Abstract
Aldehyde dehydrogenase 1A1 (ALDH1A1), an enzyme that catalyzes the conversion of lipid aldehydes to lipid carboxylic acids, plays pleiotropic roles in UV-radiation resistance, melanogenesis, and stem cell maintenance. In this study, a combination of RNAi and pharmacologic approaches were used to determine which ALDH1A1 substrates and products regulate melanogenesis. Initial studies revealed that neither the UV-induced lipid aldehyde 4-hydroxy-2-nonenal nor the ALDH1A1 product all-trans retinoic acid appreciably induced melanogenesis. In contrast, both the ALDH1A1 substrate 9-cis retinal and its corresponding product 9-cis retinoic acid potently induced the accumulation of MITF mRNA, Tyrosinase mRNA, and melanin. ALDH1A1 depletion inhibited the ability of 9-cis retinal but not 9-cis retinoic acid to stimulate melanogenesis, indicating that ALDH1A1 regulates melanogenesis by catalyzing the conversion of 9-cis retinal to 9-cis retinoic acid. The addition of potent ALDH1A inhibitors (cyanamide or Angeli’s salt) suppressed Tyrosinase and MITF mRNA accumulation in vitro and also melanin accumulation in skin equivalents, suggesting that 9-cis retinoids regulate melanogenesis in the intact epidermis. Taken together, these studies not only identify cyanamide as a potential novel treatment for hyperpigmentary disorders, but also identify 9-cis retinoic acid as a pigment stimulatory agent that may have clinical utility in the treatment of hypopigmentary disorders, such as vitiligo.
Keywords: melanogenesis, retinoids, aldehyde dehydrogenase 1A1 (ALDH1A1), cyanamide (cya), 9-cis retinoic acid (9-cis RA)
Introduction
Melanin, a pigment produced by the epidermal melanocyte, protects the skin from the harmful effects of UV irradiation and is aberrantly regulated in many human skin diseases including vitiligo and melasma (1, 2). Genetic and biochemical studies have identified over 150 genes that regulate melanogenesis in mouse and human skin (3). This knowledge has led to the identification of pigment inhibitory agents, such as hydroquinone, that can effectively inhibit melanin production in vivo (3). Unfortunately, the clinical use of hydroquinone has been limited by its potential teratogenicity (4–7). Despite the fact that many genes that regulate melanogenesis have been identified, effective pharmacologic agents that can stimulate melanogenesis in hypopigmented skin are currently unavailable (8, 9). These observations highlight the continuing need to develop safe pigment modulatory agents for the treatment of pigmentary disorders.
Our group recently utilized a genome-wide RNAi screening approach to identify pharmaceutically tractable drug targets for the rational design of pigment modulatory agents (10). This approach identified 92 novel regulators of melanin production in human cells, including a number of genes that control the expression of microphthalmia-associated transcription factor (MITF), the central transcriptional regulator of melanogenesis, and tyrosinase (TYR), the enzyme that catalyzes the rate-limiting step in melanogenesis (1, 10–12). One of the novel regulators of MITF and TYR expression identified in our screen was ALDH1A1 (10), a well-characterized NAD (P)+-dependent enzyme that catalyzes the irreversible oxidation of highly reactive aliphatic and aromatic aldehydes to their corresponding non-toxic carboxylic acids (EC number 1.2.1.36) (13). The ALDH1A subfamily (ALDH1A1, ALDH1A2, ALDH1A3) is known to catalyze, with an equal efficiency, the oxidation of all-trans retinal and 9-cis retinal to all-trans retinoic acid and 9-cis retinoic acid, respectively (14, 15). Additionally, ALDH1A1 functions in the lens and cornea to detoxify 4-hydroxy-2-nonenal (4-HNE), a highly reactive lipid aldehyde that is generated from ultraviolet radiation induced lipid peroxidation reactions (16, 17). Other studies have revealed that ALDH1A1 is a marker of stem cells (18). In summary, ALDH1A1 is an enzyme that is able to regulate diverse processes ranging from stem cell maintenance (18), to UV-radiation resistance, to melanogenesis (10) by catalyzing the conversion of specific aldehydes to their corresponding carboxylic acids (1, 10, 16–20).
Previous work demonstrated that ALDH1A1 depletion in primary melanocytes and MNT-1 melanoma cells inhibited the accumulation of both TYR and MITF mRNA and protein (10). Additionally, two potent inhibitors of the ALDH1A family (cyanamide and Angeli’s salt) were shown to inhibit both TYR protein accumulation and melanin accumulation in normal human melanocytes (10). Recent studies have suggested that human melanocytes can catalyze the light-induced isomerization of 9-cis retinal and revealed that the addition of 9-cis retinal in conjunction with UVA radiation is sufficient to induce melanin accumulation in normal human melanocytes (21). In this study, we utilize a combination of RNAi and pharmacologic approaches to identify 9-cis retinal as the ALDH1A1 substrate that regulates melanogenesis.
Materials and Methods
Cell culture
Human MNT-1 melanoma cells were a gift from M. Marks (University of Pennsylvania). These cells were cultured in DMEM (CellGro) supplemented with 15% fetal bovine serum (CellGro), sodium pyruvate (Invitrogen), L-glutamine (Invitrogen), MEM vitamin solution (Invitrogen), antibiotic-antimycotic (Invitrogen), and 10% AIM-V medium (Invitrogen). For the melanin quantitation experiments, MNT-1 cells were switched to DMEM minus phenol red (CellGro) supplemented with 10% fetal bovine serum, sodium pyruvate, L-glutamine and antibiotic-antimycotic as previously described (10). Human deeply-pigmented neonatal epidermal melanocytes (Invitrogen) were cultured in Medium 254 (Invitrogen) supplemented with phorbol 12-myristate 13-acetate-free Human Melanocyte growth supplement-2 (Invitrogen). As these melanocyte strains were purchased from commercial entities, no IRB approval was required prior to their use.
