and 300 ng/dl, respectively; ≥ 100 ng/dl, abnormal), but their 24 h UFC values were normal. Repeat salivary cortisol level in the subject with an initial value of 402 ng/dl was 114 ng/dl, still slightly abnormal. The second patient was lost to follow-up. On history and physical exam, neither patient had signs or symptoms of Cushing’s syndrome. Median 24 h UFC and salivary cortisol values of the combined healthy volunteer and overweight and obese group are provided in Table 2.
Table 2.
24 h UFC (ug/24 h) and salivary cortisol (ng/dl) values of healthy volunteers and overweight and obese study subjects
| BMI (kg/M2) | Test | N | Median (IQR) |
|---|---|---|---|
|
| |||
| 18.6–24.9 | salivary cortisol | 18 | 26 (20, 58) |
| 24 h UFC | 19 | 17 (14, 22) | |
|
| |||
| 25–29.9 | salivary cortisol | 56 | 20 (12, 44) |
| 24 h UFC | 60 | 16 (12, 24) | |
|
| |||
| 30–34.9 | salivary cortisol | 78 | 26 (15, 50) |
| 24 h UFC | 103 | 20 (12, 27) | |
|
| |||
| 35–83 | salivary cortisol | 174 | 24 (15, 51) |
| 24 h UFC | 222 | 18 (12, 27) | |
To convert UFC ug/24 h to nmol/24 h multiply by 2.76; to convert salivary cortisol ng/dl to nmol/l multiply by .0276.
Association of cortisol levels with BMI
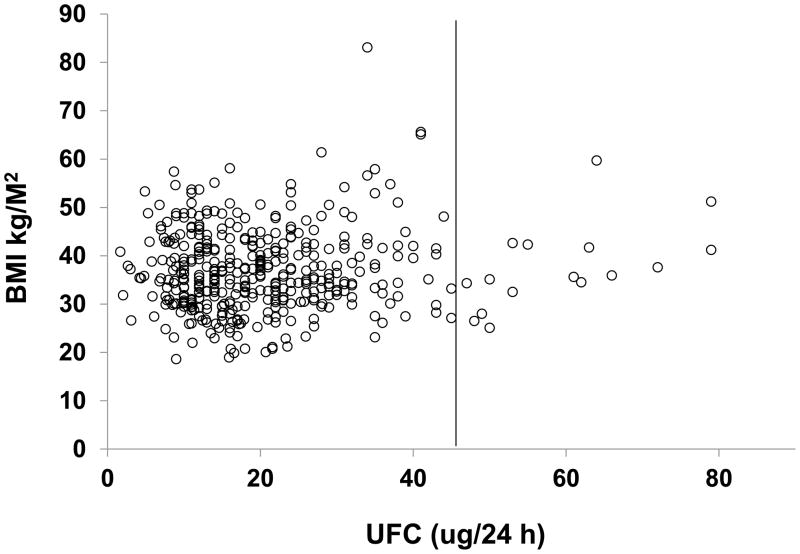
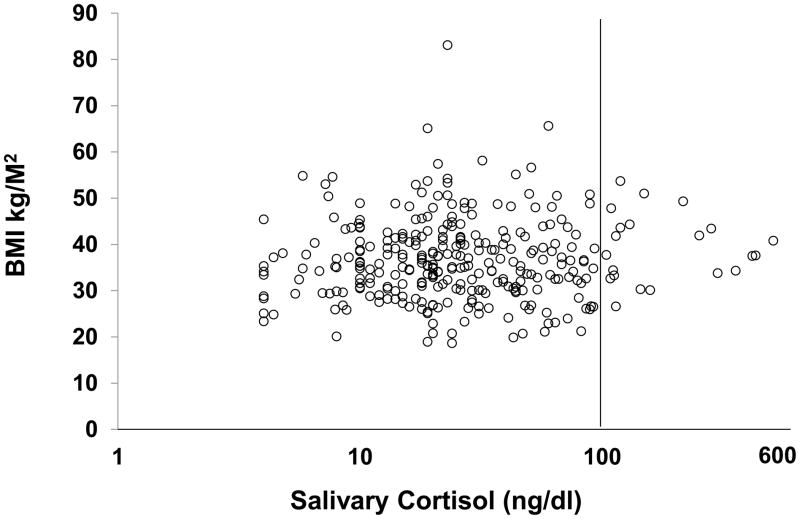
We found no correlation between BMI or weight and any cortisol parameter in the overweight and obese study population (Figures 1, 2; Table 3).
Figure 1.
24 h urine free cortisol (UFC) versus BMI in individual subjects (healthy volunteers and overweight and obese study group). The solid line represents the upper reference limit.
Figure 2.
Salivary cortisol (log scale) versus BMI in individual subjects (healthy volunteers and overweight and obese study group). The solid line represents the upper reference limit.
Table 3.
Review of published studies evaluating urinary, salivary, post-dexamethasone, and plasma cortisol levels against measures of obesity and metabolic syndrome.
