Abstract
DSPP is a large precursor protein that is proteolytically processed into three fragments: a NH2-terminal fragment [dentin sialoprotein (DSP), a proteoglycan form (DSP-PG)] and a COOH-terminal fragment [dentin phosphoprotein (DPP)]. In vitro studies indicate that DPP is a strong initiator and regulator of hydroxyapatite crystal formation and growth, but the role(s) of the NH2-terminal fragment of DSPP (i.e., DSP and DSP-PG) in dentinogenesis remain unclear. This study focuses on the function of the NH2-terminal fragment of DSPP in dentinogenesis. Here, transgenic mouse lines expressing the NH2-terminal fragment of DSPP driven by 3.6-kb type I collagen promoter (Col 1a1) were generated and crossbred with Dspp null mice to obtain mice that express the transgene but lack the endogenous Dspp (Dspp KO/DSP Tg). We found dentin from the Dspp KO/DSP Tg mice was much thinner; more poorly mineralized and remarkably disorganized compared to the Dspp KO mice. The fact that Dspp KO/DSP Tg mice exhibited more severe dentin defects than the Dspp null mice indicates that the NH2-terminal fragment of DSPP may inhibit dentin mineralization or may serve as an antagonist against the accelerating action of DPP and serve to prevent predentin from being mineralized too rapidly during dentionogenesis.
Keywords: DSPP, Proteolytic processing, Posttranslational modification, biomineralization, dentin
Genetic studies have shown that dentin sialophosphoprotein (DSPP) plays an essential role in dentinogenesis (1–3). For example, the dentin in Dspp knockout mice is hypomineralized and the predentin is widened, creating a phenotype similar to that of dentinogenesis imperfecta type III in humans (3). While studies have demonstrated the critical role of DSPP in dentin formation, the mechanism(s) by which DSPP function(s) in biomineralization remain largely unclear.
DSPP, first identified by cDNA cloning (4), is a large protein that undergoes proteolytic processing to form the NH2-terminal fragment known as dentin sialoprotein (DSP), proteoglycan form of the NH2-terminal fragment referred to as DSP-PG and a COOH-terminal fragment named dentin phosphoprotein (DPP) (5–7). The sequence of the NH2-terminal fragment (DSP or DSP-PG) lies in the 5′-portion, while the DPP sequence is located in the 3′-region of the DSPP transcript (4, 8–10). Studies done in our laboratory and others established the presence of a proteoglycan form of DSP (known as DSP-PG) (5–7). DSP/DSP-PG and DPP are abundant in the dentin extracellular matrix (ECM) whereas the protein representing the entire sequence (DSPP) is scarcely present, which led to the belief that the processed fragments may be the functional forms of DSPP. Recent work in our laboratory has shown that blocking the proteolytic fragmentation of DSPP inactivates the function(s) of this molecule during dentinogenesis (11). This observation provides strong evidence that the proteolytic processing of DSPP is an activation event which releases the functional fragments that exert distinct molecular role(s) during dentin formation (11).
DPP, discovered in 1967, is the most abundant non collagenous protein (NCP) in the dentin ECM (12, 13). The unusual feature of DPP is the presence of large amounts of aspartic acid (Asp) and phosphoserine (Pse), mostly found in the repeating sequences of (Asp-Pse-Pse)n and (Asp-Pse)n (14,15). Several in vitro mineralization studies have indicated that DPP is an important initiator and modulator for the formation and growth of hydroxyapatite (HA) crystals (16–18). It is believed that the DPP secreted by the odontoblasts is transported to the mineralization front where it binds to collagen fibrils promoting the formation of initial HA. As this mineralization process proceeds, and more predentin is converted to dentin, these mineral crystals grow in an oriented fashion under the modulation of DPP and other non-collagenous proteins (NCPs) that bind to the growing HA faces (19, 20).
DSP, discovered in 1981, is much less abundant than DPP in dentin ECM (21). DSP [protein without the glycosaminoglycan (GAG) chains]does not have a significant effect on the in vitro mineral formation and growth (22). Since there is no in vivo data concerning the role of DSP in dentinogenesis, the functions of this fragment is presently undefined. The recently discovered proteoglycan form of DSP (DSP-PG) is a major component in the proteoglycan pool of the dentin extracellular matrix (ECM) and appears to be more abundant than DSP (5, 6). We believe that the NH2-terminal fragment of DSPP (DSP/DSP-PG) must play a role in biomineralization, distinct from that of the COOH-terminal fragment of DSPP (DPP). DSP-PG from the NH2-terminal sequence of DSPP was found in the dentin ECM at higher amounts than the core protein DSP, which led us to believe that DSP-PG may be the functional form of the NH2-terminal fragment. We hypothesize that the role of DSP-PG may be to prevent the predentin from being mineralized too rapidly and to serve as an antagonist of DPP in dentinogenesis.
In order to examine the function of the NH2-terminal fragment of DSPP in dentinogenesis, we expressed the NH2-terminal fragment of DSPP in the Dspp-null background (referred as “Dspp KO/DSP Tg”) to analyze the in vivo effects of this fragment in a gain of function experimental approach. In this study, we systematically characterized the dentin of Dspp KO/DSP Tg mice and compared it to the dentin from Dspp-null (Dspp knockout or Dspp KO) mice and wild type (WT) mice. We observed that the dentin of the Dspp KO/DSP Tg mice appeared much worse compared to the Dspp-null mice. The results from this investigation support our hypothesis that the NH2-terminal fragment of DSPP has an inhibitory role in dentinogenesis.
