Abstract
The input, processing, and output characteristics of inhibitory interneurons help shape information flow through layers 2/3 of the visual cortex. Parvalbumin (PV)-positive interneurons modulate and synchronize the gain and dynamic responsiveness of pyramidal neurons. To define the diversity of PV interneurons in layers 2/3 of the developing visual cortex, we characterized their passive and active membrane properties. Using Ward's and k-means multidimensional clustering, we identified four PV interneuron subgroups. The most notable difference between the subgroups was their firing patterns in response to moderate stimuli just above rheobase. Two subgroups showed regular and continuous firing at all stimulus intensities above rheobase. The difference between these two continuously firing subgroups was that one fired at much higher frequencies and transitioned into this high-frequency firing rate at or near rheobase. The two other subgroups showed irregular, stuttering firing patterns just above rheobase. Both of these subgroups typically transitioned to regular and continuous firing at intense stimulations, but one of these subgroups, the strongly stuttering subgroup, showed irregular firing across a wider range of stimulus intensities and firing frequencies. The four subgroups also differed in excitatory synaptic input, providing independent support for the classification of subgroups. The subgroups of PV interneurons identified here would respond differently to inputs of varying intensity and frequency, generating diverse patterns of PV inhibition in the developing neural circuit.
Keywords: mouse, acute slice, multipolar bursting cell, basket cell, Ward's clustering, k-means clustering, membrane properties, action potentials, miniature excitatory postsynaptic current
gabaergic interneurons are integral to brain function (Burkhalter 2008; Gulyas et al. 2010; Jonas et al. 2004; Mann and Paulsen 2007; Marin 2012). In the cortex, the most prevalent class of interneurons express the Ca2+-binding protein parvalbumin (PV) (Gonchar and Burkhalter 1997; Gonchar et al. 2007; Lee et al. 2010; Miyoshi et al. 2007; Xu et al. 2010). PV interneurons have been implicated in schizophrenia (Lewis et al. 2012) and may be involved in other brain disorders including autism and epilepsy (Marin 2012). In the visual cortex, PV interneurons regulate gain control (Atallah et al. 2012; Ma et al. 2010; Wilson et al. 2012) and feature selectivity (Lee et al. 2012) of visual processing and underlie gamma oscillatory activity (Cardin et al. 2009; Sohal et al. 2009).
Variations in intrinsic membrane properties and firing patterns underlie an interneuron's role in neural circuit activity. The majority of PV interneurons exhibit a fast-spiking firing pattern (Blatow et al. 2003; Kawaguchi and Kubota 1993; Povysheva et al. 2008; Woodruff et al. 2009). An exception is multipolar bursting cells (Blatow et al. 2003), which are located primarily at the border between layers 1 and 2, display a relatively depolarized resting membrane potential, and do not have fast-spiking firing patterns. In terms of fast-spiking PV interneurons, there are two general groups: chandelier and basket cells. Chandelier cells target the axon initial segment (Karube et al. 2004; Somogyi 1977) and can have a depolarizing effect on the postsynaptic axon (Szabadics et al. 2006; Woodruff et al. 2009). Chandelier cells can be electrophysiologically distinguished from other fast-spiking PV interneurons on the basis of their short delay to action potential (AP) onset and linear or superlinear subthreshold voltage responses to current input (Woodruff et al. 2009). Basket cells target soma and proximal dendrites (Karube et al. 2004; Kisvarday 1992; Kubota and Kawaguchi 2000; Tamas et al. 1998; Wang et al. 2002). Basket cells show heterogeneity in morphology (Wang et al. 2002), electrophysiology (Goldberg et al. 2008; Karagiannis et al. 2009), and receptive field (Runyan et al. 2010). At present, the variations in membrane and firing properties in the basket group of fast-spiking PV interneurons remain undefined.
Here we targeted PV interneurons expressing either EGFP or tdTomato in layers 2/3 of the visual cortex, focusing on fast-spiking “basket” cells; on the basis of anatomical location and membrane properties, we tried to minimize the inclusion of chandelier (Taniguchi et al. 2013; Woodruff et al. 2009) and multipolar bursting (Blatow et al. 2003) cells. Our goal was to characterize variations in membrane properties (passive membrane properties, AP shape and firing pattern) of PV interneurons to identify possible functional subgroups. To that end, we used multidimensional clustering, which can distinguish pyramidal neurons and major interneuron groups from each other (Cauli et al. 2000; Dumitriu et al. 2007; Guerra et al. 2011; Karube et al. 2004; Krimer et al. 2005; Nowak et al. 2003). When applied to an already restricted group of interneurons (for example, those interneurons that express somatostatin), it can identify subgroups (Halabisky et al. 2006; Ma et al. 2006; McGarry et al. 2010). For these experiments, we used juvenile mice, focusing on the period between eye opening (Gordon and Stryker 1996) and the onset of the critical period, the timing of which is dependent on maturation of PV basket cells (Fagiolini et al. 2004; Huang et al. 1999).