| Article | N, gender, mean age ± SD, age range, race if not Caucasian | Mean BMI±SD or BMI(range) | HPA axis Evaluation & Statistical Analysis | BMI | Measure of Abdominal Adiposity | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Insulin | Fasting Glucose | HOMA-IR | Triglyc | HDL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UNSTIMULATED 24 H URINE FREE CORTISOL (UFC) | ||||||||||||
| Abraham et al., 2012 DM |
102 M 50±12 267 W 48±12 |
41.0±9 38.0±7 |
24 h UFC Spearman correlation |
r = 0.11 P = 0.26 r = −0.04 P = 0.50 |
r = 0.14 P = 0.16 r = −0.04 P = 0.52 (WC) |
r = 0.15 P = 0.15 r = −0.09 P = 0.13 |
r = 0.01 P =0.90 r = −0.02 P = 0.74 |
r = 0.02 P = 0.85 r = −0.018 P = 0.80 |
r = −0.20 P = 0.09 r = −0.09 P = 0.20 |
r = 0.03 P = 0.82 r =−0.03 P = 0.68 |
r =−0.16 P =0.12 r =−0.09 P = 0.15 |
r= −0.04 P =0.66 r = 0.06 P = 0.27 |
|
Duclos et al., 2005 EX-DM |
22 W, P-BFD 33.6±8.9 31 W, A-BFD 35.4±8.9 (PREM) |
39.1±7.0 40.1±4.5 |
24 h UFC to Urine Creatinine Ratio ANOVA |
- | NS P-BFD vs A-BFD |
- | - | - | - | - | - | - |
|
Duclos et al., 2001 EX-DM |
27 W, P-BFD 38±7.1 23 W, A-BFD 38±5.3 (PREM) |
39.7±9.4 39.8±1.4 |
24 h UFC to Urine Creatinine Ratio ANOVA |
- | NS P-BFD vs A-BFD |
- | - | - | - | - | - | - |
|
Stewart et al., 1999 EX-DM |
18 M, 18 W (PREM) Group A (n=12) 27.9±5.2 Group B (n=12) 33.1±6.9 Group C (n=12) 33.9±10.7 |
Group A 22.3±1.4 Group B 27.9±1.6 Group C 35.0±2.9 |
24 h UFC Student’s t-test |
NS difference b/w groups | - | - | - | - | - | - | - | - |
|
Andrew et al., 1998 EX-DM |
31 M 51.9±2.6 37 W 52.0±2.5 (POST) |
26.6±3.4 25.2±4.1 |
24 UFC Pearson correlation |
- | r = 0.09 NS r = −0.05 NS (abd circ) |
- | - | - | - | - | - | - |
|
Pasquali et al., 1993 EX-DM |
25 W 12 A-BFD 34.0±4.6 13 P-BFD 29.1±7.1 |
35.0±4.1 35.9±4.0 |
24 h UFC ANOVA |
- |
Higher in A-BFD vs P-BFD P<0.05 |
- | - | - | - | - | - | - |
|
Marin et al., 1992 EX-DM |
87 W 41±3.7 (PREM) |
30.9±14 | 24 h UFC Pearson correlation |
- |
r = 0.68 P ≤0.01 Sagittal abdominal diameter |
- | - | - | - | - | - | - |
| UNSTIMULATED SALIVARY CORTISOL STUDIES | ||||||||||||
| Article | N, gender, mean age ± SD, age range, race if not Caucasian | Mean BMI±SD or BMI(range) | HPA axis Evaluation & Statistical Analysis | BMI | Measure of Abdominal Adiposity | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Insulin | Fasting Glucose | HOMA-IR | Triglyc | HDL |
| Abraham et al., 2012 DM |
102 M 50±12 267 W 48±12 |
41.0±9.0 38.0±7.0 |
Bedtime salivary cortisol (LC- MS/MS) Spearman correlation |
r = 0.14 P = 0.25 r = −0.001 P = 0.98 |
r = 0.28 P = 0.02 r = 0.02 P = 0.83 (WC) |
r = −0.06 P = 0.64 r = 0.24 P=0.0008 |
r = −0.16 P=0.19 r = 0.10 P= 0.16 |
r = −0.10 P = 0.45 r = 0.12 P = 0.11 |
r = −0.02 P= 0.91 r = 0.02 P = 0.84 |
r =−0.08 P = 0.59 r = 0.11 P = 0.14 |
r =−0.16 P = 0.19 r =−0.08 P = 0.27 |
r = 0.002 P = 0.99 r = 0.04 P = 0.62 |
|
Oltmanns et al., 2006 DM |
190 M+W Born 1939–1958 N=63, high cortisol group only; 21%W; 53.9±4.8 all have type 2 diabetes |
31.2±5.6 | Pre-lunch salivary cortisol determined low, med, high cortisol groups; difference between high vs other groups presenteda ANOVA |
- | - | P = 0.029 | P =0.015 | - | P = 0.001 | - | NS | NS |
|
Duclos et al., 2005 EX-DM |
22 W P-BFD 33.6±8.9 31 W A-BFD 35.4±8.9 (PREM) |
39.1±7.0 40.1±4.5 |
0800 fasting salivary cortisol ANOVA |
- |
Higher in P-BFD vs. A-BFD P=0.01 |
- | - | - | - | - | - | - |
|
Steptoe et al., 2004 EX-DM |
89 M 52.5±2.6 83 W 51.9±2.7 |
25.6±3.3 25.4±4.0 |
Cortisol evening minimum Partial product- moment correlationsb |
r = 0.1 NS NS r-values not given |
r = 0.07 NS NS r-values not given (WHR) |
- | - | - | - | - | - | r=0.11 NS NS r-values not given |
|
Duclos et al., 2001 EX-DM |
27 W, P-BFD 38±7.1 23 W, A-BFD 38±5.3 (PREM) |
39.7±9.4 39.8±1.4 |
0800 fasting salivary cortisol ANOVA |
NS P-BFD vs A-BFD |
- | - | - | - | - | - | - | |
| DEXAMETHASONE SUPPRESSION TEST | ||||||||||||
| Article | N, gender, mean age ± SD, age range, race if not Caucasian | Mean BMI±SD or BMI (range) | HPA axis Evaluation & Statistical Analysis | BMI | Measure of Abdominal Adiposity | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Insulin | Fasting Glucose | HOMA-IR | Triglyc | HDL |
| Abraham et al., 2012 DM |
102 M 50±12 267 W 48±12 |
41.0±9 38.0±7 |
1mg DST Spearman correlation coefficient |
