Abstract
Adult living donor liver transplant (LDLT) recipients have a higher incidence of biliary complications than deceased donor liver transplant (DDLT) recipients. Our objective was to define the intensity of intervention and time to resolution after diagnosis of biliary complications after liver transplantation. We analyzed the management and resolution of post-transplant biliary complications and investigated the comparative effectiveness of interventions in LDLT and DDLT recipients. Analysis of biliary complications (leak or stricture) used a retrospective cohort of liver transplant recipients at 8 centers between 1998–2006 (median follow-up was 4.7 years from onset). Number, procedure types, and time to resolution were compared between LDLT and DDLT recipients. Post-transplant biliary complications occurred among 47/189 [25%] DDLT recipients and 141/356 [40%] of LDLT recipients. Biliary leaks comprised 38% (n=18) of post-DDLT and 65% (n=91) of post-LDLT biliary complications. Median times to first biliary complication were similar (DDLT vs. LDLT: leak: 11 vs. 14 days, p=0.6; stricture: 69 vs. 107 days, p=0.3, respectively). There were 1225 diagnostic and therapeutic procedures performed, including re-operation and retransplant (mean: 6.5±5.4 per recipient; DDLT: 5.4±3.6 vs. LDLT: 6.8±5.8, p=0.5). The median number of months to resolution of a biliary complication (tube/stent/drain-free) was not significantly different between DDLT and LDLT for leaks (DDLT: 2.3; LDLT: 1.3; p=0.3) or strictures (DDLT: 4.9; LDLT: 2.3; p=0.6). Although the incidence of biliary complications after LDLT is higher than after DDLT, treatment requirements and time to resolution after development of a biliary complication are similar in LDLT and DDLT recipients.
Keywords: outcomes, observational, living donation, deceased donation, liver
Introduction
The disparity between the numbers of available donor organs and potential liver transplant recipients led to the development of adult to adult living donor liver transplantation (LDLT)(1). As experience has accumulated, it has become evident that biliary complications comprise a large proportion of post-transplant recipient morbidity (2,3). Several factors have been identified that may contribute to biliary leak or stricture following LDLT, including center volume, number of graft bile ducts, and type of anastomosis performed (4). Furthermore, while endoscopic therapies can be successfully employed to treat the majority of biliary problems in most recipients, LDLT recipients may have less favorable responses (5).
LDLT recipients have a higher overall incidence of complications than deceased donor liver transplant (DDLT) recipients. Despite a lower acuity of pre-transplant disease, hospitalization requirements for medical and surgical complications are higher after LDLT than after whole organ DDLT (6). Biliary complications contribute importantly to the excess morbidity of this procedure. Although most biliary complications do not lead to graft loss or patient death, detailed analyses of the management, course of treatment, and resolution of biliary complications after LDLT and DDLT across multiple dedicated transplant programs might contribute to understanding differences and similarities of biliary morbidity based on allograft type. In this study, we report on the comparative effectiveness of management of post-transplant biliary complications among LDLT and DDLT recipients who participated in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL).
Methods
Data Sources
Data for this study were derived from the retrospective cohort component of the A2ALL study from 8 of the 9 A2ALL centers. Data were collected based on detailed chart reviews and supplemented with data from the Scientific Registry of Transplant Recipients (SRTR) obtained through a data use agreement. Seven hundred twenty-six transplant candidates who had a potential living donor and had completed a history and physical examination between January 1, 1998 and February 28, 2003 were eligible for inclusion in the study. A total of 545 recipients that received a transplant between May 1998 and May 2006 for non-fulminant indications were included in the analysis (DDLT: 189; LDLT: 356). Recipients of domino transplants were included in the DDLT group.
Information from the study database was supplemented by abstraction of additional data (between September 2010 and May 2011) on the course of treatment for all 188 recipients who experienced a biliary complication, as identified by the presence of a leak or stricture recorded in the patient’s chart. A bile leak was defined as a persistent bilious drainage beyond seven days after surgery identified by a radiological study or surgical exploration. A biliary stricture was defined as a radiologically identified narrowing of the intrahepatic or extrahepatic bile ducts occurring at any time after donation. Detailed serial data on specific diagnostic and therapeutic interventions related to biliary complications were collected, including hospitalizations, antibiotic courses, CT scan or ultrasound (CTUS), endoscopic retrograde cholangiopancreatography (ERCP), magnetic resonance cholangiopancreatography (MRCP), percutaneous transhepatic cholangiography (PTC), reoperation, and retransplantation.
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each of the transplant centers.
