Abstract
Loss of lean body mass is a characteristic feature of the septic response, and the mechanisms responsible for this decrease and means of prevention have not been fully elucidated. The present study tested the hypothesis that in vitro treatment of skeletal muscle with lithium chloride (LiCl), a glycogen synthase kinase (GSK)-3 inhibitor, would reverse both the sepsis-induced increase in muscle protein degradation and inhibition of protein synthesis. Sepsis decreased GSK-3β phosphorylation and increased GSK-3β activity, under basal conditions. Sepsis increased muscle protein degradation, with a concomitant increase in atrogin-1 and MuRF1 mRNA and 26S proteasome activity. Incubation of septic muscle with LiCl completely reversed the increased GSK-3β activity and decreased proteolysis to basal nonseptic values, but only partially reduced proteasome activity and did not diminish atrogene expression. LiCl also did not ameliorate the sepsis-induced increase in LC3-II, a marker for activated autophagy. In contrast, LiCl increased protein synthesis only in nonseptic control muscle. The inability of septic muscle to respond to LiCl was independent of its ability to reverse the sepsis-induced increase in eIF2Bε phosphorylation, decreased eIF2B activity, or the reduced phosphorylation of FOXO3, but instead was more closely associated with the continued suppression of mTOR kinase activity (e.g., reduced phosphorylation of 4E-BP1 and S6). These data suggest that in vitro lithium treatment which inhibited GSK-3β activity: a) effectively reversed the sepsis-induced increase in proteolysis, but only in part by a reduction in the ubiquitin-proteasome pathway and not by a reduction in autophagy; and b) was ineffective at reversing the sepsis-induced decrease in muscle protein synthesis. This lithium resistant state appears mediated at the level of mTOR and not eIF2/eIF2B. Hence, use of GSK-3β inhibitors in the treatment of sepsis may not be expected to fully correct the imbalance in muscle protein turnover.
Keywords: protein synthesis, protein degradation, mTOR, proteasome, LC3
INTRODUCTION
Sepsis produces a profound negative nitrogen balance which occurs secondary to a net catabolism of skeletal muscle proteins. Sepsis-induced muscle wasting results from both a sustained decrease in protein synthesis as well as an increase in protein degradation (1–3), and it characterizes the cachexia of critical illness. Not only does this erosion of lean body mass adversely affect morbidity and mortality in septic and critically ill patients, but it has also proven difficult to ameliorate with either growth factor treatment or nutritional supplementation (2).
The glycogen synthase kinase (GSK)-3 family members, consisting of the two highly homologous α- and β-isoforms, function as serine (Ser)/threonine (Thr) kinases and lie distal to Akt in the canonical phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway (4). In contradistinction to other protein kinases, GSK-3 is catalytically active under basal conditions and inhibited by growth factor stimulation (5). Moreover, as the phosphorylation of its substrates generally leads to substrate inactivation, GSK-3 inactivation (i.e., phosphorylation) stimulates diverse cellular functions, including gene expression, cytoskeletal integrity, and metabolism (4), which have been implicated in a wide array of disease states (6).
The functions of the two GSK isoforms are not entirely redundant (7). Although the relative importance of each isoform in regulating GSK-3 activity in skeletal muscle is poorly defined, there is 3- to 4-fold more GSK-3β in muscle and, by using mutant GSK3β9A/9A knock-in mice, the β-isoform appears to be the predominant regulator of glycogen synthase in muscle (8). In addition to the large number of metabolic studies which have focused on the role of GSK-3β in glucose homeostasis (9), a change in GSK-3 activity can also modulate cellular protein turnover by potentially influencing both rates of protein synthesis and degradation. This is exemplified in studies where hypertrophy was observed in myotubes cultured with the GSK-3 inhibitor lithium chloride (LiCl) (10). In addition to LiCl, other pharmacological inhibitors of GSK-3 prevent the elevated rate of muscle proteolysis seen in response to burn injury, sepsis, and excess dexamethasone (1,11,12). However, there are no data pertaining to the effect of LiCl on the depressed rates of muscle protein synthesis in sepsis, and our current study addresses this gap in understanding.
The sites limiting protein synthesis during sepsis are beginning to be unraveled. One such regulatory site is the formation of the 43S preinitiation complex, which is markedly reduced in skeletal muscle during more sustained septic insults (13). The step involving the binding of met-tRNAmeti to the 40S ribosomal subunit, forming the 43S preinitiation complex is mediated by eukaryotic initiation factor (eIF)-2 and is regulated by the activity of another initiation factor, eIF2B (14). eIF2B is a guanine nucleotide-exchange factor with five subunits (α-ε) which catalyzes the exchange of guanosine diphosphate (GDP) bound to eIF2 for guanosine triphosphate (GTP). We previously reported that sepsis, endotoxin (LPS) and excess tumor necrosis factor (TNF)-α decrease eIF2B activity in skeletal muscle, and thereby impairs 43S preinitiation complex formation (13,15). Importantly, the sepsis-induced reduction in eIF2B activity occurs only in those muscles which exhibit a concomitant decrease in the rate of protein synthesis, i.e., muscles composed of mixed fast-twitch fibers (e.g., gastrocnemius and epitrochlearis). Sepsis reduces the content of the catalytic ε-subunit of eIF2B (eIF2Bε) over a 3–5 day period (15). Moreover, alterations in the expression and phosphorylation state of neeIF2Bε correlate with the changes in protein synthesis and translation efficiency after induction and recovery from the septic insult. GSK-3 phosphorylation of eIF2Bε on Ser535 is involved in the regulation of eIF2B activity during initiation and progression of the septic process (15). Thus, the sepsis-induced decrease in GSK-3β phosphorylation (and inactivation) is consistent with the increased phosphorylation and inactivation of eIF2Bε, which ultimately contributes to the sepsis-induced reduction in muscle protein synthesis.
Various formulations of lithium and other GSK-3 inhibitors have been used or tested as possible treatments for a wide range of diseases (4). While two chemically distinct GSK-3 inhibitors have proven efficacious in reducing LPS-induced liver and renal damage as well as acute lethality when used as a pre-treatment (16,17), there are no studies of GSK-3 inhibitors in more chronic sepsis models. Given this background, the purpose of the present study was to determine whether lithium could reverse the sepsis-induced decrease in muscle protein synthesis. To accomplish this goal we incubated the epitrochlearis from either nonseptic or septic rats in the absence or presence of LiCl and quantified the effect on protein synthesis (primary) and degradation (secondary). Additionally, various signal transduction pathways were examined to determine potential mechanisms of action.
MATERIALS AND METHODS
Animals
Adult male specific-pathogen free Sprague-Dawley rats (Charles River Breeding Laboratories, Cambridge, MA; 150–225 g) were maintained on a 12 h light: 12 h dark cycle and were fed standard rat chow ad libitum for 1 wk prior to study. Chronic abdominal sepsis was created by implantation of a fecal-agar pellet (1.5 ml) inoculated with Escherichia coli [104 colony forming units (CFU)] and Bacteroides fragilis (108 CFU) into the peritoneal cavity, as previously described (15,18). The animals develop an abdominal abscess resulting in a well-characterized hyperdynamic, hypermetabolic septic condition. A separate group of rats underwent the intra-abdominal implantation of a sterilized fecal agar pellet to which sterile saline was added instead of bacteria. Subsequently, these animals are referred to as the “nonseptic” group. While the sterile pellet produces a mild nonseptic inflammation, we have previously reported there is no difference in the in vivo rate of muscle protein synthesis between these nonseptic animals and naive control rats (19). Likewise, our preliminary data (n = 8) demonstrate that in vitro-determined rates of protein synthesis (naive control = 0.288 ± 0.011 vs nonseptic control = 0.310 ± 0.015 nmol Phe incorporated/mg protein/h) and degradation (naive control = 2.45 ± 0.15 vs nonseptic control = 2.37 ± 0.18 nmol tyrosine/mg protein/h) are not statistically different (P > 0.1) between these two control groups. Nonseptic control rats were pair-fed to match food consumption of septic animals. The Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine approved the experiments described herein.
Epitrochlearis incubation
Three days after induction of sepsis, rats were anesthetized with pentobarbital (100 mg/kg body wt), and the skin on both forelimbs was removed. The epitrochlearis muscle from each forelimb was excised intact and immediately placed in Krebs-Henseleit bicarbonate (KHB) buffer and incubated, exactly as described previously (20). The epitrochlearis incubations were performed in a 37C environmental chamber with gentle shaking and were continuously gassed with 95% O2-5% CO2. The KHB buffer consisted of (in mM) 110 NaCl, 25 NaHCO3, 3.4 KCl, 1 CaCl2, 1 MgSO4, and 1 KH2PO4 (pH 7.4), supplemented with 5.5 glucose, 5 HEPES, 0.2 valine, 0.17 leucine, 0.1 isoleucine, and 0.01% (wt/vol) BSA, either in the absence or presence of added LiCl. At the dose used, LiCl was soluble in media; for media without added LiCl, an equal molar concentration of NaCl was added to balance osmolality.