r = −0.17 P = 0.10 r = −0.04 P = 0.54 |
r = −0.12 P = 0.26 r = 0.02 P = 0.72 (WC) |
r = −0.01 P = 0.91 r = 0.18 P = 0.02 |
r = −0.09 P = 0.37 r = 0.20 P = 0.01 |
r = −0.31 P = 0.01 r = −0.08 P = 0.33 |
r = −0.20 P = 0.10 r = −0.03 P = 0.70 |
r =−0.31 P = 0.01 r =−0.09 P =0.28 |
r =−0.03 P = 0.76 r = 0.08 P = 0.25 |
r = 0.15 P = 0.14 r = 0.09 P = 0.22 |
|
Duclos et al., 2005 EX-DM |
22 W, P-BFD 33.6 ± 8.9 31 W, A-BFD 35.4± 8.9 (PREM) |
39.1±7.0 40.1±4.5 |
0.25mg DST ANOVA |
- | NS P-BFD vs A-BFD |
- | - | - | - | - | - | - |
|
Pasquali et al., 2002 EX-DM |
13 M, nl wt 35.6±13.3 36 M, obese 39.7±13.6 21 W, nl wt 31.6±8.1 51 W, obese 34.7±11.0 |
22.4±1.8 37.3±9.0 20.6±1.7 36.2±5.7 |
1mg DST→ 0.0035mg DST→ 0.007mg DST→ 0.015mg DST→ ANOVA |
nl wt vs obese P = 0.04 (W) NS (M) nl wt vs obese P = 0.009 (W) NS (M) nl wt vs obese P = 0.002 (W) NS (M) nl wt vs obese, P = 0.04 (W) NS (M) (greater inhibition, obese W) |
- | - | - | - | - | - | - | |
|
Duclos et al., 2001 EX-DM |
27 W, P-BFD 38±7.1 23 W, A-BFD 38±5.3 (PREM) |
39.7±9.4 39.8±1.4 |
0.25mg DST 0.5mg DST ANOVA |
0.25 mg DST, A-BFD greater inhibition vs P-BFD, P = 0.002; 0.5 mg DST, A-BFD vs P-BFD, NS |
- | - | - | - | - | - | - | |
|
Stewart et al., 1999 UNK-DM |
18 M, 18 W (PREM) Group A (n=12) 27.9±5.2 Group B (n=12) 33.1±6.9 Group C (n=12) 33.9±10.7 |
Group A 22.3±1.4 Group B 27.9±1.6 Group C 35.0±2.9 |
1 mg DST Student’s unpaired t-test |
NS difference b/w groups | - | - | - | - | - | - | - | - |
| Ljung et al., 1996 EX-DM |
22 M range: 40–60 y |
8 M < 25.0 14 M >25.0 Groups divided by WHR for analysis; n in these groups unknown |
0.5mg DST → 0.25mg DST → 0.125mg DST → 0.05mg DST ANOVA→ |
WHR > 1.0 less inhibition than WHR <1.0, P <0.05 No difference b/w WHR groups No difference b/w WHR groups No difference b/w WHR groups No correlation b/w BMI and inhibition |
- | - | - | - | - | - | - | |
|
Marin et al., 1992 EX-DM |
87 W 41±3.7 (PREM) |
30.9±14 Subgroup 10 W WHR≥0.87 10 W WHR<0.87 |
1 mg DST Analysis not stated |
- | NS b/w WHR groups | - | - | - | - | - | - | - |
| MORNING PLASMA CORTISOL STUDIES INCLUDING DIABETICS | ||||||||||||
| Article | N, gender, mean age ± SD, age range, race if not Caucasian | Mean BMI±SD or BMI(range) | HPA axis Evaluation & Statistical Analysis | BMI | Measure of Abdominal Adiposity | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Insulin | Fasting Glucose | HOMA-IR | Triglyc | HDL |
|
Reynolds et al., 2010 DM |
483 M 68.0±4.1 436 W 67.7±4.3 |
30.2±4.8 32.4±6.2 |
0800–0830 fasting cortisol Pearson correlation or multiple linear regressionc |
M r =−0.159 P <0.001 W r = −0.037 P = 0.442 |
M r =−0.166 P<0.001 W r = −0.001 P = 0.979 (WC) |
M+W β= 0.5±0.4 95%CI (−0.2 to 1.3) P = 0.183 |
M+W β= 0.5±0.7 95%CI (−0.9 to 1.9) P =0.485 |
- |
M+W β = 19.3±3.1 95%CI (13.3 to 25.3) P <0.001 |
- | - | M+W β= −4.7 ±19.3 95%CI (−43.6 to 33.1) P=0.806 |
|
Travison et al., 2007 DM |
999 M 62.6±8.3 |
27.7±4.4 | nonfasting cortisol 4 h post-waking multiple linear regressiond |
−0.51 95% CI (−0.7 to − 0.26)b P = 0.001 |
- | - | - | - | - | - | - | - |
|
Maggio et al., 2006 DM |
389 M (−MS)e 75±7 73 M (+MS) 74±6 results presented for combined group |
26.0±3 30.0±3 |
0700–0800 fasting cortisol generalized linear modelse |
- | NS (WC) |
NS | NS | - | NS | - | NS | NS |
|
Ward et al., 2003 DM |
258 M 47±4.7 251 W 47±4.7 Asian Indian Population |
22.9±4.1 24.8±4.9 |
0900 fasting cortisolf partial correlation coefficients & multiple linear regression |
Cortisol fell by 2.5 (95% CI 0.4–4.6) nmol/l per unit rise BMI P=0.02 |
NS (WHR) |
r = 0.25 P <0.001 |
r = 0.24 P <0.001 |
r = 0.12 P = 0.08 |
r = 0.26 P <0.001 |
r = 0.20 P<0.001 |
r = 0.17 P<0.001 |
- |
|
Walker et al., 2000 DM |
105 M 52±15.4 (25–74) 110 W 48.4±13.6 (25–74) |
26.2±3.3 25.2±4.2 |
AM fasting cortisol simple regression analysisg |
r = 0.03 P = 0.78 r = −0.12 P = 0.20 |
r = −0.04 P = 0.73 r = −0.26 P <0.01 (WHR) |
r = 0.17 P = 0.11 r = −0.06 P = 0.56 |
r = 0.19 P = 0.07 r = 0.07 P = 0.53 |
r = −0.12 P = 0.21 r = 0.05 P = 0.59 |
r = 0.12 P = 0.22 r = 0.03 P = 0.75 |
- - |
r = 0.10 P = 0.33 r = 0.15 P = 0.12 |
r = 0.10 P = 0.29 r= −0.07 P = 0.45 |
| MORNING PLASMA CORTISOL STUDIES EXCLUDING OR UNKNOWN DIABETIC STATUS | ||||||||||||