Statistical Methods
Study subjects were followed from the time of transplant to the earliest of retransplant, death, or date of biliary complication chart review. Descriptive statistics are given as means and standard deviations for continuous variables or as proportions for categorical variables. T-tests were used to compare differences between DDLT and LDLT for continuous characteristics. Chi-square tests were used to compare differences between DDLT and LDLT for categorical characteristics. The number of procedures performed for DDLT and LDLT recipients was compared using a t-test after a log transformation of the procedure counts.
The distributions for the numbers of procedures performed per person overall and for each procedure individually were compared graphically for DDLT and LDLT recipients using boxplots. The percentage of outpatient procedures that were performed was examined. A procedure was considered an outpatient procedure if there was no corresponding hospitalization record that included the date of the procedure. We compared the percentage of procedures performed as an outpatient for DDLT versus LDLT recipients with a logistic regression model to adjust for a potential time trend. This model allowed us to examine the association between type of transplant (DDLT vs. LDLT) and the probability of a procedure being performed as outpatient while adjusting for year of transplant.
To study the time to resolution of the biliary complications, the time from placement of a biliary tube, stent, or drain until the removal of all tubes, stents, and drains was examined with survival models. Follow-up for this analysis started at the time of initial placement of a tube, stent, or drain and continued until the earliest of: becoming tube, stents, and drain-free, retransplant, death, or end of study. The earliest date where all tubes, stents, and drains had been removed was considered the time of resolution (event). Follow-up was censored at retransplant, death, and end of study. The time from onset to resolution of biliary complications was examined using Kaplan-Meier survival curves by transplant type (DDLT vs. LDLT) and by complication type (leak and/or stricture). Differences between DDLT and LDLT recipients were compared for each type of complication using log-rank tests. Potential factors associated with resolution were tested by fitting multivariable Cox proportional hazards regression models stratified by complication type. Time dependent Cox regression models were used to examine the effects of developing a biliary complication on long term graft and patient outcomes. Each of the variables in Table 1 was tested in the survival models, and the best subset selection method was used to look for a parsimonious model.
Table 1.
Characteristics of recipients with biliary complications
| DDLT (n=47) mean ± SD or n (%) |
LDLT (n=141) mean ± SD or n (%) |
p-value* | |
|---|---|---|---|
| Recipient age at transplant (years) | 49.4 ± 10.3 | 49.5 ± 10.0 | 0.950 |
| Recipient Sex | 0.666 | ||
| Male | 30 (63.8) | 85 (60.3) | |
| Female | 17 (36.2) | 56 (39.7) | |
| Recipient Race | 0.385 | ||
| White | 39 (83.0) | 124 (87.9) | |
| Non-White | 8 (17.0) | 17 (12.1) | |
| Recipient BMI | 27.1 ± 4.9 | 26.3 ± 5.5 | 0.373 |
| Diagnosis (multiple diagnoses possible) | |||
| Hepatitis C (HCV) | 18 (38.3) | 63 (44.7) | 0.444 |
| Hepatocellular carcinoma (HCC) | 10 (21.3) | 20 (14.2) | 0.250 |
| Alcohol | 7 (14.9) | 19 (13.5) | 0.807 |
| Cholestatic liver disease | 8 (17.0) | 31 (22.0) | 0.467 |
| Noncholestatic cirrhosis other than HCV/alcohol | 14 (29.8) | 33 (23.4) | 0.382 |
| Other | 7 (14.9) | 13 (9.2) | 0.794 |
| MELD at Transplant | 22.0 ± 9.5 | 15.4 ± 6.3 | <0.0001 |
| Donor age (years) | 37.5 ± 13.4 | 37.1 ± 9.9 | 0.832 |
| Donor Type | N/A | ||
| Donation after brain death | 45 (95.7) | ||
| Donation after cardiac death | 2 (4.3) | ||
| Number of bile ducts from donor graft | <0.0001 | ||
| Missing | 6 (4.3) | ||
| 1 | 47 (100) | 65 (46.1) | |
| 2 | 56 (39.7) | ||
| >2 | 14 (9.9) | ||
| Number of biliary anastomoses | <0.0001 | ||
| Missing | 12 (25.5) | ||
| 1 | 35 (74.5) | 88 (62.4) | |
| 2 | 45 (31.9) | ||
| 3 | 8 (5.7) | ||
| Biliary anastomosis type | <0.0001 | ||
| Missing | 12 (25.5) | ||
| Not-All Roux | 28 (59.6) | 78 (55.3) | |
| All Roux | 7 (14.9) | 63 (44.7) | |
| Cold ischemia time (minutes) | 446.4 ± 167.7 | 93.7 ± 99.1 | <0.0001 |
| Duration of recipient operation (minutes) | 386.0 ± 119.1 | 533.5 ± 129.9 | <0.0001 |
| Year of Transplant | 0.076 | ||
| 1998 – 1999 | 6 (12.8) | 23 (16.3) | |
| 2000 – 2001 | 19 (40.4) | 76 (53.9) | |
| 2001 – 2003 | 21 (44.7) | 42 (29.8) | |
| 2004 – 2006 | 1 (2.1) |
p-values are from two sample t-tests for continuous characteristics and from chi-square tests for categorical characteristics
The rates of procedures performed by month since transplant were compared for DDLT and LDLT recipients. The rates were calculated as the number of procedures performed during the month divided by the number of patients in the risk set during that month. To test factors that might be associated with the number of procedures performed per unit time, negative binomial regression models were fit to the data. Since the rate of procedures decreased rapidly soon after transplant and changed very little after about two years post-transplant, two separate models were fitted. The first model was fitted for the first two years after transplant and included a quadratic term for time. The second model was fitted for the time beyond two years post-transplant and modeled time as a linear term.