Epitrochlearis muscles were first preincubated for 30 min, with one muscle preincubated in media containing LiCl (10 mM), and the contralateral epitrochlearis preincubated in buffer without LiCl. Comparable doses of LiCl have been reported to antagonize the increased rate of muscle proteolysis observed in other catabolic conditions (1,11,12). After preincubation, the muscles were quickly rinsed in buffer and transferred to 3 ml of fresh buffer (± LiCl) and incubated for 2 h with a change of buffer after the first hour. The buffer was supplemented with 1.2 mM of 5 µCi/ml of L-[2,3,4,5,6-3H]phenylalanine (Amersham Life Science, Arlington Heights, IL) during the final hour of incubation. At the end of the incubation, the muscles were quickly removed from the buffer, blotted dry, weighed, and frozen in liquid nitrogen. Muscles and incubation media were stored at −80 °C.
Protein synthesis and degradation
In vitro rates of protein synthesis, expressed as nanomoles phenylalanine incorporated per milligram protein per hour, were measured by the incorporation of radioactive phenylalanine from the incubation medium into the epitrochlearis, as previously described (20,21). The rate of protein synthesis was calculated by dividing the amount of radioactive phenylalanine incorporated into TCA-precipitable protein over the final incubation by the specific radioactivity of phenylalanine in the incubation medium.
The In vitro rate of protein degradation was were measured by the accumulation of tyrosine in the incubation medium, as described previously (20,22). Because tyrosine is not synthesized or metabolized by muscle, except for use by protein synthesis, tyrosine release from the epitrochlearis into the incubation medium reflects net protein balance. Tyrosine release was linear in muscles from both nonseptic and septic rats over the 2-h incubation period (data not shown). Total protein degradation was estimated simultaneously with the rate of protein synthesis as the sum of the accumulated tyrosine in the buffer over a 2-h period plus the amount of tyrosine equivalents incorporated into protein via protein synthesis during the same time interval. To obtain the amount of tyrosine incorporated into mixed muscle proteins, we multiplied the value for incorporation of radioactive phenylalanine into protein by 0.77 which is the molar ratio of tyrosine to phenylalanine in mixed proteins from skeletal muscle (22). Hence, values for tyrosine equivalents incorporated into mixed muscle protein were estimated for individual muscles. Tyrosine in the incubation medium was measured fluorometrically (20).
In vitro eIF2B and GSK-3β activity
The activity of eIF2B in muscle was measured in supernatants using a [3H]GDP-GDP exchange assay, as previously described by our laboratory (13). Tissue was homogenized in buffer consisting of (in mM) 20 triethanolamine (pH 7.0), 2 magnesium acetate, 150 KCl, 0.5 DTT, 0.1 EDTA, 250 sucrose, 5 EGTA, and 50 β-glycerolphosphate. The homogenate was then centrifuged and the supernatant assayed immediately for eIF2B activity by measuring the decrease in eIF2-[3H]GDP complex bound to nitrocellulose filters. The GSK-3β activity assay was performed as previously described by our laboratory for other IP-kinase assays (23). Briefly, GSK-3β was immunoprecipitated by incubating muscle homogenate with an excess of anti-GSK-3β antibody or appropriate IgG control at 4° C. Protein A Sepharose was added and immune complexes were washed twice with buffer (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 0.2 mM sodium vanadate, and 1 µM microcystin LR) and twice with kinase reaction buffer (25 mM HEPES, pH 7.4, 50 mM KCl, 10 mM MgCl2 and phoshatase inhibitors). Kinase reactions were initiated by resuspending immune complexes in KHB containing phosphatase inhibitors, 25 µM ATP, 5 µCi [γ-32P]-ATP and 20 mM phospho-glycogen synthase peptide-2 (Upstate Biotechnology, Lake Placid, NY). Samples were incubated for 15 min at 30° C, and 20 µl of supernatant was spotted onto Whatman P81 phosphocellulose paper. Filters were washed three times with 175 mM orthophosphoric acid, rinsed in acetone, air dried, and 32P incorporation was measured in a liquid scintillation counter. Nonspecific 32P incorporation was subtracted from values obtained using the phospho-glycogen synthase peptide.
Proteasome activity
In vitro proteasome activity was assessed by quantifying the chymotryptic-like peptidase activity in epitrochlearis. Muscle was homogenized in buffer containing 50 µM Tris-HCl (pH 7.4), 5 mM MgCl2, 250 mM sucrose, 2 mM ATP, and 1 mM DTT. The homogenate was first centrifuged at 400 × g (5 min) and then clarified by sequential centrifugation steps (10,000 × g for 20 min followed by 100,000 × g for 5 h) to isolate the 20S and 26S proteasomes, respectively, as previously described (24). After resuspension, proteasome chymotryptic-like activity was determined using a fluorometer (Molecular Devices SpectraMax Gemini EM) to measure the release of 7-amino-4-methylcoumarin (AMC) from the fluorogenic peptide substrate LLVY-AMC (Chemicon International, Temecula, CA).
Western blot analysis
The phosphorylated and total amounts of various proteins important for control of protein synthesis and degradation were quantified by protein immunoblotting (15,25,26). Total protein was determined by bicinchoninic acid assay (Pierce, Rockford, IL) after centrifugation, and equal amounts of protein per sample were subjected to standard SDS-PAGE, using antibodies obtained from Cell Signaling (Beverly, MA), unless otherwise indicated. Specifically, Western blot analysis was performed using antibodies for total and phospho-specific eIF2Bε (Ser535), GSK-3β (Ser9), GSK-3α (Ser21), Akt (Ser473), S6 (Ser240/244), TSC2 (tuberous sclerosis complex 2; Thr1462), FOXO3 (Forkhead box O; Thr32; Upstate), and total tubulin, LC3B (microtubule-associated protein 1 light chain 3; MAP1-LC3), and 4E-BP1 (eIF4 binding protein-1; from Bethyl Laboratories, Montgomery, TX). Blots were developed with enhanced chemiluminescence Western blotting reagents (Supersignal Pico; Pierce, Rockford, IL). Dried blots were exposed to X-ray film to achieve a signal within the linear range, and film was then scanned (Microtek ScanMakerIV) and quantified using Scion Image 3b software (Scion, Frederick, MD). The signal densities for phosphorylated proteins were normalized to the respective total protein or tubulin.
Ribonuclease protection assays
Total RNA was extracted from tissues in a mixture of phenol and guanidine thiocyanate (TRI Reagent; Molecular Research Center, Cincinnati, OH) using the manufacturer’s protocol. Riboprobes were synthesized from a multi-probe mouse cytokine template set (rCK-1; Pharmingen, San Diego, CA) using an in vitro transcription kit (Pharmingen). Primer sequences for insulin-like growth factor (IGF)-I, TNFα, interleukin (IL)-6 and nitric oxide synthase (NOS)-2 have been previously reported (27), as have those for the two muscle-specific E3-ubiquitin ligases [i.e., atrogin-1 and MuRF1 (muscle ring finger-1)] (21) which are collectively referred to as “atrogenes.” The labeled riboprobe was hybridized with RNA overnight using a ribonuclease protection assay (RPA). Protected RNAs were separated using a 5% acrylamide gel, and dried gels were exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, CA). The resulting data were quantified using ImageQuant and normalized to L32.
Statistical analysis
Values shown are means ± SEM. Statistical evaluation of the data when two comparisons were made was performed using Student's t-test. For multiple comparisons, a two-way ANOVA was performed followed by the Sidak test to identify individual differences if ANOVA indicated a significant difference. Differences among the means were considered significant when P < 0.05.