Drug Treatment
Briefly, MNT-1 cells or deeply-pigmented (DP) melanocytes were plated at a density of 1.5 × 104 cells per well of a 96-well microtiter plate and allowed to re-attach overnight. Subsequently, cells were incubated with varying concentrations of cyanamide (Sigma Aldrich), Angeli’s salt (Sodium α-oxyhyponitrite, chemical name; disodium diazen-1-ium-1,2,2-triolate, formal name; Cayman Chemical), 4-HNE (4-hydroxy-2E-nonenal, formal name; Cayman Chemical), 9-cis retinal, 9-cis retinoic acid, all-trans retinal or all-trans retinoic acid at the indicated concentrations (Sigma Aldrich). The cells were incubated with the retinoids for merely 30–45 minutes to avoid cellular toxicity. In the case of cyanamide, Angeli’s salt and 4-HNE, cells were incubated in each drug for at least 24 hours. For the UV experiments, cells were incubated in media containing 9-cis retinal for 30 minutes before being irradiated with the indicated dose of UV light. Media was immediately refreshed after UV irradiation, and RNA was isolated from these cells 24 hours later. For the melanin quantitation experiments, cells were allowed to grow to confluency post 9-cis retinal/UVA treatment.
Real-time quantitative PCR for mRNA
A Cells-to-Ct kit was utilized to lyse the cells after the appropriate drug treatment (Applied Biosystems). A high capacity RNA-to-cDNA kit was then employed to generate cDNA (Applied Biosystems). Solaris qPCR gene expression assays for MITF (AX-008674-00-0100), TYR (AX-012555-00-0200), ALDH1A1 (AX-008722-00-0100), β-actin (AX-003451-00-0100) and GAPDH (AX-004253-00-0100) were obtained from Thermo Scientific (Dharmacon RNAi technologies) and used with TaqMan Gene Expression Master Mix (Applied Biosystems) to complete the PCR reaction. A 7900HT Fast Real-Time PCR system (Applied Biosystems) and SDS 2.4 (Applied Biosystems) were used to determine Ct values for each sample. Values were normalized to either β-actin or GAPDH using the relative quantification mathematical model (Pfaffl) as previously described (10). A two-tailed Student’s t-test was employed to determine statistical significance.
Western blotting
MNT-1 or DP melanocytes were plated in 96-well plates at a concentration of 2 × 104 cells per well, allowed to re-attach overnight, and then incubated with drug for 30 hours. After treatment, the number of cells in each well was quantified and lysates were prepared using the RIPA lysis buffer system (RIPA supplemented with protease inhibitor, PMSF and sodium orthovanadate; Santa Cruz Biotechnology). Lysates were then clarified by centrifugation (3,000 rpm for 10 minutes at 4°C). The relative concentration of protein in each lysate was quantified using both a BCA Protein Assay Kit (Thermo Scientific) and a Coomassie (Bradford) protein assay (Thermo Scientific) to eliminate the effects of interfering melanin. A total of 20 μg of protein per sample was separated on a 4–20% SDS-polyacrylamide gel (Bio-Rad) under reducing conditions and transferred onto to a 0.45-μm nitrocellulose membrane (Bio-Rad). Subsequently, membranes were blocked in a blocking buffer solution comprised of TBS (Fisher Scientific), 0.1% Tween-20 (Fisher Scientific) and 5% non-fat milk powder (Apex).
The following antibodies were used: rabbit polyclonal α/β-tubulin, #2148, Cell Signaling Technology; goat anti-rabbit IgG, HRP-linked, #7074, Cell Signaling Technology; mouse monoclonal tyrosinase, sc-20035, Santa Cruz Biotechnology; bovine anti-mouse IgG-HRP sc-2371, Santa Cruz Biotechnology. To assess immunoreactivity, either a SuperSignal West Dura Extended Duration Substrate or a SuperSignal West Pico Chemiluminescent Substrate was used according to manufacturer’s directions (Pierce Protein Biology Products). Protein levels were assessed using densitometry analysis (ImageJ).
RNA interference
1.5 × 104 MNT-1 melanoma cells were reverse-transfected in 96-well microtiter plates with 50 nM ALDH1A1 or control siRNAs using a Dharmafect 2 transfection reagent as previously described (10). A non-targeting control (SMARTpool; D-001810-10-20; Thermo Scientific, Dharmacon RNAi Technologies) was used as the negative control, while three different siRNA oligos purchased from Ambion (s1236, s1237, s1238) were pooled for the ALDH1A1 depletion experiments. Transfected cells were allowed to remain in the transfection media for 72 hours before drug treatment to ensure protein knockdown. Real-time quantitative RT-PCR was used to verify that ALDH1A1 siRNA effectively inhibited the expression of ALDH1A1.
Pigment Measurement
MNT-1 cells were plated at a density of 1 × 104 cells per well in a 96-well plate, allowed to re-attach overnight, and incubated in the indicated concentration of drug for four days. Cells were then lysed using a Cell-Titer Glo reagent (Promega), and relative melanin accumulation was quantified by measuring the absorbance of each well at 405 nm and normalizing this value to the relative cell number in each well as determined by the Cell-Titer Glo assay (10). For the UVA experiments, cells were incubated with the indicated concentration of 9-cis retinal for 30 minutes, and then irradiated with the indicated dose of UVA. Media was changed immediately after UVA treatment to avoid toxicity. Cells were then lysed four days after UVA treatment according to the protocol described above. The relative pigment index (PI) was calculated by dividing the absorbance values at 405 nm by the Cell-Titer Glo luminescence values, and then normalizing these values to the experimental control (vehicle-treated wells). To calculate the % pigment induction, the following equation was used: (PI of test – PI control (vehicle)) x 100%. A Student’s two-tailed t-test was used to calculate the statistical significance of each value compared to the vehicle-treated control.
Maintenance and treatment of three-dimensional skin equivalents
MelanoDerm deeply-pigmented three-dimensional skin equivalents were obtained from MatTek Corporation (Ashland, MA) and maintained according to the manufacturer’s instructions. The equivalents were maintained in a humidified incubator at 37°C with 5% CO2 in EPI-100-NMM-113 media as prepared by MatTek (DMEM with 5 μg/mL gentamicin, 0.25 μg/mL amphotericin, α-MSH, β-FGF, and KGF). Both the media and the drug were refreshed every other day for three weeks before the samples were harvested for a solvable melanin assay. Cyanamide (600 μM) and kojic acid (2%) were dissolved in water before being added to the equivalents at a total volume of 20 μL. The relative melanin content of each skin equivalent after drug treatment was determined using a solvable melanin assay (22). A Student’s two-tailed t-test was utilized to determine whether cyanamide or kojic acid significantly inhibited the accumulation of melanin in skin equivalents.