| Article | N, gender, mean age ± SD, age range, race if not Caucasian | Mean BMI±SD or BMI(range) | HPA axis Evaluation & Statistical Analysis | BMI | Measure of Abdominal Adiposity | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Insulin | Fasting Glucose | HOMA-IR | Triglyc | HDL |
|
Phillips DI et al., 1998 UNK-DM |
370 M 64±na (59–70) |
26.9–27.6 (range only) | 0900 fasting cortisol (↑) linear regression P for trendh |
P = 0.049 (↓) | P = 0.52 (WHR) |
Pg =0.02 (↑) | Pg= 0.03 (↑) | - | Pg = 0.0002 (↑) | Pg = 0.006 (↑) | Pg = 0.009 (↓) | P = 0.06 (↓) |
|
Duclos et al., 2005 EX-DM |
22 W, P-BFD 33.6 ± 8.9 31 W, A-BFD 35.4 ±8.9 (PREM) |
39.1±7.0 40.1±4.5 |
0800 fasting cortisol ANOVA |
- | P = 0.27 P-BFD vs A-BFD |
- | - | - | - | - | - | - |
|
Duclos et al., 2001 EX-DM |
27 W, P-BFD 38±7.1 23 W, A-BFD 38±5.3 (PREM) |
39.7±9.4 39.8±1.4 |
0800 fasting cortisol ANOVA |
- | NS P-BFD vs A-BFD |
- | - | - | - | - | - | - |
|
Rask et al., 2001 EX-DM |
11 M; 46.8±8.7 11 M; 49.6±8.5 12 M; 51.9±12.1 |
22.9±1.4 26.4±0.7 31.7±4.0 |
0830 cortisol (fasting status unknown) | NS difference b/w groups | - | - | - | - | - | - | - | - |
|
Stewart et al., 1999 UNK-DM |
18 M, 18 W (PREM) Group A (n=12) 27.9±5.2 Group B (n=12) 33.1±6.9 Group C (n=12) 33.9±10.7 |
Group A 22.3±1.4 Group B 27.9±1.6 Group C 35±2.9 |
0900 fasting cortisol Student’s t-test |
NS | - | - | - | - | - | - | - | - |
|
Stolk et al., 1996 EX-DM |
102 M 67.7±5.7 116 W 65.8±6.1 |
26.4±3.0 26.4±4.3 |
0800–0900 fasting cortisol Pearson correlation |
r = −0.35 P <0.01 r = −0.09 P = NS |
r = 0.08 P = NS r = −0.05 P = NS (WHR) |
- | - | No association in M or W | No association in M or W | - | - | - |
|
Stolk et al., 1996 EX-DM |
102 M 67.7±5.7 116 W 65.8±6.1 |
26.4±3.0 26.4±4.3 |
0800–0900 fasting cortisol/CBG Pearson correlation & multiple linear regression |
r = −0.26 P <0.01 r = 0.10 P = NS |
r = 0.13 P = NS r = 0.05 P = NS (WHR) |
- | - | No association 9.7 per mU/L insulin [95% CI 1.9; 17.5]i |
- | - | - | - |
|
Filipovsky et al., 1996 EX-DM |
6424 M 47.1±1.9 (40–53) |
25.8±3.1 | AM fasting cortisol age adjusted partial correlation coefficients |
r = −0.040 NS |
- |
r = 0.141 P<0.0001 |
r =0.072 P<0.01 |
- | - | - | - | - |
|
Ljung et al., 1996 EX-DM |
22 M range: 40–60 y |
8 M < 25.0 14 M >25.0 |
0800 fasting cortisol Linear regression |
- |
r = 0.579 P <0.005 (WHR) |
- | - | - | - | - | - | - |
|
Marin et al., 1992 EX-DM |
87 W 41±3.7 (PREM) |
30.9±14.0 | 0800 non- fasting cortisol (2 h post breakfast) Pearson correlation |
- |
r = −0.53 P <0.001 (n=38) (WHR) |
- | - | - | - | - | - | - |
Actual statistical values presented if available; statistically significant values are in bold.
For this study, analyses presented are for obese and overweight study group only.
Triglyc, triglycerides; M, men; W, women; DM, study included diabetics; EX-DM, study excluded diabetics; UNK-DM, unknown if diabetics included; BFD, body fat distribution; P-peripheral (waist-to-hip ratio <85), A, abdominal (waist-to-hip-ratio >85); WC, waist circumference; WHR, Waist-to-Hip ratio; NS, not significant; b/w, between; abd circ, abdominal circumference; PREM, pre-menopausal; POST, post-menopausal; DST testing uses plasma cortisol; nl, normal; inhibition, i.e. percent cortisol suppression
, Cortisol levels within normal range
, Results controlled for age, socioeconomic status, smoking, alcohol consumption, and time of waking; cortisol evening minimum is the lower of the values recorded at 2000–2030 and 2200–2230
, Covariates were age, gender, BMI, duration and treatment of diabetes mellitus, anti-hypertensive treatment, lipid-lowering treatment, interaction of BMI by gender
, Adjusted for age and other significant covariates; estimated cross-sectional trend in BMI per 100 nmol/l increase in serum cortisol concentration
, MS, metabolic syndrome by ATP III criteria with exception of fasting blood glucose cut-off which was 126 mg/dl; analysis adjusted for age, smoking, alcohol use, physical activity in the year before the visit
, Except for BMI, results are partial correlation coefficients corrected for age, sex and BMI
, Patients on anti-hypertensive meds excluded from blood pressure analyses; insulin values log transformed; when adjusted for obesity, plasma cortisol had statistically significant association with diastolic blood pressure (r=0.21, P=0.04) in men and with triglycerides and insulin (r=0.28, P=0.001; r=0.19, P=0.02, respectively) in women.