RESULTS
Muscle weight, protein synthesis and degradation
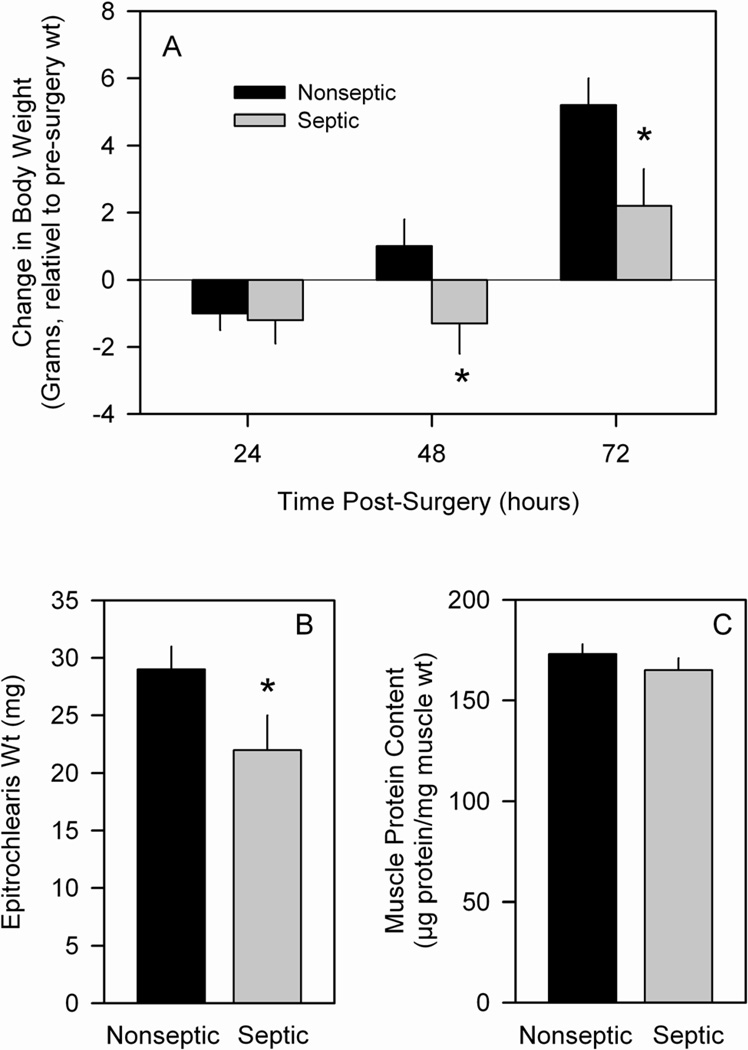
Nonseptic rats showed no change in body weight over the first 24 h but thereafter demonstrated a positive weight gain over the subsequent two days (Figure 1A). In contrast, septic rats lost body weight over the first 48 hours post surgery before gaining weight on day 3. These differences in body weight gain were independent of nutritional intake as the time-matched nonseptic rats were pair-fed to the food consumed by septic rats. On day 3, immediately upon excision from the rat (i.e., prior to incubation), the wet weight of the epitrochlearis muscle was 28% less in septic (Figure 1B). However, there was no significance difference in the protein content (µg protein/mg wet wt) of muscles between nonseptic and septic rats (Figure 1C).
Figure 1.
Weight gain and muscle weight of nonseptic and septic animals. Panel A: On day 0 rats were implanted with either a sterile (nonseptic) or infected (septic) pellet. Rats were weighed daily and the differences in weight from day 0 are plotted. Panel B: Wet weight of the epitrochlearis was determined immediately prior to incubation. Panel C: Muscle protein content was determined at the end of the 2-h incubation period. Values shown are means ± SEM for nonseptic (n = 42) and septic (n = 45) rats. *P <0.001 versus time-matched nonseptic value.
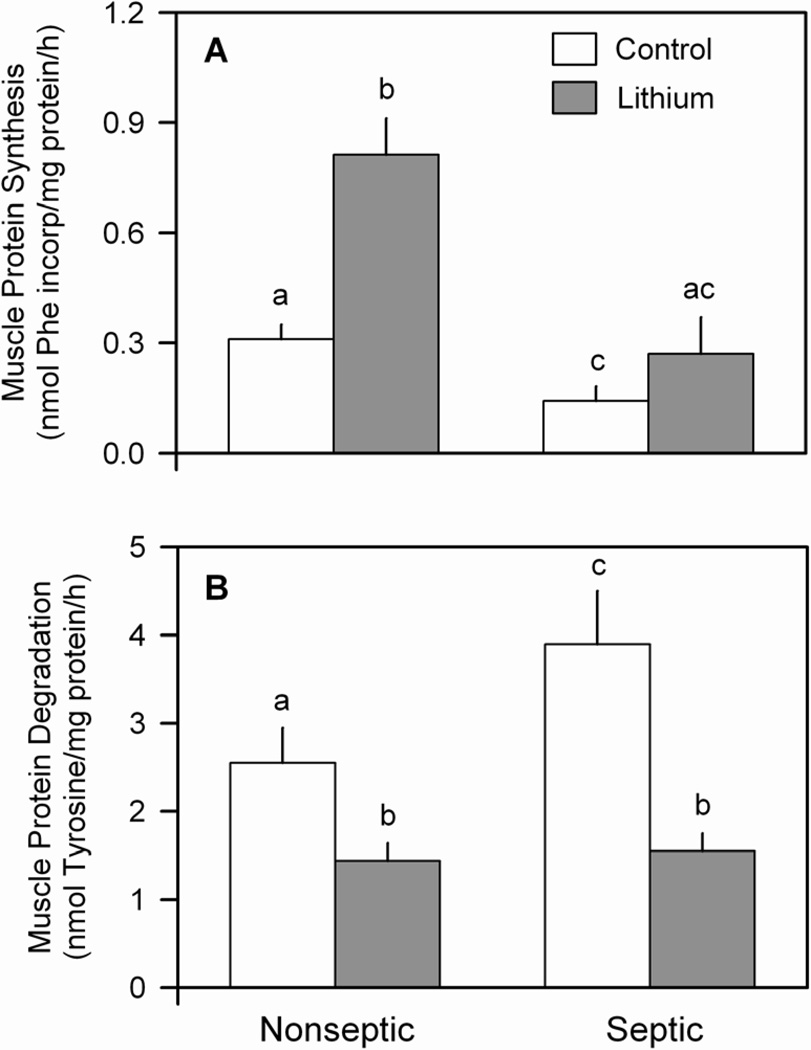
Next, epitrochlearis muscles from both groups were incubated in the absence or presence of LiCl under in vitro conditions. Sepsis reduced the rate of protein synthesis by 50% under basal conditions (e.g., no LiCl), compared with muscle from nonseptic rats (Figure 2A). The “contro" values in Figure 2 represent data pooled from muscles incubated with either 0 or 10 mM NaCl. The “extra” NaCl present in the latter group was added to the media to balance the increased osmolality incurred by the addition of an equal molar concentration of LiCl in other muscle incubations. The rates of protein synthesis (and degradation, described below) did not differ in muscles incubated with 0 and 10 mM NaCl, and therefore data were combined for purposes of graphical and statistical analysis. Lithium increased the rate of protein synthesis in epitrochlearis from nonseptic rats, with synthesis being increased 155%. In contrast, muscle from septic rats incubated with 10 mM LiCl did not show a statistically significant increase in protein synthesis. The absolute rate of synthesis in septic muscle in the presence of LiCl did not differ from that seen in nonseptic muscle under basal conditions (Figure 2A). The failure of lithium to increase protein synthesis in septic muscle was not related to a suboptimal concentration of LiCl as addition of 20 mM LiCl in the media actually decreased synthesis (0.18 ± 0.08 nmol Phe incorp/h/mg protein), compared with the response seen at 10 mM LiCl (0.27 ± 0.08 nmol Phe incorporated/h/mg protein; P < 0.05).
Figure 2.
Effect of lithium chloride (LiCl) on sepsis-induced changes in muscle protein synthesis and degradation. Epitrochlearis muscles from nonseptic and septic rats were excised on day 3 post-sepsis and incubated in vitro in the absence or presence of 10 mM LiCl. Panel A: rates of protein synthesis; Panel B, rates of protein degradation (net tyrosine release). Values are means ± SEM for 9–10 muscles in each group. Means with different letters (a, b, c) are significantly different (P < 0.05).
The rate of proteolysis under basal conditions was increased 40% in muscle from septic animals, compared with time-matched nonseptic values (Figure 2B). Inclusion of 10 mM LiCl in the media attenuated protein degradation in muscle from both nonseptic (−33%) and septic (−60%) rats. There was no significant difference in protein degradation between nonseptic and septic rats incubated with media containing 10 mM LiCl.
To assess whether LiCl may have influenced tyrosine uptake or release by incubated muscle, we also determined the tyrosine content in muscle from nonseptic and septic rats incubated in the presence and absence of LiCl. Data in Table 1 illustrate there is no difference in the tyrosine concentration among the 4 groups at time 0 (e.g., after 30 min of preincubation). Furthermore, while muscle tyrosine increased 50–70% after 2 h of incubation, the increase was comparable in all 4 groups. As such, there was no difference in the muscle tyrosine concentration among the 4 groups at this latter time point.