Using multidimensional clustering, we identified four novel PV interneuron subgroups in layers 2/3 in the visual cortex. While all subgroups typically developed continuous firing patterns with strong stimulation, subgroups differed in their firing patterns in response to near-rheobase stimuli. The subgroups also differed in passive membrane properties, AP shape, and excitatory inputs. These PV interneuron subgroups would participate differently in the wide range of inputs arriving with eye opening.
MATERIALS AND METHODS
Animals
Two transgenic mouse lines were used to identify PV interneurons in visual cortex: B13 and PV-tdTomato. B13 mice (Dumitriu et al. 2007) were generated from a bacterial artificial chromosome (BAC) clone in which EGFP cDNA and the phosphoglycerate kinase polyadenylation sequence were inserted at the translation initiation codon of a PV clone, resulting in EGFP expression driven by the PV promoter. B13 mice were used as heterozygotes in a C57BL/6 background. About 50% of PV-positive interneurons in B13 mice express GFP (Dumitriu et al. 2007), a result we have confirmed immunohistochemically (data not shown). PV-tdTomato (PV-Cre; LSL-tdTomato) mice were generated by the Cre/loxP-based strategy. PV-Cre mice (Hippenmeyer et al. 2005; Kuhlman and Huang 2008) in which Cre recombinase expression was driven by a PV promoter (an IRES-Cre-pA cassette inserted at the 3′ end of the PV gene) were crossed with the LSL-tdTomato line [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J from Jackson Labs, donated by Allen Brain Institute] in which tdTomato expression was activated upon the excision of a lox-flanked STOP cassette upstream of the tdTomato gene following Cre-mediated recombination. PV expression begins around postnatal day (P)14, and tdTomato expression follows. By adulthood, >95% of PV-expressing cells express tdTomato and all tdTomato cells express PV (data not shown), consistent with previously published results for these mice (Hippenmeyer et al. 2005; Kuhlman and Huang 2008).
Solutions
Recording solutions.
The artificial cerebral spinal fluid (ACSF) solution used for recordings consisted of (in mM) 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2 and was saturated with 95% O2-5% CO2 under all conditions. Excitatory postsynaptic currents (EPSCs) were recorded in the presence of MgCl2 unless otherwise indicated. All pharmacological agents were added to the external solution without substitution. Our standard internal solution was an ATP-generating internal (AGI) solution containing (in mM) 105 K-gluconate, 30 KCl, 3 MgCl2, 10 HEPES, 10 phosphocreatine, 4 Mg-ATP. and 0.3 GTP, pH 7.3 (KOH) and adjusted to 305 mosM with sucrose.
Pharmacological compounds.
To record miniature EPSCs (mEPSCs), APs were blocked with the Nav blocker tetrodotoxin (TTX; 1 μM). Inhibitory synaptic responses were blocked with the GABAA competitive antagonist bicuculline (50 μM) or the noncompetitive antagonist picrotoxin (50 μM). For selected recordings, AMPA receptor (AMPAR)-mediated currents were blocked with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM) to confirm glutamatergic mEPSCs. All reagents unless otherwise noted were obtained from either Sigma Aldrich (St. Louis, MO) or Tocris Cookson (Ellisville, MO).
Acute Slice Preparation
Mice between P14 and P19 were anesthetized with isoflurane and then decapitated according to protocols reviewed and approved by the institutional animal ethics committee and in keeping with guidelines established by the National Institutes of Health. The brain was dissected out in ice-cold ACSF solution with added kynurenic acid (200 μg/ml). Coronal slices were collected from each blocked hemisphere with a vibratome (Sigmann Electronik, Hüffenhardt, Germany). Slice collection began 1.0 mm rostral to the caudal cortical surface and yielded four 300-μm slices. Slices were placed into oxygenated ACSF at 32°C for 15 min and then left undisturbed at room temperature for another 40 min before use.
Electrophysiology
Membrane potentials or currents were recorded at 32–34°C with an EPC 10 USB amplifier with PatchMaster software (HEKA Elektronik, Lambrecht, Germany). Recordings were sampled at 20 kHz (APs) or 50 kHz [spontaneous EPSCs (sEPSCs)/mEPSCs] and low-pass filtered with a 4-pole Bessel filter at 10 kHz. mEPSCs were digitally refiltered at 2 kHz. Pipettes had resistances of 2–4 MΩ when filled with internal solution and measured in the ACSF bath. External solutions were bath applied at a perfusion rate of ∼3 ml/min. Membrane potentials were not corrected for liquid junction potentials.
Targeting neurons.