, P for trend adjusted for age and body mass
, Age adjusted regression coefficient
The BMI distribution of the combined healthy volunteer and overweight and obese study group was: <24.9 kg/M2,, (n = 20) 4.7 %; 25–29.9 kg/M2, (n = 69) 16%; 30–34.9 kg/M2, (n = 106) 25%; 35–39.9 kg/M2, (n = 93) 22%; 40–83 kg/M2, (n = 141) 33%. In the combined group, there were no significant differences in the median salivary cortisol or 24 UFC levels in the highest and lowest BMI groups. Specifically, the median salivary cortisol or 24 h UFC values in those with BMI < 24.9 compared to those with BMI > 40.0 were not different. However, there was a statistically significant trend in salivary cortisol but not UFC with increasing BMI values (P< 0.0001).
Association of cortisol levels with metabolic abnormalities
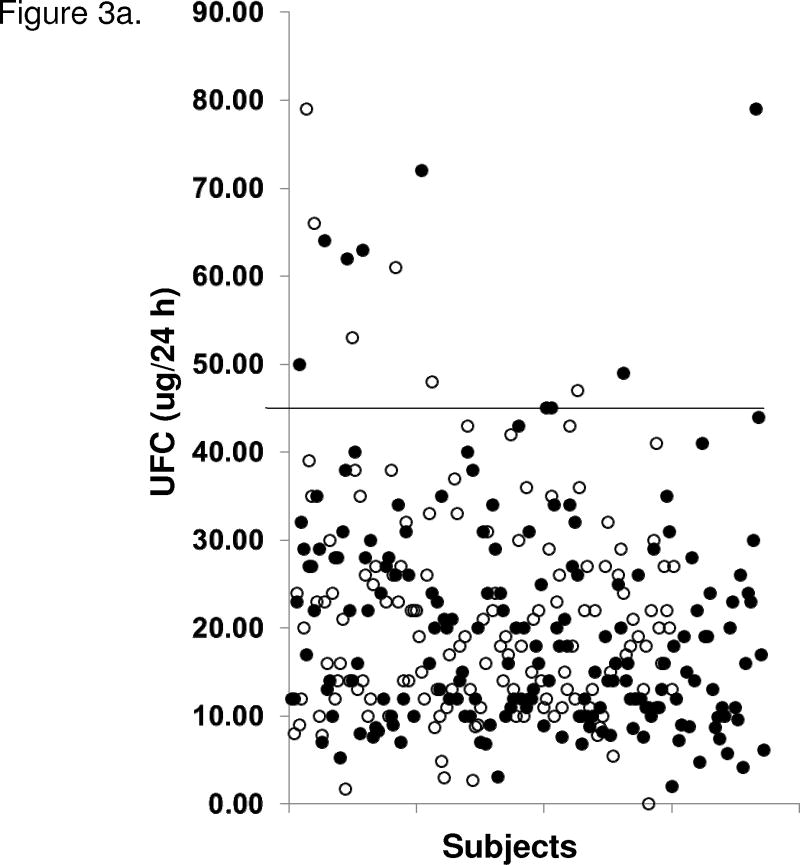
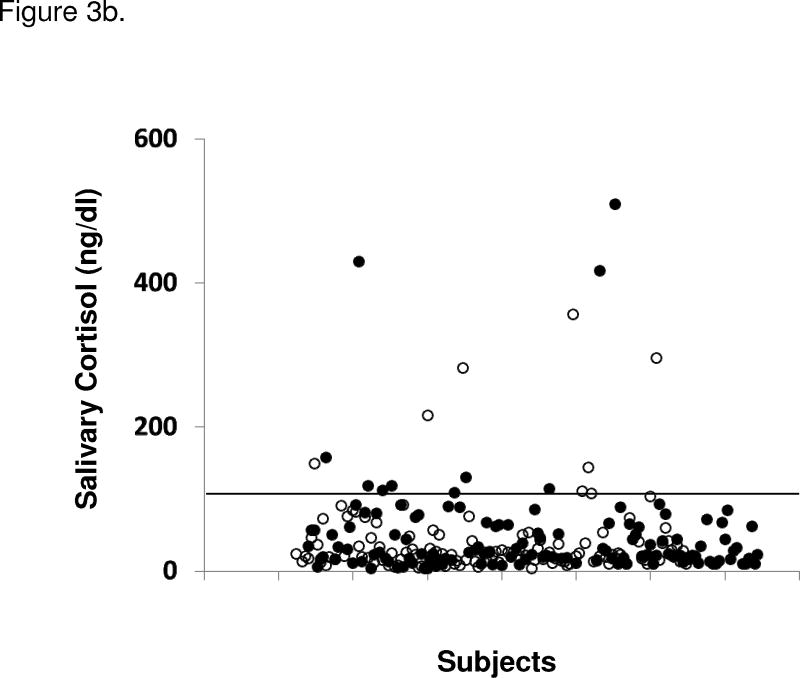
Subjects with MS, MSRx, diabetes, and/or hypertension were not more likely to have abnormal cortisol results compared to those without metabolic disorders (Figure 3). There were no significant differences in UFC, salivary cortisol levels, or post-dexamethasone cortisol levels between men and women or between men with MSRx, MS, diabetes, or hypertension compared to those without the respective disorders. The same was true for women, except that women with MSRx (14 [10–24] vs. 19 [13–27] mcg/24h), diabetes (12 [8–24] vs. 18 [12–26] mcg/24h), and hypertension (13 [10–24] vs. 20 [13–29] mcg/24h) had normal but statistically significant (all P<0.01) lower UFC levels compared to those without the disorder. There was no significant correlation between any cortisol parameter and the number of features of metabolic syndrome.
Figure 3.
Figure 3a. 24 h urine free cortisol (UFC) levels in subjects with and without metabolic syndrome (MS; using modified ATP III criteria). Unfilled circles (○) represent individuals without MS and filled circles (●) represent those with MS. Upper reference limit (solid line) for UFC is 45 ug/24 h (124 nmol/24 h). Figure 3b. Salivary cortisol levels in subjects with and without MS (using modified ATP III criteria for metabolic syndrome). Unfilled circles (○) represent individuals without MS and filled circles (●) represent those with MS. Upper reference limit (solid line) for salivary cortisol is < 100 ng/dl (2.8 nmol/l). Two patients’ salivary cortisol levels of 1300 and 643 ng/dl are not shown.