Table 1.
Tyrosine concentration in epitrochlearis incubated in the absence or presence of LiCl
| Incubation Time | |||
|---|---|---|---|
| LiCl treatment | 0 h | 2 h | |
| Nonseptic | − | 89 ± 7 | 132 ± 16* |
| + | 75 ± 6 | 128 ± 15* | |
| Septic | − | 97 ± 8 | 145 ± 21* |
| + | 81 ± 8 | 137 ± 23* | |
Values are means ± SEM (n = 6 per group), where units are nmol/g tissue. Tissue content of tyrosine was determined after the 30 min preincubation period (e.g., 0 h) and at the end of the 2-h incubation period.
P < 0.05 compared to values from same treatment group. At any given time (i.e., either 0 h or 2 h), there was no significant difference among the four experimental groups.
Signal transduction related to protein synthesis
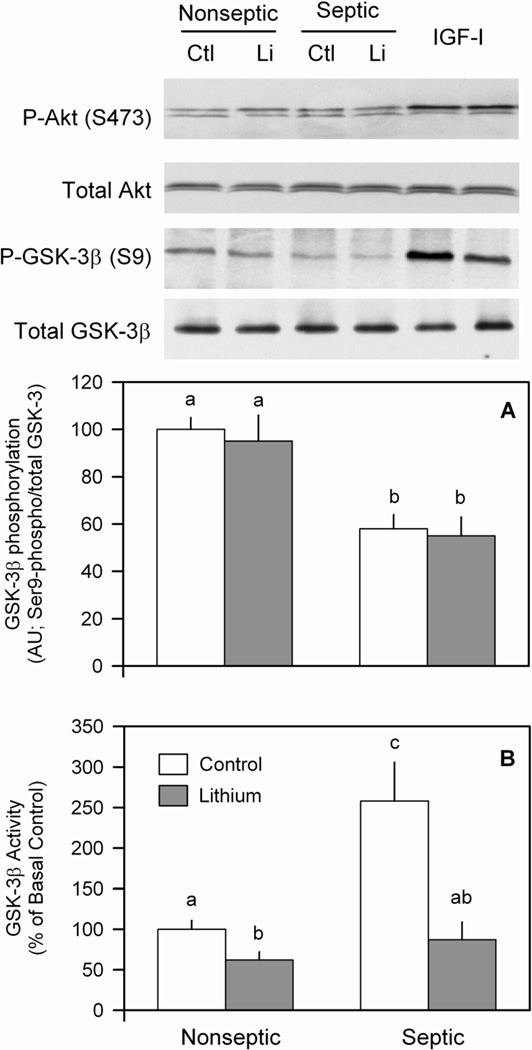
Hasselgren and co-workers have implicated GSK-3 phosphorylation as a potential regulator of protein degradation in incubated muscle following burn and acute peritonitis induced by cecal ligation and puncture (1,11,12,24). Lithium modulates GSK-3 through two mechanisms, namely dephosphorylation and direct inhibition of GSK-3 (4,24). To assess the influence of a more sustained septic insult on GSK-3 activity, first phospho-GSK-3β was semi-quantitated in muscle. The extent of GSK-3β phosphorylation was reduced 40% in muscle from septic rats under basal conditions, compared with basal nonseptic values (Figure 3A and inset). This sepsis-induced reduction was independent of a change in total GSK-3β. Furthermore, incubation of nonseptic muscle with lithium did not alter GSK-3β phosphorylation nor did it reverse the sepsis-induced decrease in this phospho-protein. In contrast, there was no sepsis-induced effect on GSK-3α Ser21-phosphorylation (data not shown). We also quantified GSK-3β activity per se using an in vitro kinase assay. In nonseptic muscle, LiCl reduced GSK-3β activity by 35% (Figure 3B). In contrast, sepsis doubled GSK-3β kinase activity under basal conditions, and in vitro treatment with lithium returned kinase activity to values not different from the two control groups. As the Ser9-residue of GSK-3β is phosphorylated by Akt, Akt activity was assessed by Ser473 phosphorylation. In contrast to the sepsis-induced decrease phosphorylation of GSK-3β noted above, we detected no sepsis- or lithium-induced change in Akt phosphorylation (Figure 3, insert). As a positive control, several control muscles were incubated with IGF-I, and we were able to detect increased Akt phosphorylation (Figure 3, insert, lanes 5,6).
Figure 3.
Effect of LiCl on Akt and GSK-3β phosphorylation as well as GSK-3 activity in muscle from nonseptic and septic rats. The phosphorylation state of Akt (Ser473) and GSK-3β (Ser9) were determined in muscle homogenates using phosphospecific antibodies. The blots were then stripped and re-probed with an antibody recognizing the respective total protein. Panel A, quantitation of all Western blot data for phosphorylated GSK-3 normalized to total GSK protein. The final 2 lanes for each Western blot are positive controls from control muscles treated with IGF-I (100 ng/ml). Panel B, GSK-3β activity was quantified in homogenates as described in Methods. Values are means ± SEM for 8–9 muscles in each group. Means with different letters (a, b, c) are significantly different (P < 0.05).
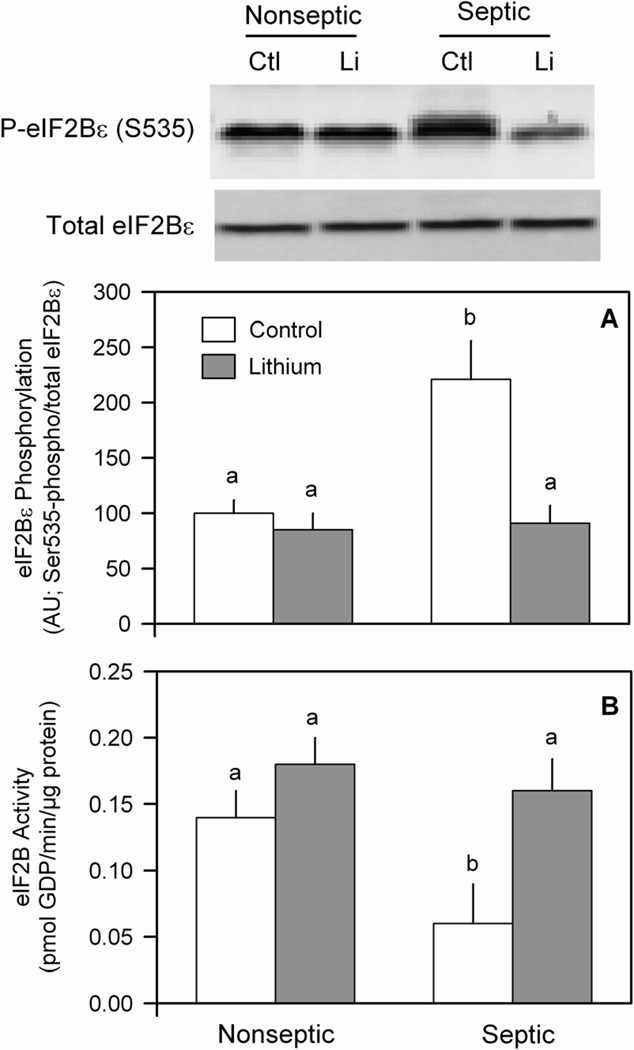
We previously reported that eIF2 phosphorylation is involved in the inhibition of eIF2Bε during sepsis and that increasing eIFBε partially overcomes the sepsis-induced decrease in muscle protein synthesis (28). Therefore, we assessed the ability of LiCl to ameliorate the effect of sepsis on eIF2Bε phosphorylation. Sepsis increased the extent of eIF2Bε phosphorylation by ~50% under basal conditions, compared to nonseptic values (Figure 4A and inset). In epitrochlearis from nonseptic rats, LiCl did not alter eIF2Bε phosphorylation. In contrast, LiCl lowered the extent of eIF2Bε phosphorylation in muscle from septic rats. There was no significant difference in the extent of eIF2Bε phosphorylation in epitrochlearis between nonseptic and septic rats incubated with lithium-containing media. Conversely, sepsis decreased eIF2B guanine nucleotide exchange activity under basal conditions, and lithium reversed this sepsis-induced decrease (Figure 4B).
Figure 4.
Lithium ameliorates the sepsis-induced increase in eIF2Bε Ser535-phosphorylation and decreased eIF2B activity in muscle. To determine the relative phosphorylation state of eIF2Bε, equal amounts of protein from homogenates of epitrochlearis from nonseptic and septic rats were immunoblotted with an anti-eIF2B antibody, specific for the phosphorylated form of eIF2Bε. The blots were then stripped of antibody and re-probed with an antibody recognizing total eIF2Bε. Panel A, bar graph indicates the amount of eIF2Bε in the phosphorylated form divided by the total eIF2Bε. Panel B, eIF2B activity was determined as in Methods. Results represent means ± SEM for 8–13 muscles in each group. Means with different letters (a, b) are significantly different (P < 0.05).