We initially identified layers 2/3 in relation to the pial edge of the slice and targeted the visual cortex, using the lateral ventricle size and shape as a reference. We then visually identified interneurons by searching for EGFP or tdTomato expression under magnification (Olympus BX50WI fitted with a ×40-W/0.80 NA objective and cooled CCD camera Quantifire XI from Optronics).
Experimental Protocols
Passive and active membrane properties.
When the whole cell mode had been achieved, resting membrane potentials (Vm) were measured in current clamp. The amplifier mode was changed to voltage clamp and the baseline holding potential set to −70 mV. We placed all cells at the same Vm so that voltage-dependent channels would be at a similar starting state and rheobase current would be independent of Vm. Pipette series resistance, neuronal membrane resistance (Rm), and neuronal membrane capacitance (Cm) were monitored at the start and regularly throughout the experiment with the LOCKIN Sine+DC protocol from HEKA (Gillis 1995), with a 20-mV (−90 to −70 mV) sine wave of 1 kHz for 100 ms. The amplifier was then switched to current-clamp mode and the current adjusted to bring the reported membrane potential to approximately −70 mV. APs were evoked with a series of depolarizing pulses of 1-s duration each, with 5 s between each stimulus. Depolarizing currents varied with cell type and Rm but ranged from 10 pA to 1 nA. Rheobase (see below) was found by applying 10-pA increments of current. The amplifier was subsequently switched to voltage-clamp mode with the holding potential set to −70 mV to record EPSCs.
sEPSC/mEPSC recording.
Neurons were voltage clamped at −70 mV and recorded in 1- to 5-min blocks. sEPSCs were recorded in the standard ACSF (containing Mg2+) used to measure APs, while mEPSCs were recorded in the same solution with added TTX (1 μM) and bicuculline (50 μM) or picrotoxin (50 μM). At the end of some experiments, CNQX (10 μM) was washed in to confirm that the observed synaptic events were AMPAR mediated.
Analysis
Passive/subthreshold membrane properties.
Vm was measured in current clamp once the whole cell mode had been achieved. Series resistance and Cm were analyzed in real time with the PatchMaster LOCKIN online analysis software. Subsequent analysis was performed with IGOR Pro (WaveMetrics). Rm is the slope of a line fit to subthreshold voltage responses to current input. Rheobase is the minimum current input over a 1-s stimulus that generates an AP.
Active/action potential shape.
The first AP evoked by rheobase was used to characterize AP shape. AP threshold was determined by the third derivative of the AP found over the AP rising phase. Smoothing of the AP waveform was performed after each derivative. AP peak and half-width were used to describe the rising and falling phases of the AP. AP peak is the absolute maximum amplitude. Half-width was measured as the time from the rising phase to the falling phase of the AP at one-half the distance from threshold to peak. Afterhyperpolarization (AHP) was characterized by the AHP peak, latency, and area. AHP peak is the difference between threshold and maximum AHP. AHP latency is the time from AP peak to AHP peak. AHP area is the area under a line defined by the AP threshold membrane potential.
Active/firing pattern.
The delay to onset of the first AP is measured from the start of a depolarizing stimulus pulse to the peak of the first AP evoked in response to rheobase. AP firing patterns, as outlined below, were analyzed in the trace that contained ∼30 APs, e.g., fired APs at 30 Hz, the low range of the gamma frequency (Jagadeesh et al. 1992; Steriade et al. 1996). In some cases the firing frequency jumped from no or a few APs to frequencies much higher than 30 Hz. In these cases, firing patterns were analyzed on the first available trace over 25 Hz. AP amplitude accommodation was measured as amplitude change (Δamplitude)—the amplitude of the first peak minus the last peak. Interspike interval (ISI) was measured as the time between successive AP peaks. Spike frequency adaptation was measured as the last ISI divided by the first ISI. Instantaneous frequency of APx is the inverse of the ISI (in s) APx to APx+1. Maximum and minimum instantaneous frequencies are the maximum and minimum instantaneous frequencies detected in the 30-AP trace. The coefficient of variation (CV) of the firing rate (CV frequency) is the standard deviation of the frequency divided by the average frequency. Input-output response (I/F slope) is measured as the slope of a line fit to a plot of the number of evoked APs vs. current input.
Cells were included in analysis only if the neurons showed fast-spiking behavior, had resting Vm < −57 mV, and had a stable baseline. To try to minimize the contribution of chandelier cells to our data set, we considered both the anatomical distribution and physiological properties of chandelier cells. Chandelier cells in superficial cortical layers are located predominantly in layer 2 adjacent to the border of layer 1 (Taniguchi et al. 2013). Assuming a soma size of ∼20 μm, this would place a significant majority of chandelier cells within 50 μm of layer 1. For our experiments, we sampled PV interneurons throughout layers 2/3. For a subset of our interneurons (15 total), we measured the distance of the soma from layer 1. None of the cell bodies was located within 50 μm, with most (12) between 200 and 400 μm away. Furthermore, only a subset of chandelier cells express PV, between 15% (prefrontal cortex) and 50% (somatosensory cortex) (Taniguchi et al. 2013). These two factors would reduce the likelihood of sampling chandelier cells. To further reduce the contribution of chandelier cells to our data set, we also considered physiological properties and did not include cells with both no delay (estimated delay to 1st AP of 150 ms or less) and linear or superlinear subthreshold I/V relationships (Woodruff et al. 2009). On the basis of this criterion, two cells were excluded as putative chandelier cells from our data set. Despite these precautions, it is possible that our data set includes a small number of chandelier cells.