MATERIALS AND METHODS
Generation of mutant mice
Transgenic mice expressing the NH2-terminal fragment of DSPP in the wild type (WT) background were generated using techniques described in our previous reports (11, 23). Briefly, a pBCKS construct containing a 3.6-kb rat Col 1a1 promoter was used to generate the transgene (24). The NH2-terminal fragment construct consisting of mouse DSPP signal peptide, three VSV epitopes, and the cDNA sequence encoding the NH2-terminal portion of mouse DSPP (25) were inserted downstream of the 3.6-kb rat Col 1a1 promoter in the pBCKS construct. A detailed description of the restriction enzymes and procedures used in generating the transgenic mice is given in our previous publication (23). We obtained eight independent founders expressing the NH2-terminal portion of mouse DSPP in the WT (C57BL/6J) background. These mouse lines expressing different levels of the transgene were crossbred with Dspp-null (Dspp-KO) mice (strain name: B6; 129-Dspptm1Kul/Mmnc; MMRRC, UNC, Chapel Hill, NC). By this crossbreeding scheme, we generated the mice expressing the NH2-terminal fragment of the DSPP transgene in the Dspp-KO background (i.e., without the endogenous Dspp gene). These mice were referred to as “Dspp-KO/DSP Tg” mice. We analyzed the effects of the transgene on dentin formation in Dspp-KO/DSP Tg mice derived from two of the eight transgenic mouse lines, line 4 (with a higher level of expression for the transgene) and line 7 (with a lower expression level of the transgene). In this report, we described in detail the data from the analyses of Dspp-deficient mice expressing the higher level of the transgene (line 4).
The polymerase chain reaction (PCR) primers for detecting the DSP transgene were: forward, 5′-CCAGTTAGTACCACTGGAAAGAGAC-3′; reverse, 5′ TCATGGTTGGTGCTATTCTTGATGC-3′; PCR amplification using this pair of primers would yield a 521 bp PCR product from the transgene and a 676 bp product for the endogenous Dspp gene when mouse genomic DNA was used template. The primers used to identify the endogenous Dspp alleles and null alleles (containing Lac Z gene) are described in our previous publication (11).
The animal protocols used in this study were approved by the Animal Welfare Committee of Texas A&M Health Science Center Baylor College of Dentistry (Dallas, TX). A multidisciplinary experimental approach was used to characterize the dentin of the three mouse genotypes: 1) Dspp-KO/DSP Tg mice, 2) Dspp-KO mice, and 3) WT (C57/BL6J) mice.
Expression levels of the DSP transgenes in teeth
Quantitative real-time PCR (qPCR) was performed to determine the DSP/DSP-PG mRNA expression level in the incisors of the eight founder mice in the wild type genetic background as previously described (11, 26). Briefly, total RNA was extracted from the mouse incisors with an RNeasy mini kit (Qiagen; Germantown, MD). The RNA (1 ug/per sample) was reverse-transcribed into cDNA using the QuantiTect Rev Transcription Kit (Qiagen; Germantown, MD). The DSP primers used for quantitative real-time PCR were: forward, 5′-AACTCTGTGGCTGTGCCTCT-3′ (in exon 3) and reverse, 5′-TATTGACTCGGAGCCATTCC-3′ (in exon 4). The real-time PCR reactions were performed using PCR conditions as we previously reported (26). The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used for standardization (26).
Extraction and isolation of noncollagenous proteins (NCPs) from mouse dentin and detection of DSPP-related proteins
For extraction of DSP/DSP-PG and other DSPP-related proteins from dentin, maxillary incisors from six 3-month-old WT and Dspp-KO/DSP Tg mice (12 incisors/group) were processed using the protocol described in our previous reports (26, 27). The extracts from the incisors were separated into 80 fractions (0.5 ml/fraction) by Q-Sepharose ion-exchange chromatography (Amersham Biosciences; Uppsala, Sweden) with a gradient ranging from 0.1 to 0.8 M NaCl in 6 M urea solution (28). The same amount of sample (60 ul) from every third fraction was treated with 3% β-mercaptoethanol (β-ME) to break the disulfide bonds of the dimeric form of DSP and was then loaded onto 10% SDS-PAGE gels. First, Stain-All staining was performed to visualize the acidic non-collagenous proteins (NCPs) in these fractions. Next, Western immunoblotting was carried out for the fractions that might contain any DSPP-related proteins. For Western immunoblotting, we used an anti-DSP monoclonal antibody (anti-DSP-2G7.3) at a dilution of 1:2000 (5). The blots were incubated in alkaline phosphatase-conjugated anti-rabbit IgG (Sigma-Aldrich; St. Louis, MO) secondary antibody at a dilution of 1:5000. This step was followed by incubation in a chemiluminescent substrate CDP-star (Ambion, Austin, TX, USA) for 5 min and exposure to X-ray films. Purified wild-type rat dentin extract (0.3 μg) previously shown to contain DSP and DPP proteins were used as positive controls for the Stains-all staining and Western immunoblotting analyses (DSP control).
Flat X-ray radiography and micro-computed tomography (μ-CT)
The mandibles from the 3-month-old and 6-month-old mice (5 mice/group) were dissected from the WT, Dspp-null and Dspp-KO/DSP Tg mice and analyzed with the Faxitron MX-20 Specimen Radiography System (Faxitron X-ray, Buffalo Grove, IL, USA). For the μ-CT analyses, we performed triplicate analyses of 3-month-old mice from the WT, Dspp-null and Dspp-KO/DSP Tg mice (n = 3). The mandibles were scanned using μ-CT35 imaging system (Scanco Medical, Basserdorf, Switzerland), to assess their structure and morphology as we previously described (11, 29).
Histology and immunohistochemistry
For histology and immunohistochemistry (IHC) analyses, the 3- and 6-month-old mice from the three groups were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The samples were fixed in 4% paraformaldehyde for 48 hr. The samples (mandibles) were then decalcified in 8% EDTA (pH 7.4) at 4°C for approximately 2 wk. The mandibular tissues were processed for paraffin embedding, and 5-μm serial sections were prepared. The sections were either stained with hematoxylin & eosin (H&E) or used for IHC analyses. The anti-DSP monoclonal antibody, referred to as “anti-DSP-2C12.3” was used at a dilution of 1:800 (30). The anti-biglycan antibody (“LF-159”; a gift from Dr. Larry Fisher of the Craniofacial and Skeletal Diseases Branch, National Institutes of Health, Bethesda, MD, USA) was used at a dilution of 1:1000. All the IHC experiments were carried out with the M.O.M. kit and DAB kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions.