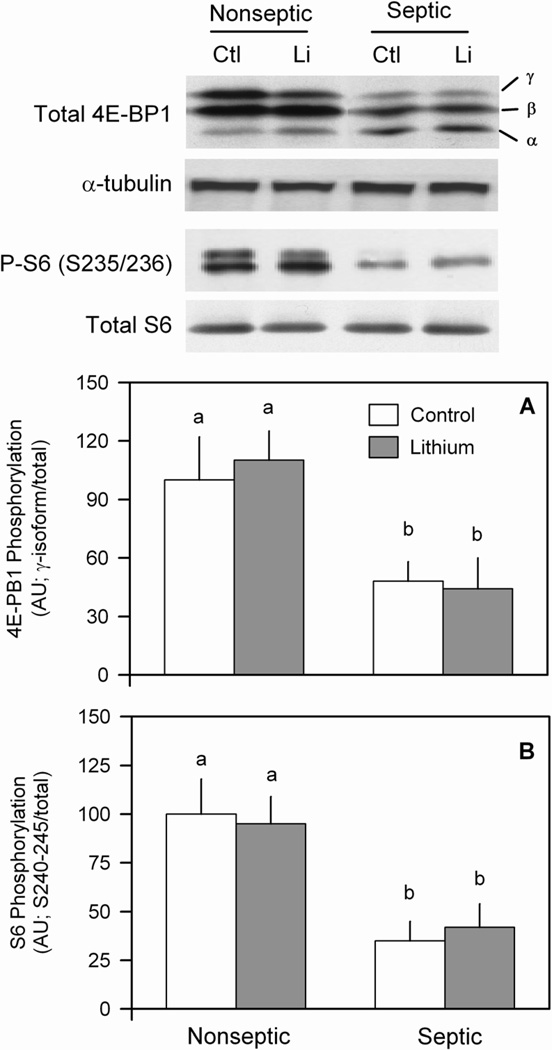
The rate of mRNA translation and protein synthesis is also regulated by mTOR (mammalian target of rapamycin) kinase activity, which we assessed by determining the phosphorylation of its downstream substrate 4E-BP1 (29). Sepsis decreased the phosphorylation of the hyper-phosphorylated γ-isoform of 4E-BP1 by 55%, compared to basal nonseptic values (Figure 5A and inset). Incubation of epitrochlearis with LiCl did not alter 4E-BP1 phosphorylation in muscle from either group. Tubulin was run as a loading control for this blot and band intensity was not different among the groups (Figure 5, inset). We also examined the S240/244-phosphorylated ribosomal protein S6, as this residue is specifically rapamycin-sensitive (i.e., mTOR-dependent) and phosphorylated by S6 kinase (S6K)-1. The basal phosphorylation of S6 was reduced in septic muscle. As with 4E-BP1, the phosphorylation of S6 was not altered in either nonseptic or septic muscles incubated with LiCl (Figure 5B).
Figure 5.
Effect of LiCl on 4E-BP1 and S6 phosphorylation in muscle of nonseptic and septic rats. Results represent means ± SEM for 8–13 muscles in each group. Total 4E-BP1 was determined and the γ-isoform (most heavy phosphorylated), β-isoform, and α-isoform (least phosphorylated) identified. α-Tubulin is also shown as a loading control. Panel A, quantitation of 4E-BP1 phosphorylation. Panel B, phosphorylated and total ribosomal protein S6 were determined and the bar graph indicates the phosphorylated form normalized to total S6. Means with different letters (a, b) are significantly different (P < 0.05).
A potentially important upstream regulator of mTOR activity is TSC2 (tuberin) and its activity can in part be regulated by both Akt and GSK-3 (30). However, we detected no sepsis- or lithium-induced change in either total or Thr1462-phosphorylated TSC2 in incubated muscle (data not shown).
Potential mechanisms for changes in protein degradation
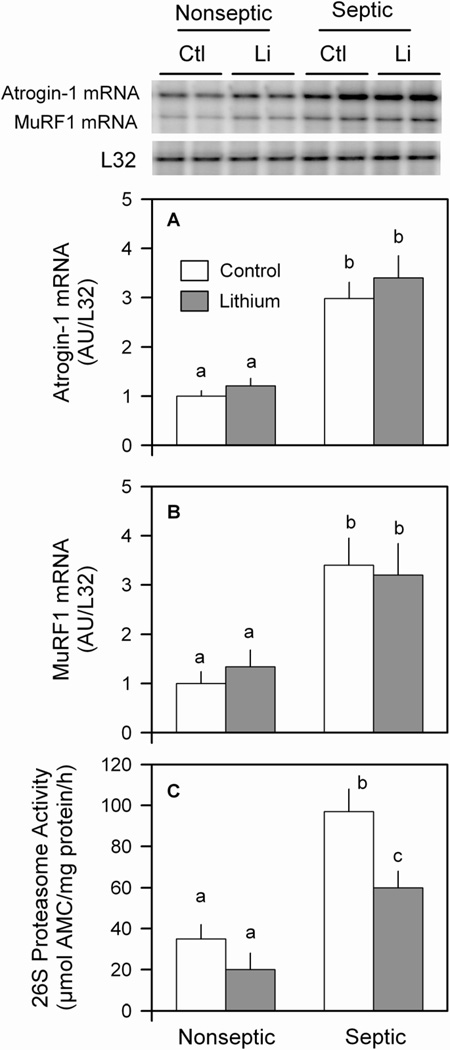
To assess whether lithium ameliorates the sepsis-induced muscle protein degradation by reduction in the ubiquitin-proteasome pathway, we assessed the mRNA content for atrogin-1 and MuRF (i.e., atrogenes) which are elevated in many catabolic conditions (31). In muscle from nonseptic rats, the LiCl-induced reduction in protein degradation appeared independent of a coordinate reduction in either atrogin-1 or MuRF1 mRNA content (Figure 6A and 6B). Furthermore, in nonseptic muscle, lithium tended to reduce 26S proteasome activity, but the change did not achieve statistical significance (Figure 6C). As expected, muscle from septic rats demonstrated a several-fold elevation in atrogin-1 and MuRF1 mRNA expression and more than a 2-fold increase in 26S proteasome activity, compared to basal nonseptic values. While incubation of septic muscle with lithium partially reversed the elevated proteasome activity, it did not blunt the sepsis-induced increase in atrogene expression (Figure 6A–C).
Figure 6.
Effect of LiCl on atrogene expression and proteasome activity in muscle from nonseptic and septic rats. The mRNA content in muscle for atrogin-1 (panel A) and MuRF1 (panel B) was quantitated by ribonuclease protection assay (RPA) and data were normalized to L32 which was unaffected by either sepsis and/or lithium treatment. The 20S and 26S proteasome activity was assessed as in Methods. Panel C, 26S proteasome activity data are presented, but the sepsis- and LiCl-induced changes in 20S proteasome activity were comparable (data not shown). Values are means ± SEM for 8–9 muscles in each group. Means with different letters (a, b, c) are significantly different (P < 0.05).