mEPSC analysis.
mEPSCs were analyzed with the MiniAnalysis program (Synaptosoft). Event detection parameters averaged a 2-ms baseline 4 ms prior to a suprathreshold peak. Amplitude threshold levels were set at 5 pA, and recordings with baseline noise root mean squared (RMS) > 5 pA were discarded. The area threshold was set at 1.5 times the amplitude threshold. Five or more events separated by maximum intervals of 5 ms were labeled as bursts and removed. Segments with high levels of noise that obscured the baseline were omitted, and event detection resumed when the baseline leveled. mEPSC events from individual cells were measured for amplitude, 10–90% rise time, half-width, area, and time to 50% decay. mEPSC frequency was measured as the inverse of the time in seconds between mEPSCx and mEPSCx+1 (for cumulative histograms) and as the total number of events recorded divided by the total time (average mEPSC frequency). To determine significant differences between the synaptic inputs to subgroups, we used two approaches. First, 500 mEPSCs were randomly sampled from each cell and combined for each subgroup and the Komogorov-Smirnov (KS) test (α = 0.05) was used to test for significance between subgroup cumulative histograms. The resulting P value was adjusted for multiple pairs with the Bonferroni correction. Second, mEPSC measurements were averaged for each cell and an ANOVA followed by Tukey test was performed for each parameter. Statistical analysis was performed in IGOR Pro (WaveMetrics).
Cluster Analysis
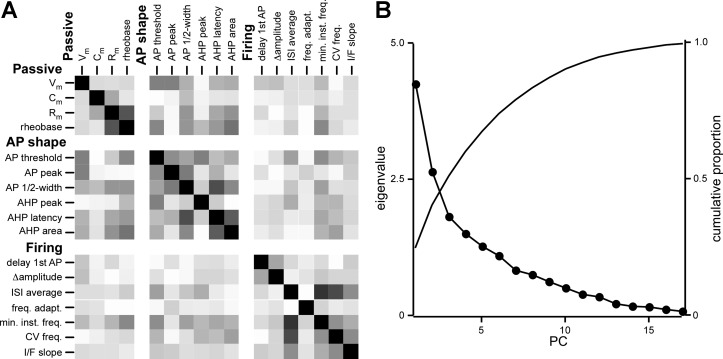
Multidimensional cluster analysis was performed on passive and active membrane properties to identify possible common groupings of PV interneurons. We started with >30 descriptive parameters of passive and active membrane properties but eliminated many of them for clustering because they were highly correlated and represented similar features of membrane properties (e.g., AP rise time and AP half-width). For clustering, we focused on 17 parameters (see Fig. 2A) that were largely unrelated. Figure 2A is a cross-correlation matrix of these 17 parameters with correlation indices shade coded, with black being perfectly correlated (correlation index of 1.0) and white being perfectly uncorrelated (correlation index of 0). Most parameters are not strongly correlated (e.g., threshold and AP peak). However, some parameters were correlated (correlation coefficient > 0.6) (Rm and rheobase; AP half-width and AHP latency; AHP latency and AHP area, ISI average and minimum frequency; minimum frequency and CV frequency) but were both retained because they encompass different features of membrane properties. For example, AP half-width is correlated to AHP latency because they both encompass the rate of AP decay; however, AP half-width also encompasses AP duration, whereas AHP latency describes the rate of AHP. Maximum instantaneous frequency and the maximum-to-minimum instantaneous frequency ratio (Max/Min) were not used in clustering but are included in the results to contrast the subgroups.
Fig. 2.
Correlation of parameters describing membrane properties of PV interneurons. A: the 17 passive and active membrane properties used for clustering (derived from 82 interneurons; see materials and methods) are arrayed against each other in a correlation matrix with the degree of correlation indicated by the shading: white is uncorrelated (correlation index of 0) and black is perfectly correlated (correlation index of 1, diagonal squares). B: principal component analysis does not greatly reduce variance. For each principal component (PC) derived from the 17 PV interneuron membrane properties in A, the variance explained by that PC (eigenvalue, circles) and the cumulative proportion of variance explained by all PCs up to and including that PC (cumulative proportion, line) are plotted.
Principal component analysis.