Resin-casted scanning electron microscopy (SEM)
Mandibles from 3-month-old WT, Dspp-null and Dspp-KO/DSP Tg mice (5 mice/group) were dissected and fixed in 2% paraformaldehyde with 2.5% glutaraldehyde in 0.1 M cacodylate buffer solution (pH 7.4) at room temperature. A detailed protocol used in processing and polishing the samples for resin-casted SEM has been described in a previous study (11). The dentin surface of all the three samples was acid-etched with 37% phosphoric acid for 2–10 s and washed with 5.25% sodium hypochlorite for 5 min. The samples were then coated with gold and palladium as described previously (31). A FEI/Philips XL30 Field emission environmental SEM (Philips, Hillsboro, OR, USA) was used to perform the SEM analyses with a digital record prepared for representative specimens.
RESULTS
Expression of the DSP transgenes in the teeth
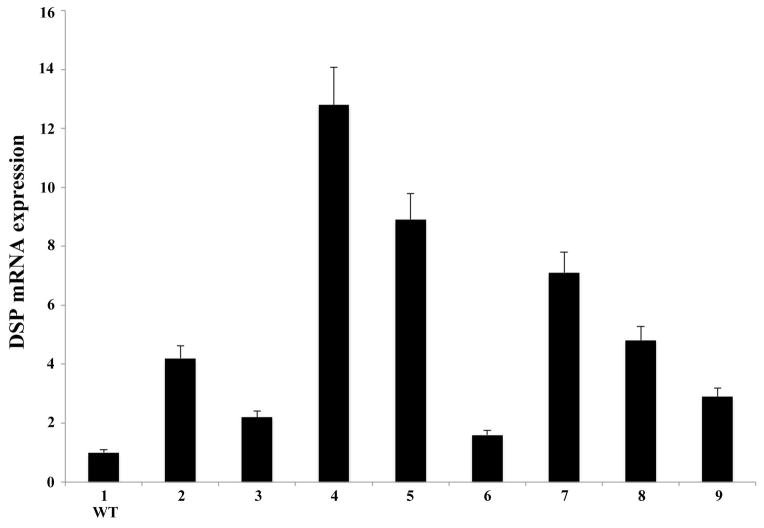
The relative mRNA expression level of the NH2-terminal fragment of DSPP in the incisors from the eight founder mice was determined by real-time PCR analyses (Fig. 1). The forward and reverse primers used in these analyses were located in exons 3 and 4 respectively, corresponding to an amplicon within the NH2-terminal region of the Dspp gene. For the purpose of calculations, the mRNA level of the WT sample (Fig. 1, line 1) was set as one. The mRNA expression in the incisors of the eight founder mice (Fig. 1, lines 2–9) was then calculated relative to that of the WT mouse incisor. Triplicate analyses were performed. Real-time analyses revealed that the founder mice of line 4 (Fig. 1) had the highest DSPP mRNA level, which was ~13 times that of the WT samples. The Dspp-KO/DSP Tg mice derived from this line of founder mice were systematically characterized and reported in detail in this report. It should be noted that although different expression levels for the transgene were observed among the different transgenic mouse lines (Fig. 1, lines 2–9), they all contained higher levels of DSPP mRNA when compared with the WT mice (Fig. 1, line 1). The transgenic mice expressing the NH2-terminal fragment of DSPP in the WT background did not show any significant difference in their teeth or their bone when compared to the WT mice (data not shown).
Figure 1. Levels of DSPP mRNA in the incisors of the transgenic founder mice.
Real-time PCR analyses showed the different DSPP mRNA levels in the eight founder transgenic mice expressing the NH2-terminal fragment of DSPP transgene in the WT background (lines 2–9). For calculation purposes, the WT sample (line 1) was set as 1 and mRNA levels in the eight transgenic mice were calculated relative to that of the WT. The results were from triplicate analyses (n = 3) for each group. The mRNA level of the transgenic mice in line 4 was the highest (approximately 13 fold of that of the endogenouse gene expression in the WT mice). This mouse line was used to crossbreed with Dspp KO mice to generate the “Dspp KO/DSP Tg” mice that expressed the NH2-terminal fragment of DSPP without the endogenous Dspp gene.
Extraction and separation of NCPs and detection of DSPP-related proteins
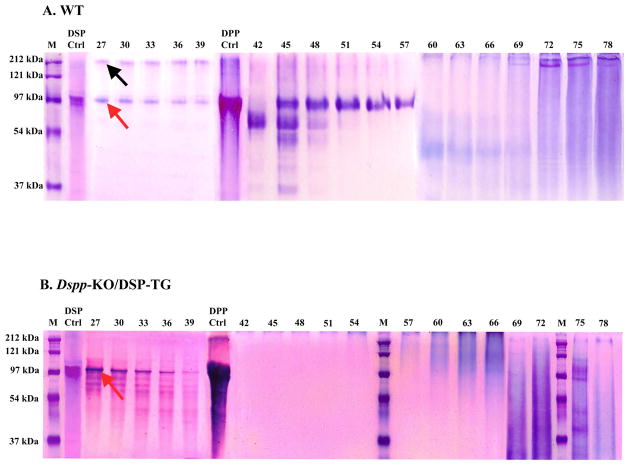
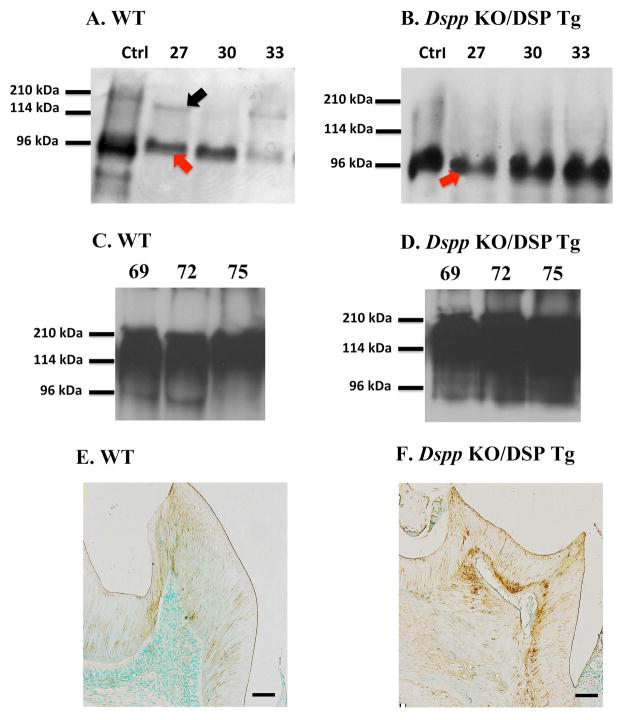
Stains-All staining and Western immunoblotting were utilized to identify the DSPP-related proteins in the dentin extracts from Dspp-KO/DSP Tg and WT mice. All the chromatographic fractions from the dentin extracts that putatively contain DSPP-related products were analyzed by SDS-PAGE with Stains-All detection (Fig. 2). In the extracts from the WT incisors, protein bands matching the migration rate of DSP, DSPP (Fig. 2A, fractions 27 to 39), as well as DPP (Fig. 2A, fractions 45 to 57), were clearly detected by affinity to the Stains-All dye. The protein bands matching the migration rate of the full length DSPP appeared to be much weaker than that of DSP in the WT samples (Fig. 2A, fractions 27 to 39). However, only large amount of DSP (Fig. 2B, fractions 27 to 39) was observed in the Dspp-KO/DSP Tg mice. No protein bands matching DSPP or DPP were detected in the dentin extracts of the Dspp-KO/DSP Tg mice. In the Western immunoblotting analyses, DSP and DSPP were clearly detected in the dentin extracts from the WT (Fig. 3A), while only DSP (without DSPP) could be identified in the dentin extracts from the Dspp-KO/DSP Tg mice (Fig. 3B). DSP-PG was detected as strong smears in the late fractions (fractions 69–75) in the extracts from the WT and Dspp-KO/DSP Tg (Fig. 3C,D). The results from both the Stains-All and Western immunoblotting analyses proved that for the Dspp-KO/DSP Tg mice, only the NH2-terminal fragment of DSPP (DSP/DSP-PG) was expressed (i.e., without DPP or full-length DSPP).