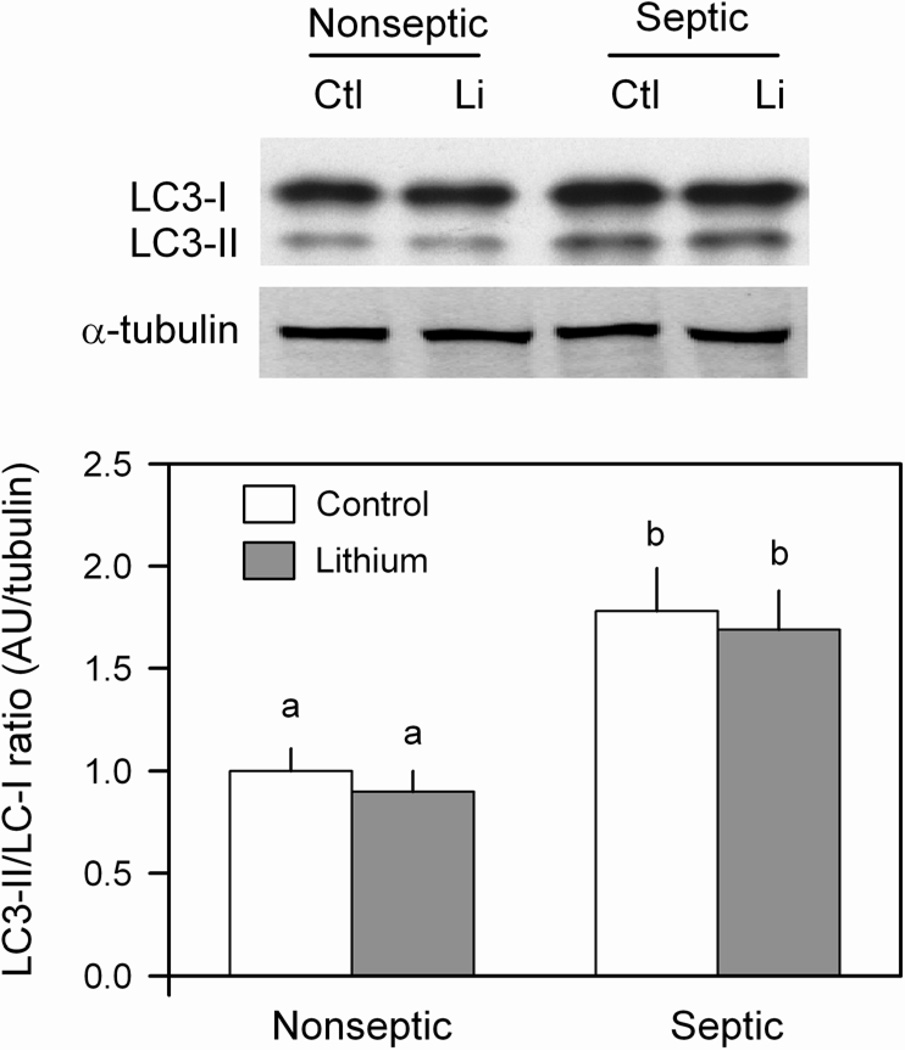
In addition to proteasome-mediated proteolysis, sepsis can also stimulate the autophagic/lysosomal pathway in skeletal muscle (32). To assess this potential mechanism, muscle homogenates were immunoblotted to detect the conversion of LC3-I to LC3-II which appears to be provide reliable index of autophagosome formation (33). As illustrated in Figure 7, sepsis increased the ratio of LC3-II/LC3-I by 60–70%, but the short-term (2–h) incubation of muscle with LiCl did not alter this ratio in either nonseptic or septic muscle.
Figure 7.
Effect of LiCl on the autophagic/lysosomal pathway as assessed by the LC3-II/LC3-I ratio in muscle from nonseptic and septic rats. LC3 was determined by Western blot analysis and the two isoforms so indicated (inset). Bar graph, represents quantitation of all data normalized to α-tubulin. Values are means ± SEM for 8–9 muscles in each group. Means with different letters (a, b) are significantly different (P < 0.05).
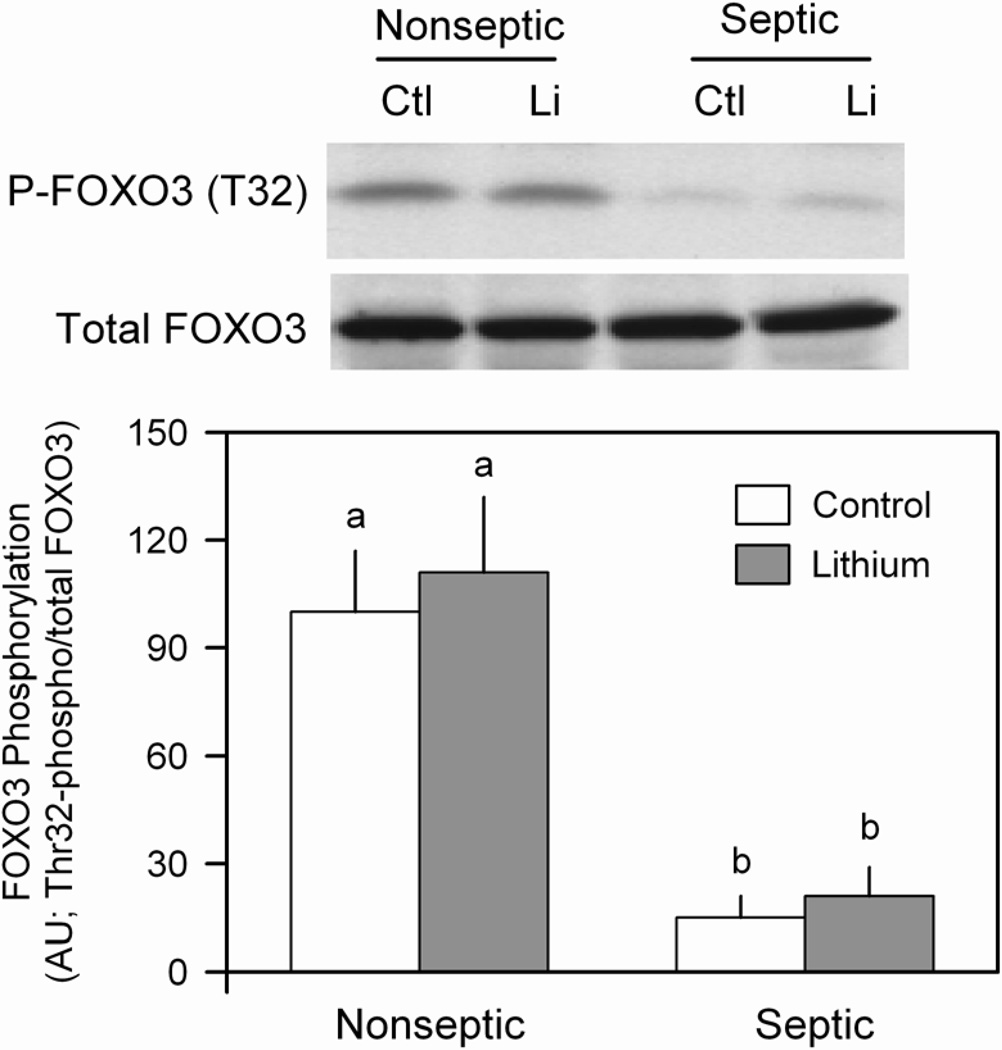
The FOXO transcription factors are implicated as regulators of muscle wasting and have been implicated in regulating both the ubiquitin-proteasome and autophagic/lysosomal pathways (3). When in the active (dephosphorylated) state, FOXO stimulates protein degradation via increased atrogene expression (34). Under basal conditions, Thr32-phosphorylated FOXO3 was decreased in muscle from septic rats, compared to nonseptic values, and incubation with LiCl did not alter the phosphorylation state of this protein in either group (Figure 8). This sepsis-induced change in FOXO3 phosphorylation was independent of a change in total FOXO3 protein in muscle.
Figure 8.
Effect of LiCl on FOXO3 phosphorylation in muscle from nonseptic and septic rats. Total and Thr32-phosphorylated FOXO3 were determined by Western blot analysis. Bar graph, represents quantitation of all data normalized to total FOXO3 protein. Values are means ± SEM for 8–9 muscles in each group. Means with different letters (a, b) are significantly different (P < 0.05).
Potential mediators of protein metabolic response
Finally, we determined the mRNA content for an important anabolic mediator (e.g., IGF-I) and several catabolic mediators (TNFα, IL-6 and NOS2) which can function in an autocrine/paracrine manner to regulate muscle protein balance (2). Sepsis increased TNFα, IL-6 and NOS2 mRNA content several-fold, compared with values from nonseptic muscle (Table 2). Incubation of muscle from septic rats with LiCl did not attenuate the sepsis-induced change in TNFα, IL-6 or IGF-I mRNA. In contrast, LiCl partially ameliorated the sepsis-induced increase in both NOS2 mRNA and protein in incubated epitrochlearis (Table 2).
Table 2.
Effect of lithium chloride (LiCl) on sepsis-induced changes in IGF-I and various inflammatory mediators
| Nonseptic | Septic | |||
|---|---|---|---|---|
| Control | LiCl | Control | LiCl | |
| IGF-I mRNA | 1.00 ± 0.08a | 0.98 ± 0.11a | 0.47 ± 0.07b | 0.55 ± 0.06b |
| TNFα mRNA | 1.00 ± 0.11a | 1.04 ± 0.09a | 3.33 ± 0.78b | 3.01 ± 0.45b |
| IL-6 mRNA | 1.00 ± 0.14a | 0.89 ± 0.12a | 2.32 ± 0.11b | 2.97 ± 0.47b |
| NOS2 mRNA | 1.00 ± 0.11a | 0.94 ± 0.11a | 4.87 ± 1.84b | 2.32 ± 1.44ab |
| NOS2 protein | 1.00 ± 0.14a | 0.88 ± 0.11a | 3.22 ± 0.43b | 1.55 ± 0.31ab |
Values are means ± SEM for 8–9 muscles in each group. Within the same row, means with different letters (a, b) are significantly different (P < 0.05); means with the same letter are not statistically different. The mRNA content for each transcript was normalized to L32. For the NOS2 protein, signal intensity was normalized to tubulin, which was not different between groups (data not shown).