Results
9-cis retinal is the ALDH1A1 substrate that stimulates melanogenesis
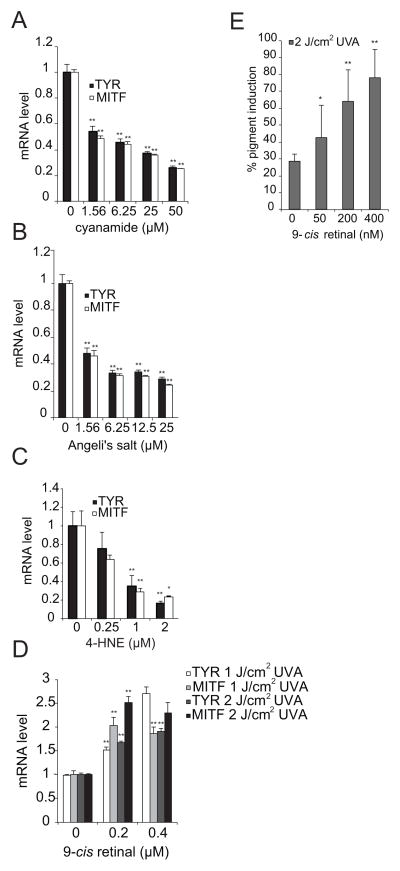
While published studies have defined lipid aldehydes that are detoxified by ALDH1A1 and identified ALDH1A1 substrates that promote cellular differentiation (17, 19, 23, 24), it is currently unclear which ALDH1A1 substrates regulate melanogenesis. Initial studies sought to verify that the ALDH1A family regulates melanogenesis by a catalytic mechanism. Two potent inhibitors of ALDH1A, cyanamide and Angeli’s salt, inhibited the accumulation of TYR and MITF mRNA in a dose responsive manner (Figures 1A–1B), consistent with previous observations that ALDH1A1 depletion inhibited the accumulation of TYR and MITF protein (10).
Figure 1. 9-cis retinal is the ALDH1A1 substrate that stimulates melanogenesis.
(A) The ALDH1A inhibitor cyanamide inhibits TYR and MITF mRNA accumulation. MNT-1 melanoma cells were treated with the indicated doses of cyanamide for 24 hours and the relative expression of TYR and MITF mRNA was quantified as described in the methods. (B) The ALDH1A inhibitor Angeli’s salt inhibits TYR and MITF mRNA accumulation. MNT-1 melanoma cells were treated with the indicated doses of Angeli’s salt for 24 hours and the relative expression of TYR and MITF mRNA was quantified as described in the methods. (C) 4-HNE is not sufficient to induce the accumulation of either TYR or MITF mRNA. MNT-1 melanoma cells were treated with the indicated doses of 4-HNE for 24 hours, and TYR and MITF mRNA expression was quantified as described in the methods. (D) In conjunction with UVA radiation, 9-cis retinal induces the accumulation of both TYR and MITF mRNA. MNT-1 melanoma cells were pre-incubated with 9-cis retinal at the indicated concentrations for 45 minutes before being irradiated with UVA light as indicated. Relative mRNA expression was quantified as described in the methods. All data shown are mean ± S.D. (n=3 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control. (E) Melanin accumulation is induced by 9-cis retinal in conjunction with UVA radiation. MNT-1 melanoma cells were treated with the indicated doses of 9-cis retinal, irradiated with UVA, and then allowed to reach confluency. Melanin accumulation was measured as described in the methods section. Data shown are mean ± S.D. (n=6 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control.
Once we had determined that ALDH1A inhibitors could block melanogenesis, we next sought to identify putative substrates of ALDH1A that regulate melanogenesis. ALDH1A1 exhibits a high affinity for 4-HNE, one of the most abundant α, β-unsaturated aldehydes generated from UV-induced lipid peroxidation reactions (17, 19, 20). Interestingly, 4-HNE not only inhibited the accumulation of melanin (Figure S1A) but also the accumulation of TYR and MITF mRNA in MNT-1 cells (Figure 1C), indicating that ALDH1A regulates melanogenesis by a mechanism that is distinct from its role in inhibiting corneal opacification (16, 17, 19).
We next sought to determine whether other known ALDH1A substrates stimulate melanogenesis. In addition to 4-HNE, ALDH1A also catalyzes the oxidation of retinal to retinoic acid (15, 25, 26). Notably, ALDH1A can metabolize both all-trans retinal and 9-cis retinal to their corresponding carboxylic acids with equal efficiency (14). Recently published studies have demonstrated that 9-cis retinal can promote melanin accumulation in the context of UVA irradiation (14, 21). Therefore, we sought to define specific retinaldehydes that stimulate melanogenesis. All-trans retinal stimulated melanogenesis at relatively high doses (Figure S1B). These observations could be secondary to a direct stimulatory effect of all-trans retinal on melanogenesis, or secondary to the fact that all-trans retinal can be isomerized to 9-cis retinal, as suggested by others (21, 27). Interestingly, 9-cis retinal in conjunction with UVA irradiation stimulated the accumulation of TYR and MITF mRNA in both MNT-1 melanoma cells and primary melanocytes (Figures 1D, S1C). Similarly, the combination of 9-cis retinal treatment and UVA exposure induced melanin accumulation in MNT-1 cells, even though this treatment also induced cellular cytotoxicity (Figures 1E, S2A, S2B).
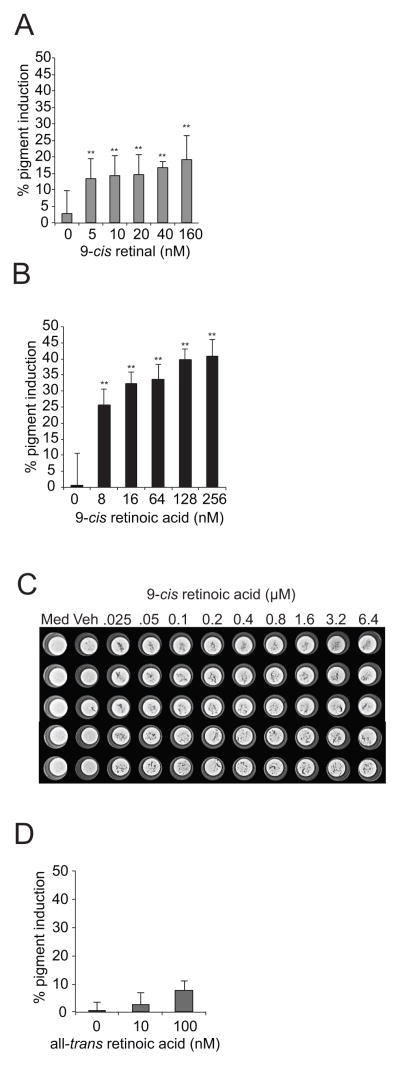
The metabolic product of 9-cis retinal oxidation, 9-cis retinoic acid, acts to stimulate melanin accumulation
While cis-retinal isomerization to trans-retinal is UV dependent, the oxidation of retinal by ALDH1A is not (28). In order to better establish whether 9-cis retinal or all-trans retinal regulates melanogenesis, we next sought to determine whether 9-cis retinal could stimulate melanin accumulation in the absence of UV-induced isomerization. 9-cis retinal induced pigment accumulation in a dose-responsive manner in the absence of UV irradiation (Figure 2A). As 9-cis retinal was able to induce pigment accumulation independently of UV-irradiation, we reasoned that the addition of 9-cis retinoic acid, the oxidation product of 9-cis retinal catalyzed by ALDH1A (15, 25), should also stimulate melanin accumulation. As expected, 9-cis retinoic acid stimulated melanin accumulation in MNT-1 cells both visually and quantitatively (Figures 2B, 2C). Interestingly, 9-cis retinoic acid stimulated melanin accumulation more effectively than 9-cis retinal, providing evidence that the product of ALDH1A catalysis is the actual species that stimulates melanogenesis (Figures 2A, 2B). In contrast, all-trans retinoic acid did not induce melanin accumulation (Figure 2D), providing further evidence that 9-cis retinoids and not all-trans retinoids stimulate melanogenesis.