Figure 2. Stains-All staining of acidic proteins in the NCP extracts from mouse incisor dentin.
The NCPs were extracted from the incisor dentin of six 3-month-old WT (A) and Dspp KO/DSP Tg mice (B). The extracted NCPs were separated into 80 fractions (0.5 ml/fraction) by a Q-Sepharose ion-exchange column. The numbers on the top of each gel image represent the fraction numbers. Equal amounts (60 μl) of every third fraction were loaded onto 10% SDS-PAGE gel. In the WT sample (A), protein bands corresponding to both DSP (~ 97 kDa, red arrow) and DSPP (~ 200 kDa, black arrow) were detected in fractions 27 to 39. The major blue bands in fractions 45 to 57 in the WT sample (A) were primarily composed of DPP. In the Dspp KO/DSP Tg mice (B), only the DSP protein bands (~ 97 kDa, red arrow) were observed in fractions 27 to 39. No DSPP or DPP proteins bands were detected. Some smaller protein bands in fractions 60–72 of the Dspp KO/DSP Tg samples (B) appeared stronger than in the wild type (A); this observation indicated that overexpressing the NH2-terminal fragment of DSPP (DSP and DSP-PG) might have a direct or indirect effect on the expression levels of other acidic proteins and proteoglycans (e.g., biglycan). M, molecular weight standard; DSP, pure DSP isolated from rat dentin; DPP, pure DPP isolated from rat dentin.
Figure 3. Western immunoblotting and anti-DSP immunohistochemistry to detect DSP in the mouse dentin.
Western immunoblotting analyses (A to D) with monoclonal anti-DSP antibody were done to detect DSPP-related molecules in the dentin extracts of WT (A, C) and Dspp KO/DSP Tg mice (B, D). The purified rat dentin extract (0.3 μg) containing rat DSPP and DSP was used as a positive control (Ctrl). The Western immunoblotting results from the chromatographic fractions (27, 30, 33, 69, 72 and 75) that had strong signals for DSP/DSPP/DSP-PG in Stains-All analyses (Fig 2) were used for Western immunoblotting analyses. While both DSP (red arrow) and DSPP (black arrow) were detected in the dentin extracts from the WT mice (A), only DSP (red arrow) was detected in the Dspp KO/DSP Tg mice (B). In fractions 69–75, DSP-PG was detected as strong smears both in the WT and Dspp-KO/DSP Tg (C and D respectively); please note the abundance of DSP-PG in the Dspp KO/DSP Tg mice. The findings from both Stains-All and Western immunoblotting analyses showed that the Dspp KO/DSP Tg mice only contained the NH2-terminal fragment of DSPP (DSP and DSP-PG), without any products from the endogenous Dspp gene.
For anti-DSP immunohistochemistry analyses (E and F), the samples from the 3-month-old WT (E) and Dspp KO/DSP Tg mice (F) were evaluated. Anti-DSP activity was observed in the matrix of the first mandibular molars in both WT (E) and Dspp KO/DSP Tg mice (F). The signal for the anti-DSP antibody in the dentin of the Dspp KO/DSP Tg mice (F) was relatively stronger than in the WT mice (E). Bar: 100 μm
Anti-DSP immunostaining
Anti-DSP immunostaining showed positive reactivity in the odontoblasts, as well as the dentin matrix of the WT and Dspp-KO/DSP Tg mice (Fig. 3E,F). In the Dspp-KO/DSP Tg mice (Fig. 3F), the anti-DSP immunostaining appeared much stronger than that of the WT mice (Fig. 3D) due to the higher expression levels of the transgene. The anti-DSP immunohistochemistry results from the Dspp-KO/DSP Tg mice also showed that the NH2-terminal fragment of DSPP derived from the transgene was secreted into the dentin ECM (Fig. 3F).
Flat X-ray radiography and micro-computed tomography (μ-CT)
Faxitron X-ray radiography (Fig. 4) revealed the dentin structure and morphology in the 3- and 6-month-old mice. The X-ray analyses showed enlarged pulp chambers with reduced dentin thickness in the Dspp KO mice compared to the WT mice, which is a characteristic feature of the KO mice (Figs. 4A,B,D,E). The expression of NH2-terminal fragment of DSPP from the engineered transgene worsened the dentin defects observed in the Dspp KO/DSP Tg mice (Figs. 4C,F). Samples from the Dspp-KO/DSP Tg mice showed extremely thin and radiolucent dentin with remarkably enlarged pulp chambers (Figs. 4C.F).