All analyses were carried out using SAS 9.2 software (SAS Publishing; SAS Institute Inc., Cary, NC).
Results
Patient Characteristics and Nature of Biliary Complications
Of the 189 DDLT recipients, 47 patients (25%) suffered a biliary complication during the follow-up period, and 141 of 356 LDLT recipients (40%) had a post-transplant biliary complication. Baseline characteristics are shown in Table 1. LDLT recipients had significantly lower Model for End-Stage Liver Disease (MELD) scores (p<0.0001), when compared to the DDLT group. The number of biliary anastomoses was significantly higher in the LDLT group (p<0.0001), as well as the proportion undergoing a complete Roux-en-Y reconstruction (p<0.0001). While the cold ischemia time was shorter in the LDLT group (p<0.0001), the duration of the recipient operation was significantly longer (p<0.0001).
Overall, a higher percentage of LDLT patients had a biliary complication (40% vs. 25%, p<0.001) (Table 2). Among those with biliary complications, biliary leaks predominated in the LDLT group (64.5% vs. 38.3% of biliary complications) (p=0.005). However, the median time from transplant to onset of a biliary leak was not different in the two groups (DDLT = 11 days, LDLT = 14 days, p=0.63). Conversely, biliary strictures were the predominant form of biliary complication in the DDLT group (59.6% vs. 32.6%). Again, the median time to onset was not significantly different (DDLT = 69 days, LDLT = 107 days, p=0.34). Although the risk of any biliary complication was higher after LDLT, the risk of stricture did not differ (14.8% after DDLT and 12.9% after LDLT, p=0.5). Five subjects had a simultaneous biliary leak and stricture (DDLT: n=1; LDLT: n=4).
Table 2.
Type and time (days) from transplant to onset of initial biliary complication
| Type of Biliary Complication | DDLT (n=189) n (%) |
LDLT (n=356) n (%)* |
|---|---|---|
| No Complication | 142 (75.1) | 215 (60.4) |
|
| ||
| Complication Type | 47 (24.9) | 141 (39.6) |
| Leak | 18/47 (38.3) | 91/141 (64.5) |
| Stricture | 28/47 (59.6) | 46/141 (32.6) |
| Both | 1/47 (2.1) | 4/141 (2.8) |
| Time to onset ** | Median (Q1 – Q3) | Median (Q1 – Q3) |
| Leak | 11 (3 – 39) | 14 (6 – 24) |
| Stricture | 69 (32 – 217) | 107 (55 – 278) |
| Both | 80 (80 – 80) | 19 (18 – 42) |
Chi-square test of proportions for each complication type among recipients with a biliary complication (DDLT vs. LDLT, p=0.0046).
Based on t-tests, there were no significant differences in mean time to onset for any of the complication types comparing DDLT and LDLT.