Figure 2. The metabolic product of 9-cis retinal oxidation, 9-cis retinoic acid, acts to stimulate melanin accumulation.
(A) 9-cis retinal alone is sufficient to induce melanin accumulation. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinal and then allowed to reach confluency. Melanin accumulation was measured as described in the methods section. (B) 9-cis retinoic acid potently stimulates melanin accumulation. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinoic acid and allowed to reach confluency. Melanin accumulation was measured as described in the methods section (C) A light micrograph of a representative opaque-walled, clear-bottomed 96-well microtiter plate containing MNT-1 cells treated with 9-cis retinoic acid is shown. Med, wells containing only media; Veh, cells treated with vehicle. (D) All-trans retinoic acid does not appreciably induce melanin accumulation. MNT-1 melanoma cells were briefly treated with the indicated doses of all-trans retinoic acid and allowed to reach confluency. Melanin accumulation was measured as described in the methods section. Data shown are mean ± S.D. (n=6 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control.
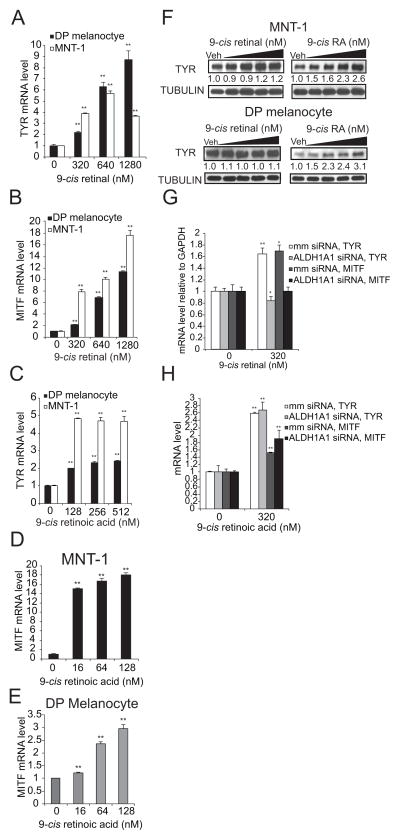
9-cis retinoic acid stimulates the accumulation of TYR and MITF mRNA and TYR protein
Based on the observation that both 9-cis retinal and 9-cis retinoic acid stimulated melanin accumulation in the absence of UVA radiation, we reasoned that the 9-cis retinoids should also induce the accumulation of TYR and MITF mRNA. As predicted, both 9-cis retinal and 9-cis retinoic acid induced the accumulation of TYR (Figures 3A, 3C) and MITF (Figures 3B, 3D, 3E) mRNAs in both primary melanocytes and MNT-1 melanoma cells, demonstrating that either species alone is sufficient to stimulate the transcription of critical regulators of melanogenesis. 9-cis retinal was not sufficient to induce TYR protein accumulation in either MNT-1 cells (upper panel) or primary melanocytes (lower panel) (Figure 3F). However, 9-cis retinoic acid induced the accumulation of TYR protein in a dose responsive manner in both skin cell types (Figure 3F). These observations could either be secondary to the fact that only a small fraction of 9-cis retinal is converted to 9-cis retinoic acid or due to the fact that the slow kinetics of the reaction prevented us from observing the stimulatory effect of 9-cis retinal on TYR protein accumulation within the experimental timeframe. Nonetheless, these results suggest that ALDH1A1 regulates melanogenesis by catalyzing the conversion of 9-cis retinal to 9-cis retinoic acid.
Figure 3. ALDH1A Regulates Melanogenesis by Converting 9-cis retinal to 9-cis retinoic acid.
(A–B) 9-cis retinal induces the accumulation of TYR and MITF mRNA independently of UVA. Primary melanocytes and MNT-1 cells were pre-incubated with 9-cis retinal at the indicated doses for 45 minutes. Relative accumulation of TYR (A) and MITF (B) mRNA was measured as described in the methods section. (C–E) 9-cis retinoic acid induces the accumulation of TYR and MITF mRNA. Primary melanocytes and MNT-1 cells were pre-incubated with 9-cis retinoic acid at the indicated concentrations for 45 minutes. Cells were harvested 20 hours post drug treatment, and the relative accumulation of TYR (C) and MITF (D, E) mRNA was quantified as described in methods. (F) 9-cis retinoic acid but not 9-cis retinal induces the accumulation of TYR protein in both primary melanocytes and MNT-1 melanoma cells. MNT-1 melanoma cells (upper panel) and primary melanocytes (lower panel) were pre-incubated with the indicated doses of 9-cis retinal or 9-cis retinoic acid for 45 minutes. 28 hours later, the relative accumulation of TYR protein was quantified by immunoblotting. The numerical values represent the relative intensity of the TYR band normalized to the Tubulin band (loading control) for each lane divided by the relative expression of the TYR band in the vehicle control. (G–H) 9-cis retinal induces the accumulation of TYR and MITF mRNAs in an ALDH1A1 dependent manner. MNT-1 melanoma cells transiently transfected with either mismatch control or ALDH1A1 siRNAs for 72 hours were briefly treated with the indicated doses of 9-cis retinal (G) or 9-cis retinoic acid (H). Cells were then harvested 20 hours post drug treatment and the relative accumulation of TYR and MITF mRNA was measured. All data are mean ± S.D. (n=3 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control.