When stratified by gender, UFC was not related to waist circumference, HDL, triglycerides, SBP or DBP. Similarly, no relationship was seen between post-dexamethasone cortisol levels and waist circumference, HDL or triglycerides. However, women showed weak correlations between post-dexamethasone cortisol levels and systolic and diastolic blood pressure (rs=0.18, P=0.02; rs=0.20, P=0.009, respectively) and a weak correlation between SBP and bedtime salivary cortisol by LC-MS/MS (rs=0.24, P=0.0008). Men showed a moderate correlation between waist circumference and bedtime salivary cortisol measured by RIA (rs=0.32, P=0.01) and LC-MS/MS (rs=0.28, P=0.02). Otherwise, salivary cortisol did not show any significant relationships with metabolic parameters (Figure 3, Table 3).
When subjects on glucose-lowering medications were excluded (n=51), there was no correlation between UFC or salivary cortisol and fasting insulin, fasting glucose or HOMA-IR. Post-dexamethasone cortisol levels were negatively associated with fasting insulin (rs=−0.31, P=0.01) and HOMA-IR (rs=−0.31, P=0.01) in men.
Associations between waist circumference and metabolic parameters
Waist circumference was correlated significantly with fasting insulin (rp= 0.37, P<0.0001), fasting glucose (rp=0.57, P=0.02), HOMA-IR (rp=0.40, P<0.0001), and weakly with SBP (rp=0.22, P<0.0001) and DBP (rp =0.19, P=0.0005). When adjusted for gender, waist circumference maintained statistically significant correlations with fasting insulin (adj rp=0.37, P<0.0001), HOMA-IR (adj rp=0.39, P <0.0001), and SBP (adj rp=0.22, P=0.004), but not with fasting glucose or DBP. When adjusted for BMI, these associations remained statistically significant (all P<0.01), except for that between waist circumference and fasting glucose.
Cortisol and Stress
PSS scores were significantly higher in study subjects than in published population controls by gender and age groups 18 to 64 years (score ranges 17–20 vs. 12–14, P<0.001 for each age group). Mean scores were similar in subjects 65 years and older and same age population controls (11 ± 5 vs 12 ± 6, P=0.61). We found no significant relationship between PSS scores and UFC, waist circumference or BMI in either sex.
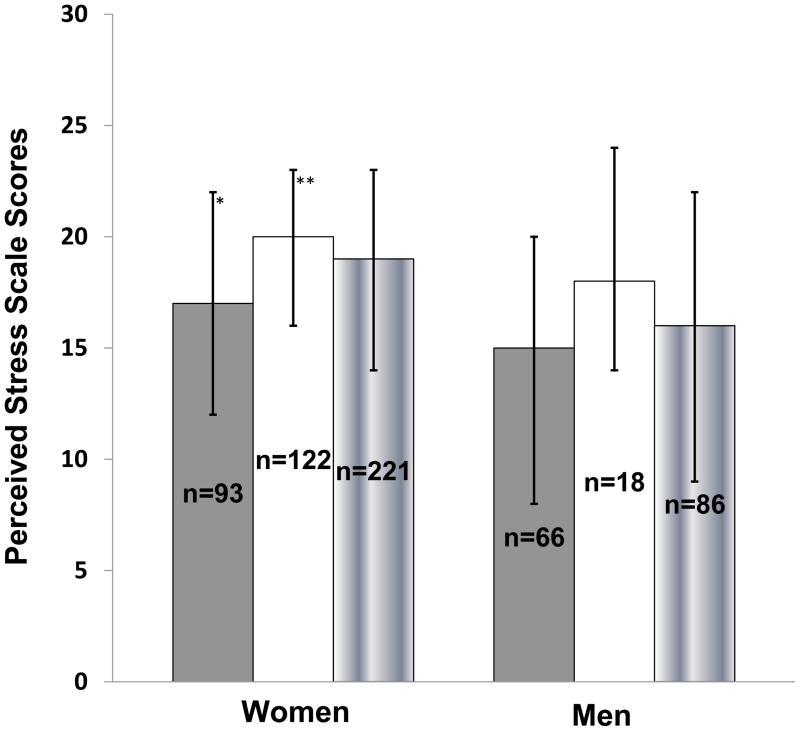
PSS scores in men (14 [8–20] vs 18 [15–24], P=0.01) and women (17 [12–21] vs 20 [15–24], P<0.02) with MS were lower than in those without MS. Women with MSRx had lower PSS scores than those without the disorder (17 [12–22] vs 20 [16–23], P=0.01) (Figure 4). In men only, we found inverse correlations between post-dexamethasone cortisol levels and PSS (rs=−0.24, P<0.03) and salivary cortisol by RIA and PSS (rs=−0.35, P=0.007).
Figure 4.
Median Perceived Stress Scale scores (PSS) in women and men with (gray bar), without (white bar) metabolic syndrome (using modified ATP III criteria), and the total group (gradient bar); Error bars represent inter-quartile range (IQR; 25th percentile, 75th percentile); *P< 0.05, PSS in women with vs. without metabolic syndrome; **P = 0.005, PSS in women vs. men.
Literature Review
Twenty studies (including this one) investigated relationships between parameters of obesity or metabolic syndrome and various cortisol measures: UFC (n=6), morning or evening salivary cortisol (n=4), morning plasma cortisol (n=14) or dexamethasone responses (n=6) (Table 3).
No study found a significant positive association between BMI and cortisol parameters including UFC (n=1) (17), salivary cortisol (n=1) (18), or the responses to 1 mg DST (2/2 studies) (17, 19). One study showed greater inhibition (percent cortisol suppression) after a lower dexamethasone dose in obese compared to normal weight women (19). Among studies evaluating relationships between morning plasma cortisol and BMI (n = 9), four showed no significant association (17, 20–22) and five showed a negative relationship (5, 6, 23–25).