Procedures for biliary complications
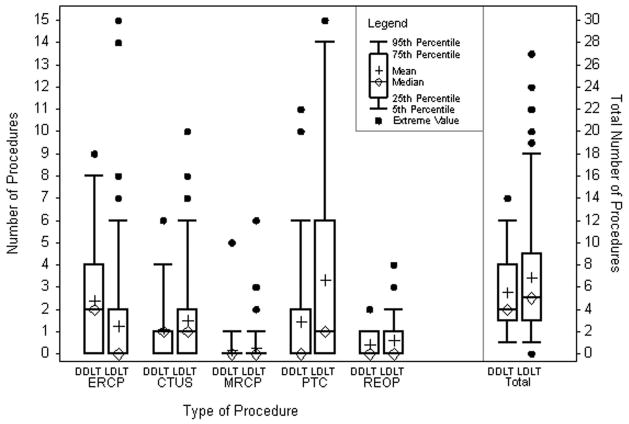
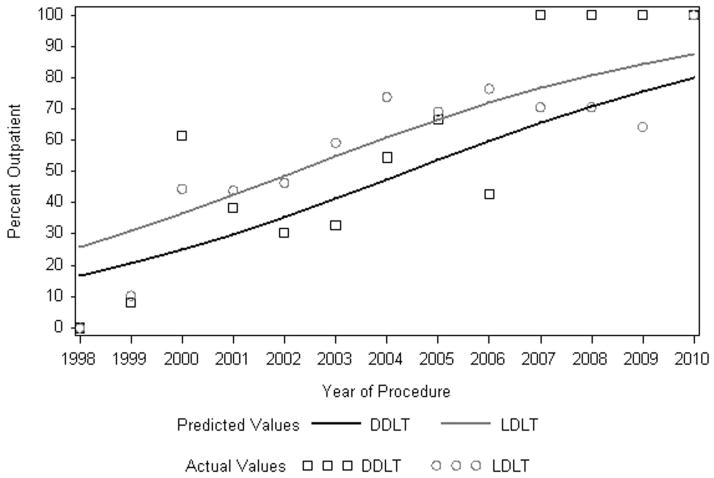
Overall, a total of 1225 diagnostic and therapeutic procedures were performed for the management of biliary complications. The proportion of procedures performed exclusively for diagnostic purposes, without a therapeutic intervention, was similar in the DDLT and LDLT groups (diagnostic only: n= 90 (34.9%) vs. n=368 (38.1%), respectively) (p=0.35). The overall number of procedures performed per patient, including diagnostic imaging, therapeutic intervention, reoperation, and retransplant, was 5.4±3.6 for DDLT recipients and 6.8±5.8 for LDLT recipients (p=0.53) (Figure 1). There were significantly more PTCs performed per patient in the LDLT group (p=0.004) and significantly more ERCPs per patient in the DDLT group (p<0.0001). Figure 2 shows that the proportion of diagnostic and therapeutic procedures performed in an outpatient setting increased dramatically over time (p<0.0001). Across the entire study period, the proportion of outpatient procedures was significantly higher in the LDLT group (p=0.0002).
Figure 1.
Number of procedures performed per patient to diagnose and/or treat biliary complications.
Figure 2. Procedures performed as an outpatient by year.
The actual values are calculated by calendar year, predicted percents are based on a logistic regression model.
Probability of resolution following a biliary complication
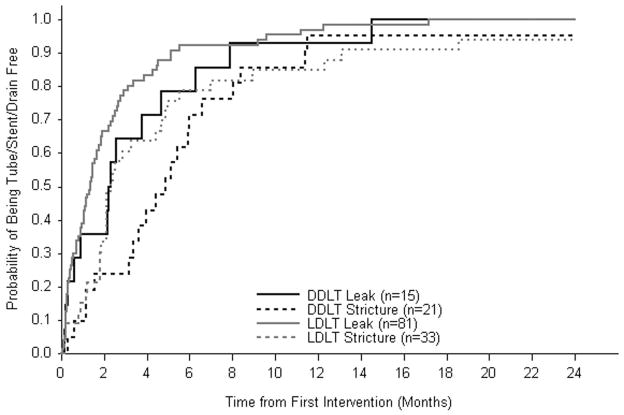
The cumulative probability of biliary complication resolution (definitive removal of a percutaneously or operatively placed tube, stent or drain, or retransplant) is shown in Figure 3. In this analysis, time to resolution was initialized on the day the biliary complication was diagnosed and/or first treated. Within six months of diagnosis, the majority (79% of DDLT and 92% of LDLT) of biliary leaks resolved and by 24 months all had resolved. Compared to biliary leak, the probability of resolution of biliary strictures was lower for recipients of both groups of transplants. Nevertheless, at 24 months following diagnosis, 95% of DDLT and 94% of LDLT recipients with biliary strictures were tube, stent, and drain-free.
Figure 3.
Probability of becoming tube/stent/drain-free after initial placement by type of biliary complication and transplant type.
The median time to tube, stent, and drain-free status after a biliary leak was one month longer among DDLT recipients (2.3 months) than among LDLT recipients (1.3 months); there was a 75% probability of resolution after 4.7 and 2.7 months for DDLT and LDLT recipients, respectively (log rank p-value 0.29). After development of a biliary stricture, the median time to tube, stent, and drain-free status was 4.9 months in the DDLT group compared to 2.3 months in the LDLT group; there was a 75% probability of resolution after 6.6 months for DDLT recipients and 5.0 months for LDLT recipients (log rank p-value 0.61). Among LDLT recipients, the median time to biliary leak resolution was not significantly different in those without (1.5 months) or with a Roux-en-Y anastomosis (1.2 months).