9-cis retinoic acid is able to stimulate the accumulation of TYR and MITF mRNAs in the absence of endogenous levels of ALDH1A1
To verify that ALDH1A regulates melanogenesis via the oxidation of 9-cis retinal, we examined whether 9-cis retinal could induce melanogenesis in an ALDH1A1 independent manner. Initial studies identified pooled ALDH1A1 siRNAs that effectively inhibited the expression of ALDH1A1 mRNA (Fig S2C). As theorized, ALDH1A1 depletion inhibited the ability of 9-cis retinal (Figure 3G) but not of 9-cis retinoic acid (Figure 3H) to stimulate the accumulation of TYR and MITF mRNA. These results confirm that the product of ALDH1A1 oxidation, 9-cis retinoic acid, stimulates melanogenesis, because the addition of 9-cis retinal in the absence of ALDH1A1 did not induce the accumulation of TYR and MITF mRNAs.
Cyanamide is able to inhibit the production of melanin in a three-dimensional model of human skin
Once we had determined how ALDH1A regulates melanogenesis in vitro, we next sought to determine whether ALDH1A activity regulates melanogenesis in a more physiologic model of human skin. For these experiments, we obtained skin equivalents containing normal human epidermal keratinocytes and melanocytes from Mattek, an equivalent model that has been used by other groups to assess the efficacy of skin depigmenting agents (22). Using doses that had been used previously to inhibit melanogenesis in primary melanocytes (10), we determined that cyanamide was able to inhibit melanin accumulation quantitatively as measured by a solvable melanin assay (Fig 4A), as well as both visually and histologically as measured by Fontana Masson staining (Figure 4B).
Figure 4. ALDH1A Controls Melanogenesis in Skin Equivalents.
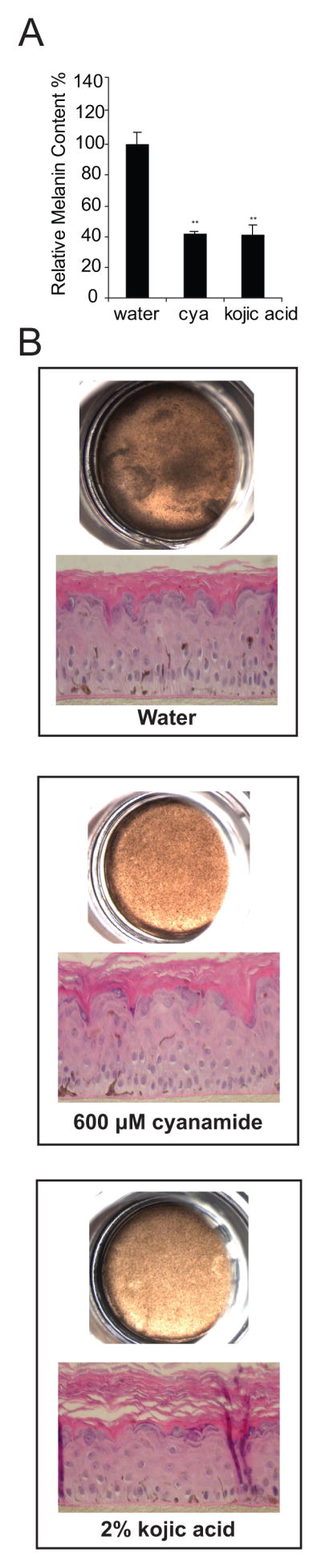
(A) Cyanamide inhibits melanin accumulation in skin equivalents as measured by a solvable melanin assay. Mattek darkly pigmented skin equivalents were incubated with 600 μM cyanamide, 2% kojic acid or water for 21 days. Melanin accumulation was quantified using a solvable melanin assay according to the manufacturer’s protocol. (B) Equivalents were photographed using a dissecting microscope and then fixed and stained with a Fontana Masson silver stain to quantify the relative accumulation of melanin.
Additionally, we sought to investigate whether 9-cis retinoic acid could induce melanogenesis in human skin equivalents. Preliminary studies revealed that neither forskolin (a well established pigment agonist) (29) nor 9-cis retinoic acid is able to quantitatively stimulate melanogenesis in lightly or darkly pigmented skin equivalents (data not shown). While an abundance of literature has documented the utility of skin equivalent models for quantifying the efficacy of melanogenesis inhibitors (22, 30–34), this system has not yet been optimized to quantify the efficacy of pigment agonists. Our preliminary results suggest that further optimization of these models is required before they can be used to quantify the efficacy of pharmacologic agents that induce melanogenesis.
Discussion
In this study, we sought to define ALDH1A substrates and products that regulate melanogenesis. Initial studies revealed that 9-cis retinal and 9-cis retinoic acid stimulated melanin accumulation, while all-trans retinoic acid was unable to stimulate melanin accumulation (Figures 1E, S2A, 2A, 2B, 2D). These results identify a novel role for the 9-cis retinoids in regulating melanogenesis. Consistent with this observation, we observed that the addition of 9-cis retinoic acid potently induced the accumulation of MITF and TYR mRNA, and TYR protein in melanocytic cells (Figures 3C–3F).
9-cis retinal was able to induce the accumulation of MITF and TYR mRNA at high doses, but was unable to induce the accumulation of TYR protein (Figures 3A, 3B, 3F). It is currently unclear what percent of the added 9-cis retinal is converted to 9-cis retinoic acid in our experiments. This makes it difficult to compare the relative effects of 9-cis retinal and 9-cis retinoic acid on the accumulation of MITF and TYR mRNA. Moreover, the interpretation of the results of these experiments is further complicated by the fact that the added 9-cis retinoic acid could also be converted to all-trans retinoic acid (35), and all-trans retinoic acid and 9-cis retinoic acid can synergistically activate RXR-RAR heterodimers (35, 36).
In light of these complications, we sought to better clarify whether 9-cis retinoic acid independently regulates melanogenesis. Depletion of ALDH1A1 inhibited the ability of 9-cis retinal but not 9-cis retinoic acid to stimulate melanogenesis (Figures 3G, 3H). This indicates that 9-cis retinoic specifically stimulates melanogenesis. Finally, ALDH1A inhibitors also inhibited the accumulation of MITF and TYR mRNAs in vitro and melanin accumulation in skin equivalents, further implicating a role for 9-cis retinoic acid in stimulating melanogenesis (Figures 1A–1B, 4A–B). Future studies will establish better models to examine the independent effects of different retinoid isomers on melanogenesis in human skin.