Principal component analysis (PCA) can be used to reduce the dimensionality (number of parameters) of a multiparameter data set by generating new uncorrelated variables (Murtagh and Heck 1987). PCA applies a multiexponential fit to the data, and each new uncorrelated variable [principal component (PC)] can describe more than one original parameter. We therefore performed PCA on the 17 parameters to reduce the dimensionality. Figure 2B shows the eigenvalues associated with the resulting PC along with the proportion of the total variance accounted for by that PC. The first seven eigenvalues are >1, indicating that they contribute more to the variance of the data set than one of the original parameters, and together account for 82% of the variance in the data set. The first 10 PCs together are required to surpass 90% of the variance. PCA is most useful when it can identify two or three PCs to describe most of the variance in a data set. Because the first three PCs accounted for only 51% of the variance, we did not use PCs for clustering and instead used the 17 original parameters accompanied by a unique ID assigned to each cell.
Clustering algorithms.
To identify potential clusters, we applied two clustering algorithms, Ward's hierarchical clustering and k-means clustering, using the statistical package R (R Development Core Team 2005). For cluster analysis, we normalized each parameter to a mean of 0 and a standard deviation of 1. Ward's hierarchical clustering builds a map (dendrogram) quantifying the similarity between samples (interneurons) and clusters of samples. It begins with each interneuron in its own cluster, where k (the number of clusters) equals the number of interneurons in the data set, and combines the two clusters with the minimum combined internal variance. The process is repeated until all interneurons are in a single cluster (k = 1). Distance between joined clusters, or fusion height, is measured as the ANOVA sum of squares (summed over all of the variables). Normalized variables were used to generate a distance matrix based on Euclidean measures (R function dist), and the distance matrix was entered into Ward's hierarchical clustering algorithm (R function hclust).
k-Means is a clustering algorithm in which cells switch clusters to minimize within-cluster Euclidean distances, as opposed to Ward's clustering, which can only combine cells or clusters of cells together into new clusters. k-Means clustering was performed with the Hartigan-Wong algorithm (R function kmeans). One hundred starts were initialized with random assignment of all cells to k groups and 100 maximum iterations (additional iterations did not change the outcome; data not shown). k-Means was initialized for k = 2 through k = 9.
Clustering statistics.
After Ward's clustering, we applied the best cut test and upper tailed t-test to find the optimal number of clusters, the bootstrap test to find the reproducibility of the cluster arrangements, and silhouette analysis to examine the quality of cluster separation. The best cut test and upper tailed t-test analyze the fusion heights in the dendrogram, looking for fusion values that are significantly larger than the rest, indicating that the clusters being joined are very different. The best cut test uses the formula
where fusions is the set of all fusion heights in the Ward's dendrogram (McGarry et al. 2010). The upper tailed t-test looks for fusion heights significantly greater than the mean of all fusion heights whose test statistic exceeds the critical value for α = 0.05. Bootstrapping [R function pvclust from the pvclust package (Suzuki and Shimodaira 2009)] tests the resilience of the cluster results by comparing the clusters formed when the parameters are duplicated or removed. Each cluster found in the data is accompanied by a P value representing the probability that the cluster is not formed by chance (100 would be a perfectly reproducible cluster). Silhouette analysis (Rousseeuw 1987) (R function silhouette) evaluates clustering based on the distance from each member of a cluster to all other members in the same cluster and to the nearest different cluster.
To compare Ward's and k-means cluster results, we used the average within-cluster distance, the average between-cluster distance, and the Calinski-Harabesz index. The R cluster.stats function from the fpc package (Hennig 2010) was used to generate metrics.