When tested with multivariable Cox models stratified by complication type (leak/stricture), none of the factors, including LDLT (HR = 1.2, p=0.37) had a significant influence on the time to being tube/stent/drain-free. Analyzing the LDLT exclusively, the overall number of biliary complication cases per center influenced the time to being tube/stent/drain-free. Experience with more than 15 biliary complications at a center was associated with a significantly shorter time to resolution (HR=1.68, p=0.04).
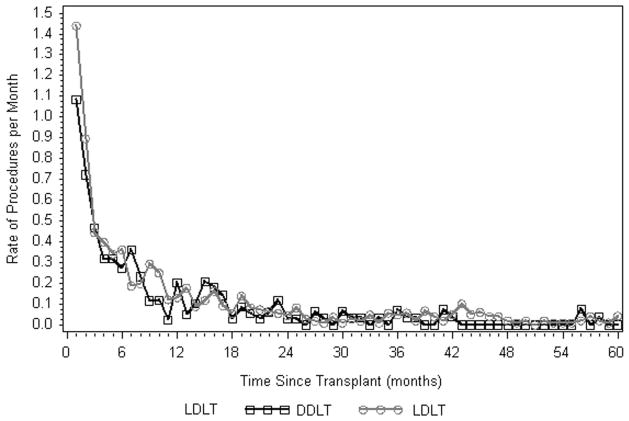
The rate of biliary related procedures declined rapidly during the first 24 months post-transplant and then remained relatively constant in both the DDLT and LDLT groups (Figure 4). Rates never fell to 0, even 10 years following transplant. During the first 24 months, procedure rates for DDLT and LDLT recipients were similar while older recipient age, higher recipient BMI, and recipient diagnosis of hepatitis C virus (HCV) were associated with a higher rate of procedures in all patients (Table 3). Beyond 24 months from the time of transplant, procedure rates for LDLT recipients were higher than for DDLT recipients, but not to the point of reaching statistical significance by conventional standards (p=0.06). The rate of procedures was significantly higher for recipients with at least one Roux type biliary anastamosis or whose initial biliary complication was a stricture or simultaneous leak and stricture, RR=2.97 (p=0.015) and RR=2.90 (p=0.019), respectively.
Figure 4.
Rate of procedures by time since transplant.
Table 3.
Models of rates of biliary complication procedures per month
| Parameter* | Estimate | Risk Ratio | p-value |
|---|---|---|---|
| Rates within the first two years post-transplant | |||
| Time Since Transplant (per month) | −0.28 | 0.76 | <.0001 |
| Time Since Transplant (per month2) | 0.01 | 1.01 | <.0001 |
| LDLT (ref. = DDLT) | 0.19 | 1.21 | 0.147 |
| Initial Biliary Complication = Stricture or Stricture + Leak (ref. = Leak only) | 0.26 | 1.30 | 0.045 |
| Recipient Age (per 10 year increase) | 0.13 | 1.14 | 0.045 |
| Recipient BMI (per unit increase) | 0.02 | 1.02 | 0.013 |
| Recipient Diagnosis of HCV | 0.28 | 1.33 | 0.025 |
| Rates beyond two years post-transplant | |||
| Time Since Transplant (per month) | −0.02 | 0.98 | 0.024 |
| LDLT (ref. = DDLT) | 1.05 | 2.85 | 0.064 |
| Initial Biliary Complication = Stricture or Stricture + Leak (ref. = Leak only) | 1.07 | 2.90 | 0.019 |
| At least 1 Roux Type Biliary Anastamosis (ref. = no Roux type) | 1.09 | 2.97 | 0.015 |
Following variables were tested in the models, but were not statistically significant: recipient sex, race, MELD, medical condition at transplant, encephalopathy, ascites, donor age, and number of arterial anastomoses.
Association of biliary complications with subsequent graft failure and death
In the absence of a biliary complication, the risk of graft failure for LDLT recipients was not significantly different from DDLT recipients (HR=1.26, p=0.25). Once a biliary complication occurred, the risk of subsequent graft loss, adjusted for recipient age, diagnosis, donor age, and packed red blood cell use, increased (DDLT: HR = 2.78, p=.0002; LDLT: HR=1.41, p=0.06). Following a biliary complication, the rate of graft failure or death among DDLT recipients was approximately twice that of LDLT recipients (graft failure HR=1.98, p=0.04; mortality HR=1.99, p=0.05). However, after performing the analysis excluding LDLT cut-surface leaks, the rate of graft failure or death was no longer significantly different in LDLT and DDLT recipients (graft failure HR=1.75, p=0.096; mortality HR=1.69, p=0.152). All patients in this cohort who developed a biliary leak were at increased risk of developing a subsequent stricture (HR = 1.79, p=0.01).