A multitude of studies to date have sought to determine whether all-trans retinoic acid inhibits or stimulates melanogenesis. While a number of studies demonstrate that all-trans retinoic acid inhibits melanogenesis (37–40), an equal number of studies propose that it stimulates melanogenesis or augments UV-induced melanogenesis in a variety of mouse and human cell lines (41–44). In this study, we approached this question by attempting to identify ALDH1A1 substrates and products that regulate melanogenesis. Our results revealed that all-trans retinoic acid was able to stimulate melanogenesis at high doses, while 9-cis retinoic acid was able to potently stimulate melanogenesis. It is particularly intriguing that 9-cis retinoic acid, which is not thought to have a functional role in the physiology of epidermal keratinocytes (45), appears to have a specific role in regulating the differentiation of epidermal melanocytes. Future studies will determine whether 9-cis retinoic acid related agents (rexinoids) are selective agents that can induce melanogenesis.
Extensive studies have sought to better define how retinoids and rexinoids regulate transcription. The biological effects of 9-cis retinoic acid are primarily mediated by its cognate receptor, the retinoid X receptor (RXR), of which there are three isoforms (α, β, and γ) (23, 46). While the retinoic acid receptor (RAR) can be activated by either 9-cis retinoic acid or all-trans retinoic acid, RXRs are activated exclusively by 9-cis retinoic acid (46, 47). Along with RARs, RXRs are members of the steroid hormone receptor superfamily of ligand-activated transcription factors (24, 48). RXRs are known to dimerize with a variety of nuclear receptors, including the vitamin D receptor (VDR), the peroxisome proliferator-activated receptor (PPAR), and the thyroid hormone receptor (T3R) (49–51). RXR homodimers act as potent inhibitors of transcription in the absence of ligand and only activate transcription upon ligand binding (52).
In the absence of ligand, RXR interacts with co-repressor proteins including silencing mediator for retinoid and thyroid hormone receptors (SMRT) and nuclear receptor co-repressor (N-CoR) (52, 53). These homologous proteins mediate the repressive effect of un-liganded receptors by recruiting histone deacetylase complexes to effectively silence the chromatin (52, 53). Upon ligand binding, RXR recruits such proteins as CBP/p300, p300/CBP-associated factor and members of the SRC/p160 family (SRC-1/NCoA-1, TIF-2/GRIP-1/NCoA-2/SRC-2 and pCIP/ACTR/A1B1/TRAM1/RAC3/SRC-3), which possess intrinsic histone acetylase transferase activity and potentiate the transcriptional activity of ligand-bound receptors (52, 54, 55).
Interestingly, when we searched the MITF promoter region for putative RXR binding sites, we discovered that while this promoter did not contain RXR-VDR or RXR-T3R binding sites, it did contain a highly conserved RAR-RXR heterodimer binding site located proximal to the transcription start site (56, 57). While only RAR agonists are required to activate transcription downstream of RAR-RXR heterodimers, it has been demonstrated that the addition of RAR and RXR agonists can synergistically stimulate transcription from these sites (58, 59). Other studies have determined that the vitamin D receptor (VDR) binds its enhancer element as a heterodimer with RXR in the human epidermis, while the thyroid hormone receptor, another potential binding partner of RXR, is expressed in the epidermis (60–62). Taken together, these studies indicate not only that RXR can play pleiotropic roles in regulating the differentiation of the epidermis but also that agents that can stimulate both RAR and RXR may be more effective at inducing melanogenesis as compared to RAR agonists alone.
Although there are three unique isoforms of RXR (α, β, and γ) RXRα is the most abundant isoform expressed in the epidermis (24, 50, 51). Interestingly, RXRα mutant mice demonstrate a premature greying phenotype, which is first visible in the snout hairs and then spreads to the truncal hair (63). Subsequent to the hair graying, these mice develop a rapidly progressive alopecia characterized by the widespread accumulation of keratinous cysts (63). Keratinocyte lineage-specific RXRα knockout mice develop progressive alopecia and accumulate keratinous cysts but do not exhibit premature hair graying (64, 65), suggesting that RXRα may have independent effects on melanogenesis and keratinocyte differentiation.
Vitiligo is characterized by the destruction of melanocytes (66). New melanocytes must migrate into the de-pigmented area of skin before re-pigmentation can occur (66). The major reservoir for melanocyte replacement is within the outer root sheath or bulge area of the hair follicle (66). Previous studies have demonstrated that retinoic acid stimulates the differentiation of melanoblasts to melanocytes as characterized by the synthesis of pigment in the melanoblasts (67). When coupled with our observations that RXR agonists can specifically stimulate melanogenesis in melanocytes, our studies implicate a role for RXR agonists as drugs with the potential to re-induce melanogenesis in vitiliginous skin. Future studies will optimize existing skin equivalent models to quantitatively determine whether RXR agonists can stimulate melanogenesis in skin equivalents with the goal of developing selective agents that can induce melanogenesis in vitiliginous skin.
Supplementary Material
(A) UV-induced oxidation products do not stimulate melanogenesis. MNT-1 melanoma cells were treated with the indicated doses of 4-HNE and then allowed to reach confluency. Melanin accumulation was measured as described in the methods. (B) All trans-retinal does not induce melanogenesis. MNT-1 melanoma cells were briefly treated with the indicated doses of all-trans retinal and then allowed to reach confluency. Melanin accumulation was measured as described in the methods. (C) In conjunction with UVA, 9-cis retinal induces the accumulation of TYR and MITF mRNA in primary melanocytes. Normal human deeply-pigmented melanocytes were incubated with 9-cis retinal at the indicated concentrations for 45 minutes and then immediately irradiated with the indicated dose of UVA light. Cells were then harvested 24 hours post UVA treatment and the relative accumulation of TYR and MITF mRNA was quantified. Data shown in Figures S1A, and S1B are mean ± S.D. (n=6, as indicated by the error bars). Data shown in Figure S1C is mean ± S.D. (n=3 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control.
(A) 9-cis retinal induces the accumulation of melanin in MNT-1 melanoma cells in conjunction with UVA radiation. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinal and then irradiated with the indicated dose of UVA. Cells were then allowed to reach confluency. Melanin accumulation was measured as described in methods.