No consistent relationship was found between abdominal obesity and cortisol parameters. Positive associations were found with UFC (3/6 studies) (3, 19, 26), morning (1/2 studies) (27) but not evening salivary cortisol (1 study) (18), or the response to 1 mg dexamethasone (n=1) (3). One of three studies evaluating lower dexamethasone suppression doses (28) reported increased cortisol suppression in women with an abdominal vs. peripheral body fat distribution while one showed no difference in suppression (27). One of the three studies showed less suppression of cortisol after 0.5mg dexamethasone dose in men with a WHR > 1.0 vs WHR < 1.0 (29). Nine studies found inconsistent relationships between morning plasma cortisol and abdominal obesity: no relationship (n=5),(5, 6, 24, 27, 28) positive association (n=1) (29) and negative association (n=3) (3, 22, 23). One study did not find a correlation between 24 h UFC and abdominal circumference (30).
The relationships between cortisol parameters and features of the metabolic syndrome also were variable. Positive associations were found between blood pressure and higher pre-lunch salivary cortisol (1/1 study) (31) and between insulin and free cortisol (1/1 study) (24). Morning plasma cortisol had inconsistent associations with glucose (3/6 positive) (5, 6, 23), blood pressure (3/6 positive) (5, 6, 20) and triglycerides (positive, negative and no relationship in 1 study each) (5, 6, 32). There was no relationship between morning plasma cortisol and HDL (4/4 studies) (5, 22, 23, 32) or insulin (3/3 studies) (6, 22, 24). No study analyzed relationships of UFC or dexamethasone suppression testing and metabolic syndrome.
Discussion
This study evaluated three parameters of activity of the hypothalamic-pituitary-adrenal (HPA) axis: its nadir (by salivary cortisol levels), its integrated daily cortisol production (by UFC), and its sensitivity to negative feedback (by the post-1mg dexamethasone cortisol level). Though some studies report positive associations between cortisol and obesity and metabolic syndrome (3, 5, 6) and cortisol was implicated as a major causal factor in a recent review (33), our data, along with a critical review of relevant literature, do not reveal strong associations between cortisol and weight or features of the metabolic syndrome.
In the combined group there was a significant trend in salivary cortisol values by increasing BMI. This was an interesting finding given that there was no significant difference between median salivary cortisol values in those with the lowest (<24.9 kg/M2) versus highest (>40 kg/M2) BMI. Other than this significant trend, our study and others found no significant relationships between weight or BMI and any cortisol parameter (17–19).
It is possible that visceral or abdominal adiposity (as measured by waist circumference) reflect metabolic risk better than BMI. As expected, waist circumference correlated strongly with insulin resistance and weakly with blood pressure (34). However, apart from a weak correlation with salivary cortisol in men, we found no relationship between waist circumference and any cortisol parameter. Along with the data reported in Table 3, these results do not support an association between increased systemic cortisol levels and obesity or abdominal adiposity.
Other features of the metabolic syndrome might be associated with cortisol. Interestingly, we observed significantly lower UFC levels in women with MSRx, hypertension, and diabetes, a finding counter to the hypothesis that cortisol may be causal. Our results and the previous data (Table 3) do not show consistent relationships between basal or suppressed levels of cortisol, and obesity or components of the metabolic syndrome. Of note, most studies with significant P-values had weak correlations (r values < 0.3).
One might argue that dynamic testing is a more appropriate tool to assess HPA axis activity. A few small studies reported increased cortisol responses to ACTH or CRH stimulation or to metabolic or psychological challenges in subjects with abdominal versus peripheral fat distribution (3, 26–28). Unfortunately, these findings have not been replicated.
Given the intense interest in the area yet conflicting results, an obvious question is: why has it been so difficult to define HPA function in metabolic syndrome? One reason is the different study designs. Previous studies are heterogeneous in terms of gender, age, cortisol parameters and timing, and presence and severity of co-morbidities.
Second, assay variability, may cause variable results. In this study, the relationship between salivary cortisol and SBP in women was significant by LC-MS/MS, but not by RIA. We previously reported discrepancies between these assays (35). Calibration of the LC-MS/MS assay can lend to discrepant results between it and immunoassays. Another possible explanation is that RIA measures non-cortisol steroid metabolites whereas LC-MS/MS does not. Cross-reactivity of cortisol metabolites in immunoassays also may contribute to overestimation of UFC levels, both in normal and obese individuals (17, 21).
In addition to study design, measurement, and interpretation differences in the literature, obesity itself may confound the results. Strain et al. reported that cortisol production rates showed a significantly positive linear correlation with “relative” (percent deviation from desirable weight) weight in men and women, but the ratio was weight invariant (36). Purnell and colleagues showed that cortisol production rates increased in proportion to body weight in lean to obese healthy volunteers. However, when corrected for body surface area, there was no association with fat mass or non-fat mass. They also showed that cortisol clearance rates were significantly higher in obese vs. lean patients with adrenal insufficiency (37). Others have demonstrated increased clearance of cortisol with increasing weight in women, but not in men (38). Thus, it is possible that seemingly elevated UFC levels in an obese patient may be due to increased cortisol production with increased excretion of metabolites that cross-react in the cortisol assay.
This study could not evaluate subtle dysregulation of cortisol in obesity and metabolic syndrome. The increasing trend of salivary cortisol levels by BMI hints at the idea that perhaps very slight increases in nadir cortisol levels contribute to obesity. One must also consider the idea that cortisol dysregulation may occur only in various sub-populations, which still need to be identified.
In addition, although the data are inconsistent, there is a suggestion that local glucocorticoid action may be amplified by up-regulated activity of 11 βHSD in visceral and hepatic tissue of obese subjects (39). In addition, serum free cortisol levels and cortisol production rates, adjusted for body surface area, are associated with accumulation of visceral fat and insulin resistance in men (39). Perceived stress and psychiatric disorders may influence HPA axis function, obesity and metabolic syndrome. In the current study, overweight and obese individuals had higher perceived stress scores than population-based gender and age-matched controls up to age 64. However, no clinically relevant associations were found between perceived stress and any cortisol parameter, or weight. Perceived stress scores were significantly lower in subjects with versus without metabolic syndrome, which was unexpected. Similar findings have been reported: in a longitudinal study of 425 middle-aged women, questionnaires reflecting depression, tension and anger measures were associated with metabolic syndrome. However, PSS scores were not significantly associated with metabolic syndrome (40). This suggests that specific trait questionnaires may be more useful than general stress questionnaires in understanding the role of stress in metabolic syndrome.