Discussion
Biliary complications are the major cause of morbidity following LDLT (7). While these complications can be intractable and potentially fatal, the incidence varies widely among transplant centers (8,9). The overall incidence of biliary complications ranges from 5% to 40% and they are associated with aberrant donor anatomy and biliary ischemia (10,11). At present, most biliary complications are diagnosed and treated using non-operative techniques (8). Several studies have retrospectively analyzed long-term complete resolution of biliary leak and/or stricture following therapeutic intervention (5,12,13,15). However, the cumulative morbidity to the recipient in the form of total number of procedures (invasive and non-invasive) and the time from onset of the complication to resolution have not been well documented. Most studies to date have evaluated clinical efficacy of a specific procedure type and do not quantify all procedures required per patient as a result of a biliary complication. In this study, we sought to document the pathway to resolution of biliary complications and quantify the related procedures in LDLT and DDLT recipients.
Percutaneous strategies can be used to repeatedly dilate strictures, place and remove biliary stents, perform sphincterotomies, and update ductal imaging. Unfortunately, the cumulative morbidity to the recipient is generally not studied in a comparative timeline based on graft type, using anatomic and clinical management details to provide a comparison of disability and/or human cost. Shah and colleagues recently reported a series of 41 LDLT recipients who had biliary complications (14). They noted that all but four patients with strictures were managed with non-operative interventions and 96% were free of any biliary complication at the time of publication. However, 13 of 19 patients with a bile leak required reoperation. A similar study reported on outcomes of 1,062 recipients, including 106 LDLTs, of which 224 developed a biliary complication treated by ERCP (5). During the ten year study period, over 700 ERCPs were performed with definitive success achieved in only 64%. Patients who received an LDLT graft or had both a leak and stricture were less likely to respond to endoscopic therapy. Finally, a recent review from Korea reports the success rate of endoscopic treatment for biliary stricture post-LDLT ranges from 37% to 71% (15).
We believe that the current study is the first to quantify the cumulative morbidity of biliary complications following LDLT in terms of diagnostic and therapeutic procedures performed and the time to complication resolution. In the A2ALL cohort, the incidence of biliary complications was higher in the LDLT group. However, the average number of procedures performed per patient was similar for DDLT and LDLT recipients as well as the average time from transplant to complication and the time from complication onset to resolution. The majority of biliary complications in all recipients were resolved within six months. However, two LDLT recipients had persistent unresolved biliary leak after 24 months of treatment and one DDLT recipient had an unresolved stricture after two years.
In this cohort, the incidence of biliary complications was higher in the LDLT group. Biliary stricture was the predominant manifestation of biliary complications in the DDLT group. Conversely, among LDLT recipients, nearly two-thirds of these complications comprised a combination of cut-surface bile leaks, which are a unique feature of the LDLT procedure, in addition to anastomotic bile leaks. In DDLT as well as LDLT recipients, the occurrence of biliary leaks was associated with a significantly increased risk of subsequent stricture.
It is not surprising that the majority of invasive and non-invasive procedures were performed in the first two years post-transplant. In fact, this study revealed a learning curve of biliary complication management as increased experience was directly associated with a shorter time to resolution. While there is a marked decline in the monthly rate of procedures after 24 months, it is important to note that it is not zero. Several LDLT and DDLT patients continued to require interventions for biliary complications up to 10 years after transplant. As experience has accumulated over time, the rate of procedures performed to treat these complications on an outpatient basis has dramatically increased for both LDLT and DDLT.
We have identified several factors associated with a significantly higher rate of procedures in the first two years, including older recipient age, higher BMI, and a primary diagnosis of HCV. However, donor source (DDLT vs. LDLT) was not significantly associated with procedure rates in this model. The occurrence of a biliary complication per se was associated with a higher risk of graft loss in both groups. This effect was greater in DDLT recipients when all biliary complications are considered, but was not statistically significant when cut-edge leaks – which occur in LDLT but not whole-organ DDLT grafts – were excluded from the analysis.
While these data were derived from eight large transplant centers across North America via detailed chart review at each center, as well as the SRTR database, we acknowledge several limitations. The current dataset is derived from a retrospective component of the A2ALL study and is of moderate size. Over the 13 year study period, clinical practice patterns may have changed with regard to how these difficult problems are treated. Furthermore, the diagnostic/therapeutic approach to a specific problem, as well as surgical techniques employed, may vary across centers. These differences may have influenced our results, but they are difficult to study.