(B) The combination of 9-cis retinal treatment and UVA stimulation is toxic to MNT-1 melanoma cells. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinal and then immediately irradiated with 2 J/cm2 of UVA light. Cells were then allowed to reach confluency and then subsequently lysed with the addition of Cell-Titer Glo reagent as previously described in the methods. The relative percentage of surviving cells was calculated by normalizing the luminescence value of drug treated cells to that of vehicle-treated control cells. (C) ALDH1A1 siRNAs inhibit ALDH1A1 mRNA expression. MNT-1 melanoma cells were transiently transfected with either mismatch control or ALDH1A1 siRNAs. Target gene expression was measured 72 hours post transfection by RT-qPCR. Data shown in Figure S2A and S2B is mean ± S.D. (n=6). Data shown in Fig S2C is mean ± S.D. (n=3, as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus mismatch-transfected control.
Acknowledgments
We thank Jonathan Schilling and Priya Vasudeva for their technical assistance. This work was supported by a grant from the National Institutes of Health (1K08AR056001) to Anand K. Ganesan.
E. Paterson, H. Ho and R. Kapadia performed the research
E. Paterson, H. Ho and A. Ganesan designed the research study
E. Paterson, H. Ho and R. Kapadia analyzed the data
E. Paterson and A. Ganesan wrote the manuscript
References
- 1.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 2.Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis--controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 4.Grimes P, Nordlund JJ, Pandya AG, Taylor S, Rendon M, Ortonne JP. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol. 2006;54:S255–261. doi: 10.1016/j.jaad.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Brenner M, Hearing VJ. Modifying skin pigmentation - approaches through intrinsic biochemistry and exogenous agents. Drug Discov Today Dis Mech. 2008;5:e189–e199. doi: 10.1016/j.ddmec.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang CH. Ov-16 [4-(3,4-dihydroxybenzoyloxymethyl)phenyl-O-β-D-glucopyranoside] inhibits melanin synthesis by regulating expressions of melanogenesis-regulated gene and protein. Exp Dermatol. 2011;20:743–748. doi: 10.1111/j.1600-0625.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahn KS, Moon KY, Lee J, Kim YS. Downregulation of NF-kappaB activation in human keratinocytes by melanogenic inhibitors. J Dermatol Sci. 2003;31:193–201. doi: 10.1016/s0923-1811(03)00039-2. [DOI] [PubMed] [Google Scholar]
- 8.Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419–431. doi: 10.1111/j.1346-8138.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaidou E, Antoniou C, Stratigos A, Katsambas AD. Narrowband ultraviolet B phototherapy and 308-nm excimer laser in the treatment of vitiligo: a review. J Am Acad Dermatol. 2009;60:470–477. doi: 10.1016/j.jaad.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan AK, Ho H, Bodemann B, et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, Yoon HD, Kim JI, Choi JS, Byun DS, Kim HR. Dioxinodehydroeckol inhibits melanin synthesis through PI3K/Akt signalling pathway in α-melanocyte-stimulating hormone-treated B16F10 cells. Exp Dermatol. 2012;21:471–473. doi: 10.1111/j.1600-0625.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 13.Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 15.Labrecque J, Dumas F, Lacroix A, Bhat PV. A novel isoenzyme of aldehyde dehydrogenase specifically involved in the biosynthesis of 9-cis and all-trans retinoic acid. Biochem J. 1995;305 (Pt 2):681–684. doi: 10.1042/bj3050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassen N, Bateman JB, Estey T, et al. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knockout mice. J Biol Chem. 2007;282:25668–25676. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassen N, Black WJ, Estey T, Vasiliou V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100–112. doi: 10.1016/j.semcdb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Koppaka V, Thompson DC, Vasiliou V. Focus on molecules: ALDH1A1: from lens and corneal crystallin to stem cell marker. Exp Eye Res. 2012;102:105–106. doi: 10.1016/j.exer.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black W, Vasiliou V. Ocular Metabolism and Disposition of 4-Hydroxy-2-nonenal. Cutaneous and Ocular Toxicology. 2005;24:165–176. [Google Scholar]
- 20.Vasiliou V, Pappa A, Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact. 2000;129:1–19. doi: 10.1016/s0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 21.Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21:1906–1911. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni-Komatsu L, Tong C, Chen G, Brindzei N, Orlow SJ. Identification of quinolines that inhibit melanogenesis by altering tyrosinase family trafficking. Mol Pharmacol. 2008;74:1576–1586. doi: 10.1124/mol.108.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangelsdorf DJ, Borgmeyer U, Heyman RA, et al. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 24.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 25.Molotkov A, Duester G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J Biol Chem. 2003;278:36085–36090. doi: 10.1074/jbc.M303709200. [DOI] [PubMed] [Google Scholar]
- 26.Fan X, Molotkov A, Manabe S, et al. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futterman S, Rollins MH. The catalytic isomerization of all-trans-retinal to 9-cis-retinal and 13-cis-retinal. J Biol Chem. 1973;248:7773–7779. [PubMed] [Google Scholar]
- 28.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 30.Jain P, Sonti S, Garruto J, Mehta R, Banga AK. Formulation optimization, skin irritation, and efficacy characterization of a novel skin-lightening agent. J Cosmet Dermatol. 2012;11:101–110. doi: 10.1111/j.1473-2165.2012.00610.x. [DOI] [PubMed] [Google Scholar]
- 31.Smiles KA, Dong KK, Canning MT, Grimson R, Walfield AM, Yarosh DB. A hydroquinone formulation with increased stability and decreased potential for irritation. J Cosmet Dermatol. 2007;6:83–88. doi: 10.1111/j.1473-2165.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 32.Goh MJ, Park JS, Bae JH, Kim DH, Kim HK, Na YJ. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother Res. 2012;26:1107–1112. doi: 10.1002/ptr.3682. [DOI] [PubMed] [Google Scholar]
- 33.Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 34.Kondo S, Takahashi T, Yoshida K, Mizoguchi H. Inhibitory effects of autolysate of Leuconostoc mesenteroides isolated from kimoto on melanogenesis. J Biosci Bioeng. 2012;114:424–428. doi: 10.1016/j.jbiosc.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Shih TW, Lin TH, Shealy YF, Hill DL. Nonenzymatic isomerization of 9-cis-retinoic acid catalyzed by sulfhydryl compounds. Drug Metab Dispos. 1997;25:27–32. [PubMed] [Google Scholar]
- 36.Minucci S, Leid M, Toyama R, et al. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol Cell Biol. 1997;17:644–655. doi: 10.1128/mcb.17.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985;45:1474–1478. [PubMed] [Google Scholar]