Figure 4. Flat X-ray radiography analyses of mandibles from 3- and 6-month-old mice.
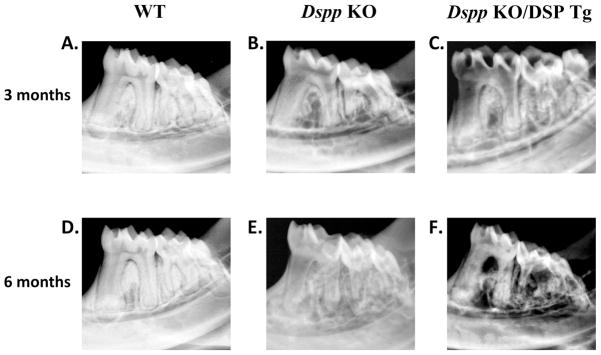
At 3 and 6 months of age, the WT mice (A and D) showed evenly distributed and well mineralized radio-opaque dentin with small pulp chamber. The mandibular molars in the Dspp KO mice (B and E) had an enlarged pulp chamber and thinner dentin compared to the WT mice. The tooth defects in the Dspp KO/DSP Tg mice (C and F) were worse than those of the Dspp KO mice, with much thinner, radiolucent dentin and a more significant loss of the alveolar bone.
Similar findings were observed from the μ-CT (Fig. 5) analyses of 3-month old mice, in which the alveolar bone and dentin in the Dspp-KO/DSP Tg mice (Figs. 5C,F) appeared considerably more porous and thinner than those of the Dspp KO mice (Figs. 5B,E). The μ-CT analyses showed that the dentin and alveolar bone defects in the 6-month-old Dspp-KO/DSP Tg mice (data not shown) were similar those observed in flat X-ray radiographic images of mice at the same age.
Figure 5. μ-CT analyses of mandibles from 3-month-old mice.
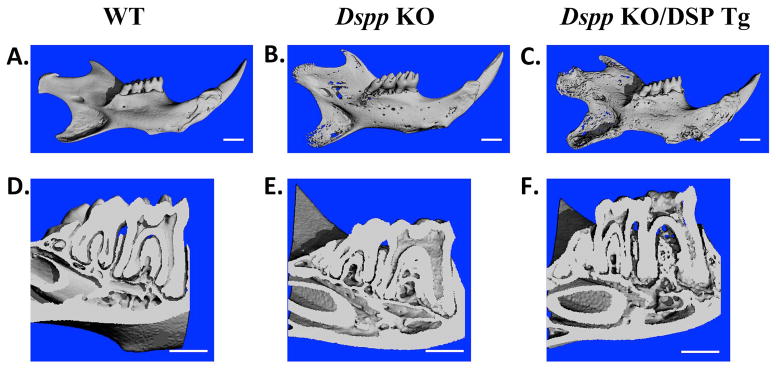
WT (A and D), Dspp KO (B and E) and Dspp KO/DSP Tg mice (C and F) were evaluated for their overall morphology (A, B and C) and dentin phenotypes in the molar cross sections (D, E and F). The WT mice showed evenly distributed and well mineralized dentin (A and D). The mandible of the Dspp KO mice (B) had porosities when compared to the WT sample. Molar cross section of the Dspp KO mice (E) showed an enlarged pulp chamber and thinner dentin compared with the WT mice (D). The defects in the Dspp KO/DSP Tg mice (C and F) were even more pronounced with marked increase in porosities and reduction in dentin thickness compared to the Dspp KO mice. Bar: 1 mm
Histological Analysis
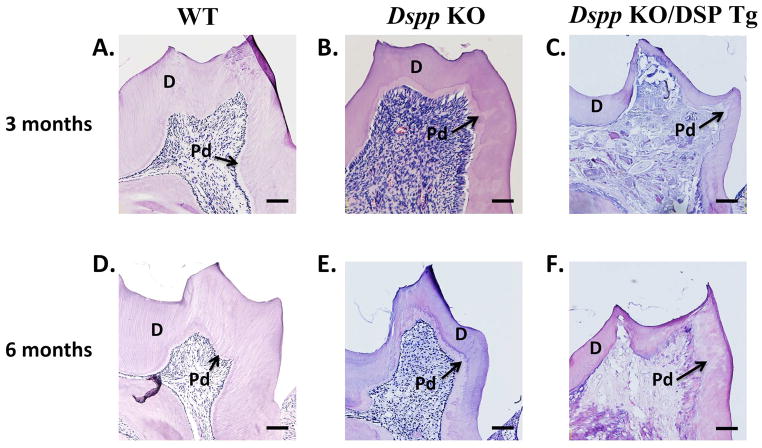
In the histological analyses, the pulp chamber in the mandibular molars of the Dspp KO mice appeared significantly larger with widened predentin zones as compared to the WT mice at both ages (Figs. 6A,B,D,E). The defects of the predentin-dentin complex in the Dspp-KO/DSP Tg mice (Figs. 6C,F) appeared noticeably worse than those of the Dspp-KO mice (Figs. 6B,E) with the former mice demonstrating extremely thin dentin. The predentin zone in the Dspp-KO/DSP Tg mice had dramatically expanded to replace almost the entire predentin-dentin complex at both 3 and 6 months of age (Figs. 6C,F).
Figure 6. H&E staining in 3- and 6-month-old mice.
At 3 and 6 months, the Dspp KO mice (B and E) had wider predentin (denoted as “Pd”) and thinner dentin (denoted as “D”) compared to the WT mice (A and D). The Dspp KO/DSP Tg mice (C and F) displayed more severe dentin abnormalities compared to those of the Dspp KO mice (B and E). The dentin of the Dspp KO/DSP Tg (C and F) mice was almost non-existent with almost the entire layer consisting of the unmineralized predentin structure. The pulp showed evidence of ectopic calcification (C and F), which appeared worsened at 6-months (F) than 3 months of age. This could possibly be due to chronic inflammation arising from pulp exposure. All the six sections showed in this figure were processed, sectioned, stained and visualized using the same settings and protocols. The differences in the staining intensity seen in the sections were probably due to the difference in the matrix composition (e.g., increased amounts of proteoglycans in the Dspp KO/DSP Tg and Dspp KO mice). Bar: 100 μm.