Biliary complications are a formidable problem in liver transplantation whose incidence is higher following LDLT. However, once a complication has occurred, we have shown that the number of required interventions and the time to complete resolution were similar in the LDLT and DDLT recipients. These findings highlight the ongoing challenges of biliary complications after liver transplantation regardless of donor source. Overall, these data refute the common impression that biliary complications following LDLT are a more protracted problem and less likely to resolve compared to DDLT.
Acknowledgments
The authors acknowledge the contributions of the following study coordinators, coinvestigators, and administrative assistants at each of the participating institutions: Andrea Herman, R.N., Carlos Garcia, Michelle Jaramillo, and Rita Lerner at the University of Colorado Denver, Aurora, CO; Janet Mooney at the University of California, Los Angeles, CA; Dulce MacLeod, R.N., at the University of California, San Francisco, LA; Colleen Green and Royanne Dell, R.N., at the University of Virginia, Charlottesville, VA; Patrice Al-Saden at Northwestern University, Chicago, IL; Scott Heese at Columbia University, New York, NY; and Charlotte Hoffman at the Virginia Commonwealth University, Richmond, VA.
Source of Funding:
The patients participating in this trial were enrolled in the National Institutes of Health (NIH)-sponsored Adult-to-Adult Living Donor Liver Transplantation Cohort Study. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through cooperative agreements (NIDDK grant nos.: U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, and U01-DK62531). The trial was also funded by research grants from Schering-Plough, through a cooperative research and development agreement with NIH-NIDDK, and through a clinical trial agreement between Ortho-Biotech and NIH-NIDDK. This study was supported by the NIDDKD through cooperative agreements (listed in parentheses). Additional support was provided by the Health Resources and Services Administration and the American Society of Transplant Surgeons.
The A2ALL Study Group includes Northwestern University, Chicago, IL; University of California – Los Angeles, CA; University of California – San Francisco, CA; University of Colorado Health Sciences Center, Denver, CO; University of North Carolina, Chapel Hill, NC; Epidemiology and Clinical Trials Branch, Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD; University of Michigan, Ann Arbor, MI; Department of Surgery, Columbia Presbyterian Medical Center, New York, NY; University of Pennsylvania, Philadelphia, PA; Department of Internal Medicine, University of Virginia, Charlottesville, VA; and Virginia Commonwealth University, Richmond, VA. The following individuals were instrumental in the planning, conduct, and/or care of patients enrolled in this study at each of the participating institutions. Columbia University Health Sciences, New York, NY (DK62483): principal investigator, Jean C. Emond, M.D.; coprincipal investigator, Robert S. Brown, Jr., M.D., M.P.H.; study coordinators, Scott Heese, B.A., and Taruna Chawla, M.D. Northwestern University, Chicago, IL (DK62467): principal investigator, Michael M.I. Abecassis, M.D., M.B.A.; coprincipal investigator, Laura M. Kulik, M.D.; study coordinator, Patrice Al-Saden, R.N., C.C.R.C. University of Pennsylvania Health System, Philadelphia, PA (DK62494): principal investigator, Abraham Shaked, M.D., Ph.D.; coprincipal investigator, Kim M. Olthoff, M.D.; study coordinators, Brian Conboy, P.A., M.B.A., and Mary Shaw, R.N., B.B.A. University of Colorado Health Sciences Center, Denver, CO (DK62536): principal investigator, Gregory T. Everson, M.D.; coprincipal investigator, Igal Kam, M.D.; study coordinator, Andrea Herman, R.N. University of California Los Angeles, Los Angeles, CA (DK62496): principal investigator, Johnny C. Hong, M.D.; coprincipal investigator, Ronald W. Busuttil, M.D., Ph.D.; study coordinator, Janet Mooney, R.N., B.S.N. The principal investigator for LADR was Sammy Saab, M.D. University of California San Francisco, San Francisco, CA (DK62444): principal investigator, Chris E. Freise, M.D., F.A.C.S.; coprincipal investigator, Norah A. Terrault, M.D.; study coordinator, Dulce MacLeod, R.N. University of Michigan Medical Center, Ann Arbor, MI (DK62498): principal investigator, Robert M. Merion, M.D.; data coordinating center staff, Anna S.F. Lok, M.D., Akinlolu O. Ojo, M.D., Ph.D., Brenda W. Gillespie, Ph.D., Margaret Hill-Callahan, B.S., L.S.W., Terese Howell, B.S., C.C.R.C., Lisa Holloway, B.S., C.C.R.C., Monique Lowe, M.S., Abby Smith, B.A., and Abby Brithinee, B.A. University of North Carolina, Chapel Hill, NC (DK62505): principal investigator, Paul H. Hayashi, M.D., M.P.H.; study coordinator, Tracy Russell, M.A. University of Virginia (DK62484): principal investigator, Carl L. Berg, M.D.; study coordinator, Jaye Davis, R.N., and Colleen Green, P.A. The principal investigator for LADR was Abdullah M.S. Al-Osaimi, M.D. Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): principal investigator, Robert A. Fisher, M.D., F.A.C.S.; coprincipal investigator, R. Todd Stravitz, M.D.; study coordinators, April Ashworth, R.N., Andrea Lassiter, B.S., and Charlotte Hoffman, R.N. The principal investigator for LADR was Mitchell Shiffman, M.D. Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: James E. Everhart, M.D., M.P.H., Averell Sherker, M.D., and Jay H. Hoofnagle, M.D.