- 38.Orlow SJ, Chakraborty AK, Pawelek JM. Retinoic acid is a potent inhibitor of inducible pigmentation in murine and hamster melanoma cell lines. J Invest Dermatol. 1990;94:461–464. doi: 10.1111/1523-1747.ep12874568. [DOI] [PubMed] [Google Scholar]
- 39.Roméro C, Aberdam E, Larnier C, Ortonne JP. Retinoic acid as modulator of UVB-induced melanocyte differentiation. Involvement of the melanogenic enzymes expression. J Cell Sci. 1994;107 (Pt 4):1095–1103. doi: 10.1242/jcs.107.4.1095. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Morita M, Ichikawa C, Takahashi H, Toriyama M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci Biotechnol Biochem. 2008;72:2589–2597. doi: 10.1271/bbb.80279. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes SS, Arcuri R, Morgado-Díaz JA, Benchimol M. Increase of melanogenesis by retinoic acid: an ultrastructural and morphometric study. Tissue Cell. 2004;36:95–105. doi: 10.1016/j.tice.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Ho KK, Halliday GM, Barnetson RS. Topical retinoic acid augments ultraviolet light-induced melanogenesis. Melanoma Res. 1992;2:41–45. doi: 10.1097/00008390-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Lotan R, Lotan D. Stimulation of melanogenesis in a human melanoma cell line by retinoids. Cancer Res. 1980;40:3345–3350. [PubMed] [Google Scholar]
- 44.Welsh BM, Mason RS, Halliday GM. Topical all-trans retinoic acid augments ultraviolet radiation-induced increases in activated melanocyte numbers in mice. J Invest Dermatol. 1999;112:271–278. doi: 10.1046/j.1523-1747.1999.00510.x. [DOI] [PubMed] [Google Scholar]
- 45.Calléja C, Messaddeq N, Chapellier B, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyman RA, Mangelsdorf DJ, Dyck JA, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 47.Allenby G, Bocquel MT, Saunders M, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 49.IJpenberg A, Tan NS, Gelman L, et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004;23:2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elder JT, Aström A, Pettersson U, et al. Differential regulation of retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98:673–679. doi: 10.1111/1523-1747.ep12499896. [DOI] [PubMed] [Google Scholar]
- 51.Fisher GJ, Talwar HS, Xiao JH, et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J Biol Chem. 1994;269:20629–20635. [PubMed] [Google Scholar]
- 52.Nagy L, Kao HY, Love JD, et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–3216. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagy L, Kao HY, Chakravarti D, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 54.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 55.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 56.Daily K, Patel VR, Rigor P, Xie X, Baldi P. MotifMap: integrative genome-wide maps of regulatory motif sites for model species. BMC Bioinformatics. 2011;12:495. doi: 10.1186/1471-2105-12-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie X, Rigor P, Baldi P. MotifMap: a human genome-wide map of candidate regulatory motif sites. Bioinformatics. 2009;25:167–174. doi: 10.1093/bioinformatics/btn605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pogenberg V, Guichou JF, Vivat-Hannah V, et al. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem. 2005;280:1625–1633. doi: 10.1074/jbc.M409302200. [DOI] [PubMed] [Google Scholar]
- 59.Chen JY, Clifford J, Zusi C, et al. Two distinct actions of retinoid-receptor ligands. Nature. 1996;382:819–822. doi: 10.1038/382819a0. [DOI] [PubMed] [Google Scholar]
- 60.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 61.Holick MF. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone. 1995;17:107S–111S. doi: 10.1016/8756-3282(95)00195-j. [DOI] [PubMed] [Google Scholar]
- 62.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 63.Du X, Tabeta K, Mann N, Crozat K, Mudd S, Beutler B. An essential role for Rxr alpha in the development of Th2 responses. Eur J Immunol. 2005;35:3414–3423. doi: 10.1002/eji.200535366. [DOI] [PubMed] [Google Scholar]
- 64.Li M, Indra AK, Warot X, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 65.Li M, Chiba H, Warot X, et al. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 66.Boissy RE, Nordlund JJ. Vitiligo: current medical and scientific understanding. G Ital Dermatol Venereol. 2011;146:69–75. [PubMed] [Google Scholar]
- 67.Dupin E, Le Douarin NM. Retinoic acid promotes the differentiation of adrenergic cells and melanocytes in quail neural crest cultures. Dev Biol. 1995;168:529–548. doi: 10.1006/dbio.1995.1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) UV-induced oxidation products do not stimulate melanogenesis. MNT-1 melanoma cells were treated with the indicated doses of 4-HNE and then allowed to reach confluency. Melanin accumulation was measured as described in the methods. (B) All trans-retinal does not induce melanogenesis. MNT-1 melanoma cells were briefly treated with the indicated doses of all-trans retinal and then allowed to reach confluency. Melanin accumulation was measured as described in the methods. (C) In conjunction with UVA, 9-cis retinal induces the accumulation of TYR and MITF mRNA in primary melanocytes. Normal human deeply-pigmented melanocytes were incubated with 9-cis retinal at the indicated concentrations for 45 minutes and then immediately irradiated with the indicated dose of UVA light. Cells were then harvested 24 hours post UVA treatment and the relative accumulation of TYR and MITF mRNA was quantified. Data shown in Figures S1A, and S1B are mean ± S.D. (n=6, as indicated by the error bars). Data shown in Figure S1C is mean ± S.D. (n=3 as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus vehicle-treated control.
(A) 9-cis retinal induces the accumulation of melanin in MNT-1 melanoma cells in conjunction with UVA radiation. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinal and then irradiated with the indicated dose of UVA. Cells were then allowed to reach confluency. Melanin accumulation was measured as described in methods.
(B) The combination of 9-cis retinal treatment and UVA stimulation is toxic to MNT-1 melanoma cells. MNT-1 melanoma cells were briefly treated with the indicated doses of 9-cis retinal and then immediately irradiated with 2 J/cm2 of UVA light. Cells were then allowed to reach confluency and then subsequently lysed with the addition of Cell-Titer Glo reagent as previously described in the methods. The relative percentage of surviving cells was calculated by normalizing the luminescence value of drug treated cells to that of vehicle-treated control cells. (C) ALDH1A1 siRNAs inhibit ALDH1A1 mRNA expression. MNT-1 melanoma cells were transiently transfected with either mismatch control or ALDH1A1 siRNAs. Target gene expression was measured 72 hours post transfection by RT-qPCR. Data shown in Figure S2A and S2B is mean ± S.D. (n=6). Data shown in Fig S2C is mean ± S.D. (n=3, as indicated by the error bars). *, p < 0.05 or **, p < 0.01 using a Student’s paired t-test with a two-tailed normal distribution versus mismatch-transfected control.