RESULTS
Parvalbumin-Positive Interneurons in Layers 2/3 of Visual Cortex from B13 and PV-tdTomato Mice
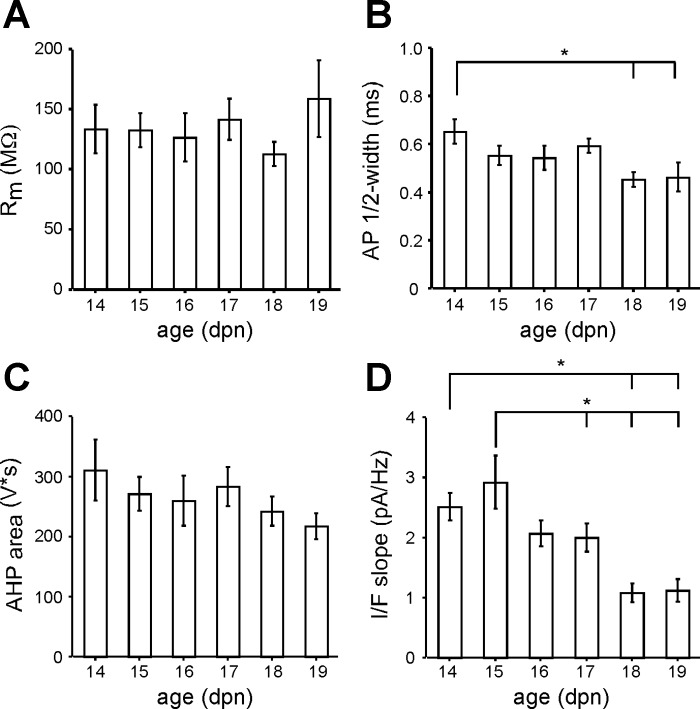
PV interneurons in layers 2/3 of the visual cortex in acute slices were identified by EGFP or tdTomato fluorescence, patched in the whole cell configuration, and stimulated to confirm fast-spiking firing patterns. We recorded passive and active membrane properties (see materials and methods) from 97 fast-spiking interneurons (86 from PV-tdtomato and 11 from B13 mice) ranging in age from P14 to P19. Several membrane properties including Rm, AP half-width, and firing frequency undergo significant developmental changes in layer 2/3 PV interneurons between P13 and P18 in the somatosensory cortex (Goldberg et al. 2011) and between P15 and P25 in the visual cortex (Lazarus and Huang 2011). In our data set, parameters measured between P15 and P19 were largely independent of age, including Rm (Fig. 1A), AP half-width (Fig. 1B), and AHP area (Fig. 1C). The only parameter that showed age dependence between P15 and P19 was the I/F slope (Fig. 1D), which at P15 was significantly greater than that at P17, P18, and P19. On the other hand, several parameters including AP half-width (Fig. 1B), AHP peak (not shown), AHP latency (not shown), and I/F slope (Fig. 1D) were significantly different between P14 and older ages. Given the age dependence of P14 features, we excluded P14 interneurons from our data set and focused only on interneurons between P15 and P19 (n = 82).
Fig. 1.
Lack of age dependence of membrane properties in parvalbumin-expressing (PV) interneurons in layers 2/3 of the visual cortex between postnatal day (P)15 and P19: average membrane resistance (Rm, A), action potential (AP) half-width (B), afterhyperpolarization (AHP) area (C), and input-output response (I/F slope, D) for each day of postnatal age (dpn). *Significantly different values (Tukey, P < 0.05). Error bars are ±SE.
We characterized PV interneuron properties by using 17 largely uncorrelated parameters (Fig. 2A) (see materials and methods). PCA can reduce the dimensionality of a multidimensional data set by generating new uncorrelated parameters accounting for the variance of the original parameters. However, PCA did not greatly reduce the variability in our data set, requiring 10 PCs to account for 90% of the variance (Fig. 2B) (materials and methods). For clustering analysis, we therefore used the 17 parameters normalized to a mean of 0 and a standard deviation of 1.
Ward's Hierarchical Cluster Analysis Identifies Four Major Clusters of PV Interneurons
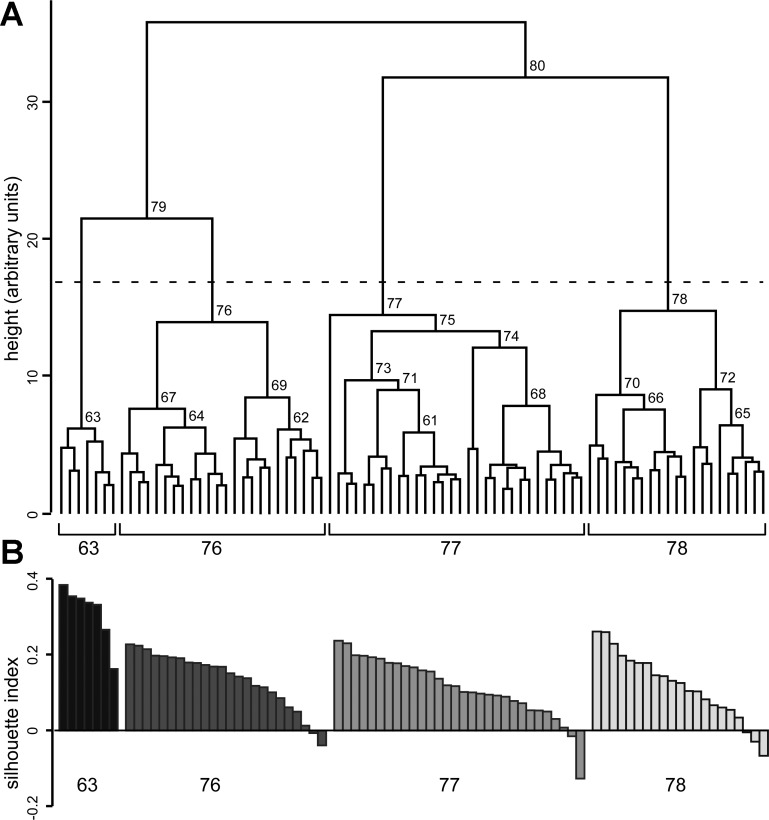
Initially, we used Ward's hierarchical clustering (see materials and methods) to identify subgroups of PV interneurons. Figure 3A illustrates a dendrogram based on Ward's clustering. Each cluster formed is given a unique ID, shown above the horizontal link forming the cluster. The relationship among interneurons in a cluster is described by the absolute fusion height (the height of the horizontal link merging 2 parent clusters) and by the difference between the cluster fusion heights of the progeny cluster and its parent clusters. A larger absolute fusion height means that the interneurons in that cluster are more heterogeneous. A larger difference between the fusion height of the progeny and parent clusters indicates that the parent clusters are more distinct from each other. The height is in arbitrary units (a.u.), as it measures distance between standardized parameter measures.