Resin-cast SEM
The resin-cast SEM analyses (Fig. 7) showed well organized and evenly distributed dentinal tubules running parallel to one another and perpendicular to the dentino-enamel junction (DEJ) in the WT sample (Fig. 7A). The Dspp KO mice (Fig. 7B) on the other hand, revealed disrupted and disorganized dentinal tubules. In the Dspp-KO/DSP Tg mice (Fig. 7C), only an extremely small number of dentinal tubules could be visualized. The few dentinal tubules that were discernable in the Dspp-KO/DSP Tg mice appeared irregular and collapsed at several places (Fig. 7C).
Figure 7. Resin-casted and acid-etched SEM analyses.
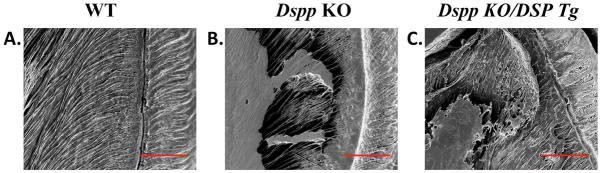
In the resin infiltration and acid-etched SEM images of the mandibular first molar, the dentinal tubules in the WT mice (A) had a uniform distribution of dentinal tubules running to parallel to one another towards the DEJ. The dentinal tubules in the Dspp KO mice (B) appeared fewer in number when compared to the WT sample and were disorganized in their orientation. The dentinal tubules in the Dspp KO/DSP Tg mice (C) were completely collapsed, tangled and could not even be distinguished. Bar: 50 μm
Anti-biglycan immunostaining
Since the proteoglycan biglycan is primarily localized in the predentin zone of the molars, we used anti-biglycan immunostaining (Fig. 8) to localize the predentin zone in the three groups of mice. A very narrow predentin zone was evident in the WT mice (Fig. 8A), whereas the predentin zone in the Dspp KO (Fig. 8B) appeared comparatively wider. The biglycan immununoreactivity in the Dspp-KO/DSP Tg mice (Fig. 8C) was much stronger and appeared over a wider area than that observed in the Dspp KO mice (Fig. 8B). In fact, the biglycan was distributed in almost the entire predentin-dentin complex, confirming the histological finding that nearly the whole predentin-dentin complex was not mineralized in the Dspp-KO/DSP Tg mice. In other words, the histology and biglycan immunostaining analyses showed that in the Dspp-KO/DSP Tg mice, the mineralized dentin was almost non-existent, composed almost entirely of the premature and unmineralized predentin. Due to this reason, we were unable to measure the thickness of dentin and the predentin in order to quantify the ratio between the two.
Figure 8. Biglycan immunostaining of predentin in 3-month-old mice.
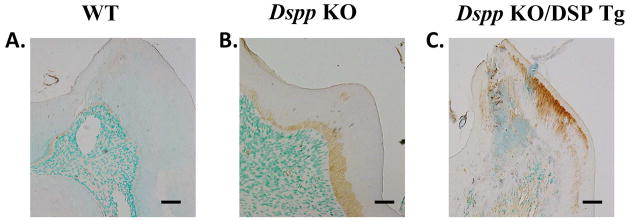
The WT mice (A) showed a narrow predentin zone demarcated by the brown positive biglycan staining. In the Dspp KO mice (B), anti-biglycan activity revealed a widened predentin layer and an irregular dentin-predentin border as compared to the WT mice (A). The results from the Dspp KO/DSP Tg mice (C) indicated that nearly the entire predentin-dentin complex in these mice was made of the unmineralized predentin with anti-biglycan staining extending and covering most of the structure. Bar: A-H = 100 μm.
We analyzed the teeth of Dspp-KO/DSP Tg mice derived from two of the eight lines of the founder mice: line 4 (with a higher level of expression for the transgene) and line 7 (with a lower expression level of the transgene). While in both cases, expressing the NH2-terminal fragment (at a higher or lower level) in the Dspp knockout background significantly worsened the Dspp-deficient dentin defects, the dentin abnormalities in the Dspp-deficient mice expressing the higher level of the transgene (line 4) were worse than in the mice expressing the lower expression level of the transgene (line 7, data not shown). These observations suggest that expression levels of the NH2-terminal fragment of DSPP correlated with its inhibitory potential in dentinogenesis.
DISCUSSION
In the dentin ECM, large amounts of the processed NH2-terminal (DSP-PG/DSP) and COOH-terminal (DPP) fragments are present, along with only trace amounts of the full-length DSPP (27). Several recent reports indicate that the cleavage of DSPP at the peptide bond corresponding to Gly451-Asp452 in mouse DSPP is essential for releasing the NH2-terminal and COOH-terminal fragments of this protein (32–34). More recently, we showed that blocking the cleavage of mouse DSPP at this site leads to the loss of its function in dentinogenesis (11). These recent findings support the belief that proteolytic processing of DSPP may be an activation process, which releases the active fragments, DSP-PG/DSP and DPP.
DSP-PG/DSP and DPP with dramatically different biochemistry features are likely to play different roles in dentinogenesis. Several excellent reports have demonstrated the role of DPP in nucleation and modulation of apatite crystal formation (16–18). However, very little is known about the role of the function of the NH2-terminal fragment of DSPP (DSP-PG and/or DSP). It has recently been discovered that DSP-PG consists of two glycosaminoglycan (GAG) chains, which are attached to Ser242 and Ser254 in the mouse DSPP sequence and the GAG chains are made of chondroitin-4-sulfate (35). It is also worth noting that DSP-PG appears more abundant than DSP (5, 6). In this study, we generated the Dspp-KO/DSP Tg mice to investigate the function of the NH2-terminal fragment of DSPP (DSP-PG and/or DSP). These mice did not contain DPP or the endogenous Dspp gene but instead only possessed DSP-PG and DSP derived from the transgene. The immunohistochemistry analyses with anti-DSP monoclonal antibody confirmed that the NH2-terminal fragment was properly secreted in the dentin ECM. We thoroughly characterized the dentin of the Dspp-KO/DSP Tg mice in comparison with the Dspp-KO and WT mice. Our analyses revealed that the dentin of the Dspp-KO/DSP Tg mice was remarkably thinner than that of the Dspp-KO mice. The mineralized dentin layer that was present in the WT mice could not be seen in the Dspp-KO/DSP Tg mice, while instead, structures resembling unmineralized predentin were observed in this region of the tooth in the Dspp-KO/DSP Tg mice. The structure of the dentinal tubules in the Dspp-KO/DSP Tg mice was more severely disrupted when compared to the Dspp-KO mice. These findings indicate that the NH2-terminal fragment of DSPP may exert an inhibitory role during dentinogenesis. We suggest that the NH2-terminal fragment of DSPP may prevent the predentin from being mineralized too rapidly. Data from this study has helped to delineate one role for the NH2-terminal fragment in tooth formation.