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- CTUS
CT scan or ultrasound
- DDLT
deceased donor liver transplant
- ERCP
endoscopic retrograde cholangiopancreatography
- HCV
hepatitis C virus
- LDLT
living donor liver transplant
- MELD
Model for End-Stage Liver Disease
- MRCP
magnetic resonance cholangiopancreatography
- PTC
percutaneous transhepatic cholangiography
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Conflicts of Interest
Dr. Zimmerman, Mr. Goodrich, Dr. Samstein, Dr. Abt, Dr. Hong, Dr. Baker, Dr. Merion, and Dr. Freise receive funding from NIH; they have no other conflicts of interests to declare. Dr. Kumer, Dr. Cotterell, and Dr. Everhart have no conflicts of interests to declare.
References
- 1.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–82. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379–92. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 3.Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776–82. doi: 10.1002/lt.21955. [DOI] [PubMed] [Google Scholar]
- 4.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569–79. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxbaum JL, Biggins SW, Bagatelos KC, Ostroff JW. Predictors of endoscopic treatment outcomes in the management of biliary problems after liver transplantation at a high-volume academic center. Gastrointest Endosc. 2011;73:37–44. doi: 10.1016/j.gie.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Shearon TH, Berg CL, Everhart JE, Abecassis MM, Shaked A, et al. Hospitalization rates before and after adult-to-adult living donor or deceased donor liver transplantation. Ann Surg. 2010;251:542–9. doi: 10.1097/SLA.0b013e3181ccb370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127–36. doi: 10.1002/lt.22381. [DOI] [PubMed] [Google Scholar]
- 8.El-Meteini M, Hamza A, Abdalaal A, Fathy M, Bahaa M, Mukhtar A, et al. Biliary complications including single-donor mortality: experience of 207 adult-to-adult living donor liver transplantations with right liver grafts. HPB (Oxford) 2010;12:109–14. doi: 10.1111/j.1477-2574.2009.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler S, Pascher A, Mittler J, Neumann U, Neuhaus P, Pratschke J. Management of biliary complications following living donor liver transplantation--a single center experience. Langenbecks Arch Surg. 2009;394:1025–31. doi: 10.1007/s00423-009-0506-8. [DOI] [PubMed] [Google Scholar]
- 10.Soin AS, Kumaran V, Rastogi AN, Mohanka R, Mehta N, Saigal S, et al. Evolution of a reliable biliary reconstructive technique in 400 consecutive living donor liver transplants. J Am Coll Surg. 2010;211:24–32. doi: 10.1016/j.jamcollsurg.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Terblanche J, Allison HF, Northover JM. An ischemic basis for biliary strictures. Surgery. 1983;94:52–7. [PubMed] [Google Scholar]
- 12.Gomez CM, Dumonceau JM, Marcolongo M, de Santibañes E, Ciardullo M, Pekolj J, et al. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation. 2009;88:1280–5. doi: 10.1097/TP.0b013e3181bb48c2. [DOI] [PubMed] [Google Scholar]
- 13.Kim TH, Lee SK, Han JH, Park do H, Lee SS, Seo DW, et al. The role of endoscopic retrograde cholangiography for biliary stricture after adult living donor liver transplantation: technical aspect and outcome. Scand J Gastroenterol. 2011;46:188–96. doi: 10.3109/00365521.2010.522722. [DOI] [PubMed] [Google Scholar]
- 14.Shah SA, Grant DR, McGilvray ID, Greig PD, Selzner M, Lilly LB, et al. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transplant. 2007;7:161–7. doi: 10.1111/j.1600-6143.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 15.Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut and Liver. 2011;5:133–42. doi: 10.5009/gnl.2011.5.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]