Fig. 3.
PV interneurons grouped with Ward's hierarchical clustering algorithm. Ward's hierarchical clustering was performed with 17 descriptive parameters of passive and active membrane properties (see materials and methods) derived from 82 interneurons. A: cell cluster merge heights were used to construct the dendrogram. Branch points (marking the formation of a new cluster from 2 smaller parent clusters) of heights >5.5 are labeled with cluster identities (unique numbers assigned when each cluster is created). All clusters converge at 36 arbitrary units (a.u.). The horizontal dashed line shows the best cut height, 17 a.u. Clusters below the best cut line are 63, 76, 77, and 78. B: the silhouette index is shown for each interneuron in the subgroups identified with Ward's clustering after a cut at the best cut line (k = 4). Positive values indicate a good cluster assignment, whereas negative values point to an interneuron that better fits an alternate cluster. Interneurons are shaded black (cluster 63), dark gray (cluster 76), gray (cluster 77), and light gray (cluster 78). Interneurons are ordered by silhouette index within each cluster and do not correspond to the order of interneurons displayed in the dendrogram in A.
The two largest clusters, 79 and 80, merge at a height of ∼36 a.u. (Fig. 3A; Table 1). This fusion height is well separated from parent cluster 79, which forms around 22 a.u., but not from parent cluster 80, which forms around 32 a.u. This means that the two parent clusters making up cluster 80 (77 and 78) are almost as dissimilar to each other as they are to cluster 79, or that clusters 77, 78, and 79 are all comparably dissimilar. Cluster 79 is well separated from its parent clusters 63 (6.1 a.u.) and 76 (14 a.u.), suggesting that 63 and 76 are separate clusters. Hence, based on the fusion heights, there appear to be at least three clusters.
Table 1.
Ward's hierarchical cluster analysis of membrane properties of PV interneurons
| Cluster ID | # | Height | t-Value | Bootstrap Probability, % | k |
|---|---|---|---|---|---|
| 81 | 82 | 35.81 | 47.49 | 1 | |
| 80 | 51 | 31.78 | 41.15 | 96 | 2 |
| 79 | 31 | 21.48 | 24.97 | 100 | 3 |
| 78 | 21 | 14.73 | 14.37 | 66 | 4 |
| 77 | 30 | 14.43 | 13.89 | 92 | 5 |
| 76 | 24 | 13.90 | 13.06 | 0 | 6 |
| 75 | 29 | 13.24 | 12.03 | 70 | 7 |
| 74 | 14 | 12.04 | 10.14 | 99 | 8 |
| 73 | 15 | 9.65 | 6.39 | 70 | 9 |
| 72 | 9 | 8.99 | 5.36 | 94 | 10 |
| 71 | 12 | 8.94 | 5.28 | 51 | 11 |
| 70 | 12 | 8.59 | 4.72 | 97 | 12 |
| 69 | 11 | 8.40 | 4.42 | 77 | 13 |
| 68 | 12 | 7.78 | 3.45 | 100 | 14 |
| 67 | 13 | 7.58 | 3.13 | 97 | 15 |
| 66 | 9 | 7.52 | 3.04 | 94 | 16 |
| 65 | 6 | 6.36 | 1.22 | 96 | 17 |
| 64 | 9 | 6.21 | 0.98 | 94 | 18 |
| 63 | 7 | 6.14 | 0.87 | 96 | 19 |
| 62 | 6 | 6.07 | 0.76 | 90 | 20 |
| 61 | 8 | 5.84 | 0.40 | 99 | 21 |
For each cluster ID, the number of interneurons (#), the height of cluster fusion (height), the t statistic for that fusion height (t value), and the bootstrap probability for that cluster are shown along with the number of clusters (k) present in the dendrogram at that height. The upper tailed t-test reveals that clusters with t values >3.20 have heights significantly larger than the mean (P > 0.001). PV, parvalbumin.