In these experiments, we also noted that the Dspp-KO/DSP Tg mice revealed more severe defects in the alveolar bone than the Dspp-KO mice; this finding is consistent with our recent observation that DSPP plays an essential role in maintaining the health of periodontal tissues including the alveolar bone and cementum (36).
In the extracellular matrix of dentin, the ratio of DPP to DSP is approximately 10:1. Even when the presence of DSP-PG is taken into account, the ratio of DPP to DSP is not close to 1:1. Therefore, there must be an intrinsic, yet unknown, mechanism to which the inequality in molar ratio between the NH2-terminal and COOH-terminal fragments of DSPP is attributed. One possibility is that NH2-terminal fragment with few or no phosphates might be degraded faster than the highly phosphorylated COOH-terminal fragment of DSPP. We envision that in the degradation process of the NH2-terminal fragment of DSPP, the two GAG chains of DSP-PG are first removed and then, the initial degradation product of DSP-PG (i.e., DSP) is broken into small fragments by proteinases such as matrix metalloproteinase-20 (MMP-20) present in the dentin extracellular matrix. Clearly, more studies are needed to address these puzzling questions.
From the in vivo data generated in this study, we cannot conclude whether DSP or DSP-PG was responsible for the observed phenotypic changes (inhibitory effects on mineralization). DSP has been shown to have no significant effects on in vitro mineralization (37). Proteoglycans are present as an amorphous gel in the predentin region of the teeth and occupy spaces between the collagen fibrils acting as ground substances providing a medium for transport and diffusion (38). Chondroitin sulphate, of which the GAG chains of DSP-PG are made, shows a decreasing gradient from the pulp towards the mineralized front of dentin (39, 40); the gradient shifting in the distribution of proteoglycan chondroitin sulphate appears to be associated with the changes in the activities of matrix metalloproteinase-3 (MMP-3, i.e., stromelysin-1 or proteoglycanase) in the pulp-predentin-dentin complex (41). Several in vitro studies have shown that chondroitin sulfate in solution inhibits the formation and growth of hydroxyapatite crystals in collagen gels (42–45). Recent in vitro mineralization analyses showed that the proteoglycan form of the NH2-terminal fragment of dentin matrix protein 1 (DMP1) inhibits the formation and growth of hydroxyapatite crystals (46). Based on the finding in this study that Dspp KO/DSP Tg mice had more severe dentin defects than the Dspp null mice, along with previous observation that DSP has no significant effects on in vitro mineralization (37) and the fact that chondroitin sulfate proteoglycans inhibit the formation and growth of hydroxyapatite crystals, we believe that DSP-PG was responsible for the observed inhibitory effects on dentinogenesis in the Dspp KO/DSP Tg mice. We hypothesize that during dentinogenesis, DSP-PG primarily present in predentin serves as an antagonist against the mineralization-accelerating action of DPP and thus this proteoglycan prevents predentin from being mineralized too rapidly. At the mineralization front where predentin is converted to dentin, DSP-PG is degraded and the loss of DSP-PG allows the mineralization process to proceed. As stated above, in the degradation process of DSP-PG, the two GAG chains are first removed and DSP-PG is converted to DSP, and then DSP is further broken into small fragments and/or amino acids by proteinases such as MMP-3 and MMP-20. While these purported roles of DSP-PG or DSP and their degradation processes in dentinogenesis are conjecture, they represent hypotheses that best fit all the data. Clearly, experiments are warranted to test the in vitro effects of DSP-PG on the formation and growth of hydroxyapatite crystals.
Additionally, in recent years, DSPP expression has also been seen in several non-mineralized tissues such salivary glands, cartilage, liver, kidney and brain (26, 47–49). Investigations to examine the roles of the cleaved fragment DSP and/or DSP-PG in these soft tissues, as well as in long bones, are also needed for a more complete understanding of DSPP in maintaining the structural integrity of these diverse tissues.
Acknowledgments
We are grateful to Jeanne Santa Cruz for her assistance with the editing of this manuscript. This work was supported by NIH Grant DE005092 (to CQ) and DE015209 (to JF).