This study shows that systemic cortisol levels are similar in the presence or absence of metabolic syndrome, hypertension and diabetes. A literature review highlighted a lack of consistency in relationships between cortisol and weight or metabolic parameters. Taken together, these data raise doubts about the role of systemic cortisol in the development and maintenance of obesity and the metabolic syndrome.
Strengths of our study include the large number of subjects and use of cortisol parameters that reflect both basal values and dynamic response. Limitations include the study’s cross-sectional design, which prevented us from evaluating whether people who become obese or develop metabolic syndrome have subtle cortisol excess over time. Selection bias may have skewed the results as the subjects enrolled were motivated to attend a weight loss clinic and may have failed other treatments. From a socioeconomic standpoint, these subjects did not have financial restrictions preventing them from participating in the weight loss program. Also, the investigation focused on systemic hypercortisolism and did not evaluate the role of cortisol within tissues or cortisol production rates.
Minor alterations in various systems – e.g., the HPA axis, sympathetic nervous system, inflammatory system, and psychological system – may contribute jointly to the pathogenesis of obesity and metabolic syndrome. A study designed to evaluate these systems collectively and longitudinally with strict attention to population characteristics, and a better understanding of cortisol metabolism, might lead to an improved understanding of the pathophysiology of these conditions.
Acknowledgments
We thank Ms. Elizabeth Saverino and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Clinical Trials Database Team, Unit on Computer Support for their invaluable support with patient recruitment and data management.
Funding:
This work was supported in part by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Author Disclosure: The authors have nothing to declare
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 2.Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am. 2005;34:327–39. viii. doi: 10.1016/j.ecl.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–6. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali R, Anconetani B, Chattat R, et al. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: effects of the corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism. 1996;45:351–6. doi: 10.1016/s0026-0495(96)90290-5. [DOI] [PubMed] [Google Scholar]
- 5.Phillips DIW, Barker DJP, Fall CHD, et al. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–60. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- 6.Ward AM, Fall CH, Stein CE, et al. Cortisol and the metabolic syndrome in South Asians. Clin Endocrinol (Oxf) 2003;58:500–5. doi: 10.1046/j.1365-2265.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol. 2007;165:828–37. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 8.Chandola T, Britton A, Brunner E, et al. Work stress and coronary heart disease: what are the mechanisms? Eur Heart J. 2008;29:640–8. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 9.Ketteler J. Want to lose fat? Chill out. Women’s Health. 2008 [Google Scholar]
- 10.FTC. Targets Products Claiming to Affect the Stress Hormone Cortisol. 2010 Aug 11; Available from: www.ftc.gov/opa/2004/10/windowrock.htm.
- 11.Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab. 2009;94:3857–64. doi: 10.1210/jc.2008-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 13.Wade M, Baid S, Calis K, Raff H, Sinaii N, Nieman L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur J Endocrinol. 2010;162:109–13. doi: 10.1530/EJE-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 16.Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome--recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem. 1997;34 (Pt 3):222–9. doi: 10.1177/000456329703400302. [DOI] [PubMed] [Google Scholar]
- 17.Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–7. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 18.Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. Int J Obes Relat Metab Disord. 2004;28:1168–73. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- 19.Pasquali R, Ambrosi B, Armanini D, et al. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: A dose-response study. J Clin Endocrinol Metab. 2002;87:166–75. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- 20.Filipovsky J, Ducimetiere P, Eschwege E, Richard JL, Rosselin G, Claude JR. The relationship of blood pressure with glucose, insulin, heart rate, free fatty acids and plasma cortisol levels according to degree of obesity in middle-aged men. J Hypertens. 1996;14:229–35. doi: 10.1097/00004872-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Rask E, Olsson T, Soderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–21. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 22.Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds RM, Labad J, Strachan MWJ, et al. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. J Clin Endocrinol Metab. 2010;95:1602–8. doi: 10.1210/jc.2009-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolk RP, Lamberts SW, de Jong FH, Pols HA, Grobbee DE. Gender differences in the associations between cortisol and insulin in healthy subjects. J Endocrinol. 1996;149:313–8. doi: 10.1677/joe.0.1490313. [DOI] [PubMed] [Google Scholar]
- 25.Travison TG, O’Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf) 2007;67:71–7. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- 26.Pasquali R, Cantobelli S, Casimirri F, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–6. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 27.Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–66. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- 28.Duclos M, Gatta B, Corcuff JB, Rashedi M, Pehourcq F, Roger P. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clin Endocrinol (Oxf) 2001;55:447–54. doi: 10.1046/j.1365-2265.2001.01384.x. [DOI] [PubMed] [Google Scholar]
- 29.Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes Res. 1996;4:277–82. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 30.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83:1806–9. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- 31.Oltmanns KM, Dodt B, Schultes B, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325–31. doi: 10.1530/eje.1.02074. [DOI] [PubMed] [Google Scholar]
- 32.Maggio M, Lauretani F, Ceda GP, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54:1832–8. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 34.Poirier P, Lemieux I, Mauriege P, et al. Impact of waist circumference on the relationship between blood pressure and insulin: The Quebec Health Survey. Hypertension. 2005;45:363–7. doi: 10.1161/01.HYP.0000155463.90018.dc. [DOI] [PubMed] [Google Scholar]
- 35.Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007;92:3102–7. doi: 10.1210/jc.2006-2861. [DOI] [PubMed] [Google Scholar]
- 36.Strain GW, Zumoff B, Strain JJ, Levin J, Fukushima DK. Cortisol production in obesity. Metabolism. 1980;29:980–5. doi: 10.1016/0026-0495(80)90043-8. [DOI] [PubMed] [Google Scholar]
- 37.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–7. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 38.Strain GW, Zumoff B, Kream J, Strain JJ, Levin J, Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism. 1982;31:209–12. doi: 10.1016/0026-0495(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 39.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11′-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296:E351–E7. doi: 10.1152/ajpendo.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–7. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]