DISCUSSION
Previous studies investigating the role of lithium and GSK-3 on the regulation of protein balance in catabolic conditions have primarily assessed the role of this protein in controlling muscle protein degradation (1,11,12). In addition, LiCl has also been demonstrated to increase protein synthesis and produce hypertrophy, albeit in neonatal cardiomyocytes (35). Our present data confirm the many reports that sepsis decreases skeletal muscle protein synthesis and that this defect can be retained under in vitro conditions (20,36). This sepsis-induced decrease in protein synthesis was associated with increased phosphorylation of eIF2Bε and a concomitant reduction in eIF2B activity. In addition, these sepsis-induced changes were associated with decreased GSK-3β phosphorylation and stimulation of GSK-3 activity. Decreased GSK-3 phosphorylation has previously been reported for muscle obtained directly from septic (15) or burned (1) rats. While we acknowledge that these many sepsis-induced changes are associations and do not prove causality, they are all internally consistent with the fact that the ε-subunit of eIF2B is necessary for full catalytic activity of the holoenzyme (37), that the phosphorylation of Ser535-eIF2Bε is inversely proportional to changes in eIF2B activity and protein synthesis (38) and that eIF2Bε is a known substrate for GSK-3β (14). Akt activity occupies a central position in regulating muscle protein synthesis and degradation, with increased Akt activity inducing hypertrophy and decreased Akt activity leading to atrophy (39). As GSK-3β is a known substrate for Akt, it was unanticipated that the sepsis-induced decrease in GSK-3β phosphorylation appeared independent of a change in Akt Ser473-phosphorylation (activity) under our experimental conditions. In contrast, we and others have reported that Akt phosphorylation is reduced in muscle from septic rats when tissue is analyzed immediately upon its removal from the rat (40,41). Therefore, we cannot exclude the possibility that a sepsis-induced change in Akt phosphorylation occurred in vivo. but it was not retained when muscles were incubated in vitro.
We hypothesized that incubation of muscle from septic rats with LiCl might reverse the decreased protein synthesis because of the coordinate change in GSK-3β and eIF2B activities. Such a lithium-induced antagonism on muscle protein degradation has been reported in incubated muscle from rats following burn injury or dexamethasone treatment (1,11,12). While we confirmed the ability of LiCl to completely reverse the sepsis-induced increase in proteolysis (discussed later), no such reversal for protein synthesis was observed. Lithium clearly stimulated protein synthesis in muscle from nonseptic rats, but this anabolic effect was essentially absent in muscle from septic rats. This finding of “lithium resistance” in septic muscle is novel and does did not appear to involve either GSK-3β or eIF2B. That is, incubation of septic muscle with LiCl was able to decrease GSK-3β activity which, in turn, was associated with a reduction in eIF2Bε phosphorylation and increased eIF2B activity. It is noteworthy that in contrast to previous results (11), we detected no change in the phosphorylation state of GSK-3β in muscle from either nonseptic or septic rats in response to LiCl, despite a demonstrable decrease in GSK-3 activity. This lack of a LiCl-induced change in GSK-3β phosphorylation is consistent with the lack of change in its upstream kinase, Akt. Moreover, this divergence between Ser9-GSK-3β phosphorylation and GSK activity per se has been previously reported using the chemically distinct GSK-3 inhibitor CT118637 (42,43), and it is consistent with other known mechanisms for the activation for this enzyme (44). Despite the complete reversal of this GSK-3/eIF2B pathway by LiCl, the sepsis-induced decrease in protein synthesis was still apparent. In contrast, LiCl did not reverse the sepsis-induced increase in 4E-BP1 phosphorylation, with the phosphorylation state of this mTOR substrate being the same in septic muscle in the absence and presence of lithium. Similarly, LiCl did not alter the mTOR-dependent phosphorylation of the S6K1 substrate S6 in muscle from septic rats. Collectively, these data suggest the decreased protein synthetic rate in muscle from septic rats is predominantly mediated via the mTOR pathway and not by defects in eIF2/eIF2B.
As mentioned above, prior to our study, essentially all of the data pertaining to the control of skeletal muscle protein turnover by GSK-3 focused on the degradation side of the protein balance equation. Inhibition of GSK-3 activity by LiCl, TDZD-8 (a highly specific GSK-3β inhibitor) and siRNA directed towards GSK-3β all decrease burn-induced muscle degradation in incubated muscles (1,12) − a response at least as great as that seen following treatment of muscle with the anabolic hormone IGF-I. Lithium also decreased the dexamethasone-induced increase in proteolysis in L6 myotubes (11). The mechanism by which LiCl and GSK-3 mediate protein degradation has not been fully elucidated. Of the proteolytic pathways, the ubiquitin-proteasome-dependent mechanism appears in part responsible for the increased degradation in skeletal muscle observed in various catabolic states and the up-regulation of the muscle-specific E3-ubiquitin ligases atrogin-1 and MuRF1 appears particularly important in this regard (31). In support of their putative role, cultured myotubes incubated with LiCl or the GSK-3β inhibitor SB415 for 24 h show complete inhibition of the dexamethasone-induced increase in atrogin-1 and MuRF-1 mRNA content (11). Moreover, LiCl can reduce proteasome activity, albeit in non-muscle cells (45). In contrast, transfection of a constitutively active GSK-3 did not alter the dexamethasone-induced increase in atrogin-1 (34). Our current data are more supportive of the latter study, indicating the lithium-induced reduction in GSK-3 activity does not blunt the sepsis-induced increase in muscle atrogene mRNA content and results in only approximately half of the elevation in proteasome activity in muscle. As muscles in our study were only incubated for 2 h with LiCl, it is possible that a longer exposure time might be required to decrease atrogen expression. Given that our data indicate that LiCl decreased the sepsis-induced increase in muscle proteolysis, it seems unlikely this anti-catabolic effect of LiCl is mediated via altered atrogene expression. However, we did note that lithium decreased the sepsis-induced increase in 26S proteasome activity. This latter conclusion is supported by previous results indicating that LiCl also ameliorates the increased proteasome activity in L6 myotubes cultured with the calcium ionophore A23817 (11). The mechanism by which LiCl decreased the sepsis-induced increase in proteasome activity was not further investigated.
In addition to the proteasome, lysosomes are also important in regulating muscle proteolysis and FOXO3 activation can coordinately upregulate both the ubiquitin-proteasome and lysosomal pathways (46). Atrophic stimuli increase transcription of a number of autophagy-related genes, including LC3 (32,33,46). LC3-I is located in the cytosol, but during autophagy it is lipidated by a series of ubiquitinization-like reactions producing LC3-II. Hence, the sepsis-induced increase in the LC3-II/LC-I ratio strongly suggests an increased autophagic activity. Although sepsis- and trauma-induced changes in autophagy have been reported for other tissues (47), we believe this is the initial report that prolonged sepsis increases autophagy in skeletal muscle per se. These data are consistent with the previously reported increase in LC3-II in atrophying muscle of dexamethasone-treated rats (48). Also, our data suggest that, at least acutely under in vitro conditions, the sepsis-induced autophagy can not be ameliorated by LiCl. The coordinated up-regulation of MuRF-1, atrogin-1, and LC3-II is internally consistent with the reduction in FoxO3 phosphorylation (46). Additional studies will be needed to more fully characterize the sepsis-induced changes in autophagy-related genes/proteins and to determine whether inhibition of this particular pathway in vivo alters the atrophic response.
The proinflammatory cytokines TNFα and IL-6 can either directly or indirectly impact both protein synthesis and degradation (2,3). But, in our current study, LiCl did not ameliorate the sepsis-induced increase in either cytokine, suggesting the over-production of these cytokines is not causally related to the ability of lithium to modulate the sepsis-induced changes in muscle protein balance. In contrast, LiCl partially reduced the elevation in NOS2 mRNA and protein in muscle from septic rats. Although increased NO production can decrease muscle protein synthesis (25,43), its effect on skeletal muscle proteolysis does not appear to have been previously studied. Our data are consistent with the ability of GSK-3 inhibitors to blunt endotoxin-induced increase in NO in other cell types (43,49).