Footnotes
GibsonMP, Liu Q, Zhu Q, Lu Y, JaniP, WangX, Liu Y, PaineML, SneadML, FengJQ, Qin C. Role of NH2-terminal fragment of Dentin Sialophosphoprotein (DSPP) in dentinogenesis. Eur J Oral Sci
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 3.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 5.Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003;111:235–242. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 7.Sugars RV, Olsson ML, Waddington R, Wendel M. Substitution of bovine dentine sialoprotein with chondroitin sulfate glycosaminoglycan chains. Eur J Oral Sci. 2006;114:89–92. doi: 10.1111/j.1600-0722.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 8.Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB. Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci. 2000;108:35–42. doi: 10.1034/j.1600-0722.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie HH, Li X. A novel rat dentin mRNA coding only for dentin sialoprotein. Eur J Oral Sci. 2001;109:342–347. doi: 10.1034/j.1600-0722.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamakoshi Y, Hu JC, Liu S, Zhang C, Oida S, Fukae M, Simmer JP. Characterization of porcine dentin sialoprotein (DSP) and dentin sialophosphoprotein (DSPP) cDNA clones. Eur J Oral Sci. 2003;111:60–67. doi: 10.1034/j.1600-0722.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Gibson MP, Liu Q, Liu Y, Lu Y, Wang X, Feng JQ, Qin C. Proteolytic Processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J Biol Chem. 2012;287:30426–30435. doi: 10.1074/jbc.M112.388587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veis A, Perry A. The phosphoprotein of the dentin matrix. Biochemistry. 1967;6:2409–2416. doi: 10.1021/bi00860a017. [DOI] [PubMed] [Google Scholar]
- 13.MacDougall M, Zeichner-David M, Slavkin HC. Production and characterization of antibodies against murine dentine phosphoprotein. Biochem J. 1985;232:493–500. doi: 10.1042/bj2320493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SL, Veis A, Glonek T. Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry. 1977;16:2971–2979. doi: 10.1021/bi00632a026. [DOI] [PubMed] [Google Scholar]
- 15.Butler WT, Bhown M, DiMuzio MT, Cothran WC, Linde A. Multiple forms of rat dentin phosphoproteins. Arch Biochem Biophys. 1983;225:178–186. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- 16.Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989;224:154–166. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- 17.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Mineral induction by immobilized phosphoproteins. Bone. 1997;21:305–311. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 19.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 20.Butler WT, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol. 1995;39:169–179. [PubMed] [Google Scholar]
- 21.Butler WT, Bhown M, Dimuzio MT, Linde A. Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981;1:187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 22.Boskey A, Spevak L, Tan M, Doty SB, Butler WT. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif Tissue Int. 2000;67:472–478. doi: 10.1007/s002230001169. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Q, Prasad M, Kong H, Lu Y, Sun Y, Wang X, Yamoah A, Feng JQ, Qin C. Partial Blocking of Mouse DSPP Processing by Substitution of Gly(451)-Asp(452) Bond suggests the presence of secondary cleavage site(s) Connect Tissue Res. 2012;53:307–312. doi: 10.3109/03008207.2011.650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303:191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paine ML, Luo W, Wang HJ, Bringas P, Jr, Ngan AY, Miklus VG, Zhu DH, MacDougall M, White SN, Snead ML. Dentin sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J Biol Chem. 2005;280:31991–31998. doi: 10.1074/jbc.M502991200. [DOI] [PubMed] [Google Scholar]
- 26.Prasad M, Zhu Q, Sun Y, Wang X, Kulkarni A, Boskey A, Feng JQ, Qin C. Expression of dentin sialophosphoprotein in non-mineralized tissues. J Histochem Cytochem. 2011;59:1009–1021. doi: 10.1369/0022155411423406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Lu Y, Chen S, Prasad M, Wang X, Zhu Q, Zhang J, Ball H, Feng J, Butler WT, Qin C. Key proteolytic cleavage site and full-length form of DSPP. J Dent Res. 2010;89:498–503. doi: 10.1177/0022034510363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Lu Y, Chen L, Gao T, D’Souza R, Feng JQ, Qin C. DMP1 processing is essential to dentin and jaw formation. J Dent Res. 2011;90:619–624. doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin DM, Hallsworth AS, Buckley T. A method for the study of internal spaces in hard tissue matrices by SEM, with special reference to dentine. J Microsc. 1978;112:345–352. doi: 10.1111/j.1365-2818.1978.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie HH, Yee CT, Tang XN, Dong Z, Fuller RS. DSP-PP precursor protein cleavage by tolloid-related-1 protein and by bone morphogenetic protein-1. PLoS One. 2012;7:e41110. doi: 10.1371/journal.pone.0041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y. Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp) J Bone Miner Res. 2011;26:220–228. doi: 10.1002/jbmr.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Marschall Z, Fisher LW. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1) Matrix Biol. 2010;29:295–303. doi: 10.1016/j.matbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Q, Sun Y, Prasad M, Wang X, Yamoah AK, Li Y, Feng J, Qin C. Glycosaminoglycan chain of dentin sialoprotein proteoglycan. J Dent Res. 2010;89:808–812. doi: 10.1177/0022034510366902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson MP, Zhu Q, Liu Q, D’Souza RN, Feng JQ, Qin C. Loss of DSPP leads to periodontal disease in mice. J Periodontal Res. 2012 doi: 10.1111/j.1600–0765.2012.01523.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boskey A, Spevak L, Tan M, Doty SB, Butler WT. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif Tissue Int. 2000;67:472–478. doi: 10.1007/s002230001169. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg M, Septier D, Escaig-Haye F. Glycoconjugates in dentinogenesis and dentine. Prog Histochem Cytochem. 1987;17:1–112. doi: 10.1016/s0079-6336(87)80001-3. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg M, Rapoport O, Septier D, Palmier K, Hall R, Embery G, Young M, Ameye L. Proteoglycans in predentin: the last 15 micrometers before mineralization. Connect Tissue Res. 2003;44 (Suppl 1):184–188. [PubMed] [Google Scholar]
- 40.Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331–349. doi: 10.1177/10454411010120040401. [DOI] [PubMed] [Google Scholar]
- 41.Hall R, Septier D, Embery G, Goldberg M. Stromelysin-1 (MMP-3) in forming enamel and predentine in rat incisor-coordinated distribution with proteoglycans suggests a functional role. Histochem J. 1999;12:761–770. doi: 10.1023/a:1003945902473. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal NC, Posner AS, Silverman LD, Rosenberg LC. Effect of proteoglycans on in vitro hydroxyapatite formation. Calcif Tissue Int. 1979;27:75–82. doi: 10.1007/BF02441164. [DOI] [PubMed] [Google Scholar]
- 43.Chen CC, Boskey AL, Rosenberg LC. The inhibitory effect of cartilage proteoglycans on hydroxyapatite growth. Calcif Tissue Int. 1984;36:285–290. doi: 10.1007/BF02405332. [DOI] [PubMed] [Google Scholar]
- 44.Hunter GK, Allen BL, Grynpas MD, Cheng PT. Inhibition of hydroxyapatite formation in collagen gels by chondroitin sulphate. Biochem J. 1985;228:463–469. doi: 10.1042/bj2280463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989;224:154–166. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- 46.Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Y, Gandhi V, Prasad M, Yu W, Wang X, Zhu Q, Feng JQ, Hinton RJ, Qin C. Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the condylar cartilage of rat mandible. Int J Oral Maxillofac Surg. 2010;39:272–281. doi: 10.1016/j.ijom.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 49.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–409. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]