In summary, the results of the present study suggest that LiCl can antagonize sepsis-induced muscle protein turnover under in vitro conditions. The ability of lithium to inhibit muscle proteolysis is pronounced, while septic muscle is resistant to lithium’s ability to stimulate muscle protein synthesis. Collectively, these data suggest the potential effectiveness of GSK-3 inhibitors in restoring muscle protein balance might be limited. However, definitive conclusions are difficult because the current study was limited by the lack of corresponding data from rats treated in vivo with LiCl or another GSK-3 inhibitor. Therefore, further studies where septic animals are treated in vivo with this general class of inhibitor and muscle protein balance assessed seem warranted. From a mechanistic perspective, the nominal stimulatory activity of lithium on muscle protein synthesis appears largely related to its inability to activate mTOR kinase activity and thereby stimulate cap-dependent translation as opposed to its ability to increase eIF2B activity. Finally, the ability of lithium to reverse the sepsis-induced increase in muscle protein breakdown is not dependent on the down-regulation of autophagy or the E3-ubiquitin ligases atrogin-1 and MuRF1. However, LiCl did partially ameliorate the sepsis-induced increase in the proteasome pathway, suggesting that lithium may directly or indirectly modulate other regulatory steps in this protein degradation pathway.
ACKNOWLEDGMENTS
The authors thank Anne Pruznak, Jay Nystrom and Gina Deiter for their expert technical assistance.
Funding source: This work was supported in part by National Institute on General Medical Sciences grant GM-39277 (TCV) and GM-38032 (CHL)
Footnotes
CONFLICT OF INTEREST
None
DEDICATION
This paper is dedicated to Thomas C. Vary, PhD, who died on 8 July 2010. All of the experimental protocols and much of the analyses were performed under his direction and he wrote the original draft of the manuscript. Dr. Vary was a long-time member of the Shock Society, a member of the editorial board of SHOCK, and an active shock and trauma researcher for more than 25 years. His work focused on the mechanisms responsible for the sepsis-induced disturbances in carbohydrate and protein metabolism in skeletal and cardiac muscle. He will be missed by his many friends and colleagues.
REFERENCES
- 1.Fang CH, Li B, James JH, et al. GSK-3beta activity is increased in skeletal muscle after burn injury in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1545–R1551. doi: 10.1152/ajpregu.00244.2007. [DOI] [PubMed] [Google Scholar]
- 2.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 3.Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab. 2008;295:E762–E771. doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayasam GV, Tulasi VK, Sodhi R, et al. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 6.Dugo L, Collin M, Allen DA, et al. Inhibiting glycogen synthase kinase 3beta in sepsis. Novartis Found Symp. 2007;280:128–142. [PubMed] [Google Scholar]
- 7.Zhou J, Lal H, Chen X, et al. GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. J Clin Invest. 2010;120:2280–2291. doi: 10.1172/JCI41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus EJ, Sakamoto K, Armit LJ, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr Drug Targets. 2006;7:1435–1441. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- 10.Vyas DR, Spangenburg EE, Abraha TW, et al. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–C551. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- 11.Evenson AR, Fareed MU, Menconi MJ, et al. GSK-3beta inhibitors reduce protein degradation in muscles from septic rats and in dexamethasone-treated myotubes. Int J Biochem Cell Biol. 2005;37:2226–2238. doi: 10.1016/j.biocel.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Fang CH, Li BG, James JH, et al. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor i and glycogen synthase kinase-3beta inhibitors. Endocrinology. 2005;146:3141–3149. doi: 10.1210/en.2004-0869. [DOI] [PubMed] [Google Scholar]
- 13.Vary TC, Jurasinski CV, Karinch AM, et al. Regulation of eukaryotic initiation factor-2 expression during sepsis. Am J Physiol. 1994;266:E193–E201. doi: 10.1152/ajpendo.1994.266.2.E193. [DOI] [PubMed] [Google Scholar]
- 14.Pavitt GD. eIF2B, a mediator of general and gene-specific translational control. Biochem Soc Trans. 2005;33:1487–1492. doi: 10.1042/BST0331487. [DOI] [PubMed] [Google Scholar]
- 15.Vary TC, Deiter G, Kimball SR. Phosphorylation of eukaryotic initiation factor eIF2Bepsilon in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 2002;283:E1032–E1039. doi: 10.1152/ajpendo.00171.2002. [DOI] [PubMed] [Google Scholar]
- 16.Dugo L, Collin M, Allen DA, et al. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Rehani K, Jope RS, et al. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svanberg E, Frost RA, Lang CH, et al. IGF-I/IGFBP-3 binary complex modulates sepsis-induced inhibition of protein synthesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1145–E1158. doi: 10.1152/ajpendo.2000.279.5.E1145. [DOI] [PubMed] [Google Scholar]
- 19.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol. 1992;262:C1513–C1519. doi: 10.1152/ajpcell.1992.262.6.C1513. [DOI] [PubMed] [Google Scholar]
- 20.Vary TC, Dardevet D, Grizard J, et al. Differential regulation of skeletal muscle protein turnover by insulin and IGF-I after bacteremia. Am J Physiol. 1998;275:E584–E593. doi: 10.1152/ajpendo.1998.275.4.E584. [DOI] [PubMed] [Google Scholar]
- 21.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–R1789. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257:1613–1621. [PubMed] [Google Scholar]
- 23.Hong-Brown LQ, Brown CR, Kazi AA, et al. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem. 2010;109:1172–1184. doi: 10.1002/jcb.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Hu Z, Hu J, et al. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 25.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock. 2009;32:416–426. doi: 10.1097/SHK.0b013e3181a034d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vary TC, Deiter G, Lang CH. Diminished ERK 1/2 and p38 MAPK phosphorylation in skeletal muscle during sepsis. Shock. 2004;22:548–554. doi: 10.1097/01.shk.0000144131.13900.ff<. [DOI] [PubMed] [Google Scholar]
- 27.Nystrom G, Pruznak A, Huber D, et al. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism. 2009;58:787–797. doi: 10.1016/j.metabol.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuckow AP, Vary TC, Kimball SR, et al. Ectopic expression of eIF2Bepsilon in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E241–E248. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 32.Voisin L, Breuille D, Combaret L, et al. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+ -activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest. 1996;97:1610–1617. doi: 10.1172/JCI118586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju JS, Varadhachary AS, Miller SE, et al. Quantitation of "autophagic flux" in mature skeletal muscle. Autophagy. 2010;6:929–935. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haq S, Choukroun G, Kang ZB, et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall-Angeras M, Angeras U, von AD, et al. Influence of sepsis in rats on muscle protein turnover in vivo and in tissue incubated under different in vitro conditions. Metabolism. 1991;40:247–251. doi: 10.1016/0026-0495(91)90105-6. [DOI] [PubMed] [Google Scholar]
- 37.Anthony TG, Fabian JR, Kimball SR, et al. Identification of domains within the epsilon-subunit of the translation initiation factor eIF2B that are necessary for guanine nucleotide exchange activity and eIF2B holoprotein formation. Biochim Biophys Acta. 2000;1492:56–62. doi: 10.1016/s0167-4781(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 38.Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294(Pt 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 40.Crossland H, Constantin-Teodosiu D, Gardiner SM, et al. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol. 2008;586:5589–5600. doi: 10.1113/jphysiol.2008.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazi AA, Pruznak AM, Frost RA, et al. Sepsis-induced alterations in protein-protein interactions with mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. . Shock. 2010 doi: 10.1097/SHK.0b013e3181ecb57c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dokken BB, Saengsirisuwan V, Kim JS, et al. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol Endocrinol Metab. 2008;294:E615–E621. doi: 10.1152/ajpendo.00578.2007. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson LS, Fabian JR, Kimball SR. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol. 1999;31:191–200. doi: 10.1016/s1357-2725(98)00141-1. [DOI] [PubMed] [Google Scholar]
- 44.Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Shim M, Smart RC. Lithium stabilizes the CCAAT/enhancer-binding protein alpha (C/EBPalpha) through a glycogen synthase kinase 3 (GSK3)-independent pathway involving direct inhibition of proteasomal activity. J Biol Chem. 2003;278:19674–19681. doi: 10.1074/jbc.M301356200. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Brault JJ, Schild A, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto D, Maki T, Herningtyas EH, et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve. 2010;41:819–827. doi: 10.1002/mus.21621. [DOI] [PubMed] [Google Scholar]
- 49.Lin CF, Tsai CC, Huang WC, et al. IFN-gamma synergizes with LPS to induce nitric oxide biosynthesis through glycogen synthase kinase-3-inhibited IL-10. J Cell Biochem. 2008;105:746–755. doi: 10.1002/jcb.21868. [DOI] [PubMed] [Google Scholar]