To define the statistical significance of the clusters identified in the dendrogram, we used the best cut test, the upper tailed t-test, and bootstrapping (Fig. 3 and Table 1). The best cut height is 17 (dashed line), identifying 4 clusters: 63, 76, 77, and 78. Based on the upper tailed t-test, clusters 68–81, including 63, 76, 77, and 78, all have statistically significant fusion heights (Table 1) (P < 0.001). Bootstrapping finds the probability that a given cluster will contain the same pool of interneurons under various conditions and uses this measure of resilience to assign it a confidence value, listed in Table 1 as bootstrap probability. The largest clusters with bootstrap values >95% (P > 0.05) (Table 1) are 61, 63, 65, 67, 68, 70, 74, 79, 80, and 81. Cluster 76 has a bootstrap probability of 0%, while cluster 77 has a bootstrap probability of 92%. Thus of the four largest clusters (63, 76, 77, 78), two (63 and 78) are validated in three separate tests and two (76 and 77) are validated in two of the three tests.
Silhouette analysis was used to examine the quality of interneuron assignments in the four major clusters (63, 76, 77, 78) from the Ward's clustering dendrogram. The silhouette index for each interneuron is grouped in descending order by cluster (Fig. 3B). All interneurons in cluster 63 (average silhouette index of 0.31) have a positive silhouette index indicating an optimal cluster placement. For cluster 76 (average silhouette index 0.14) 22 of 24 interneurons are positive, for cluster 77 (average silhouette index 0.11) 28 of 30 are positive, and for cluster 78 (average silhouette index of 0.12) 18 of 21 are positive. In total, 75 of 82 interneurons have a positive silhouette index with an overall silhouette index of 0.14 ± 0.01 (mean ± SE). The silhouette results confirm that there is a natural clustering to the data; cluster 63 is the most tightly clustered, while clusters 76, 77, and 78 are more loosely clustered.
In summary, Ward's hierarchical clustering identifies four clusters: clusters 63 (7 interneurons), 76 (24 interneurons), 77 (30 interneurons), and 78 (21 interneurons).
k-Means Cluster Analysis Identifies the Same General Clustering Arrangement
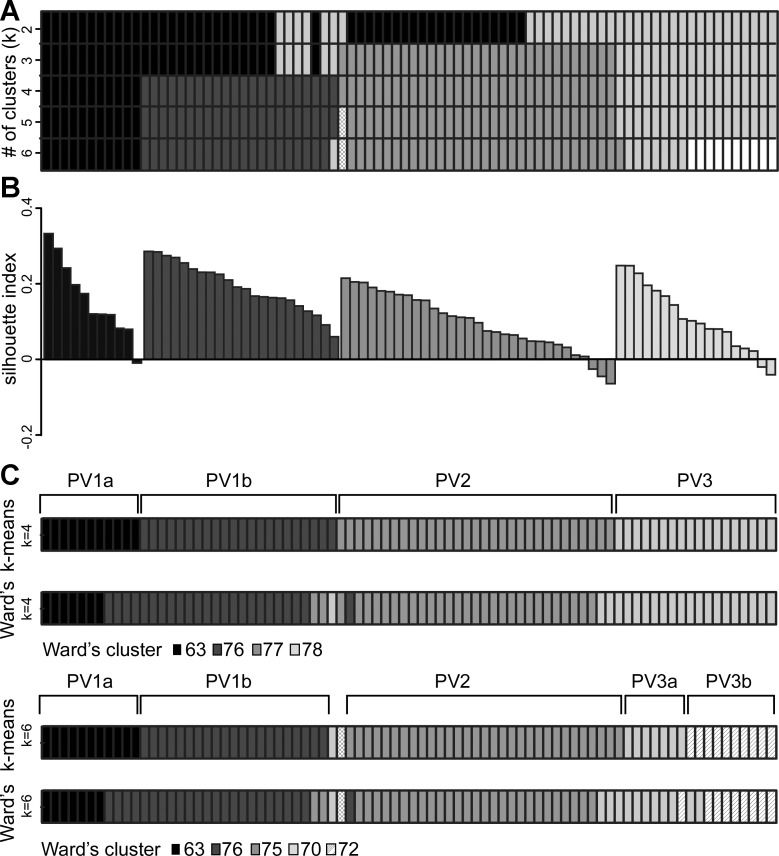
To further examine the PV interneuron subgroups, we performed k-means clustering on the same data set as the Ward's clustering described above. Solutions were generated for k = 2 through k = 8 (k = number of clusters). Solutions k = 2 through k = 6 were plotted together as a heat map (Fig. 4A) to facilitate comparisons between clusters. A number of interneurons from both the black and gray clusters at k = 2 are moved into the new clusters appearing at k = 3 and k = 4. Importantly, at k = 4 cluster assignments stabilize and show remarkable consistency even with larger k. The cluster shaded black in Fig. 4A contains the same 11 interneurons from k = 4 through k = 6; the dark, medium, and light gray clusters are identical between k = 4 and k = 5 (a single interneuron splits off from the medium gray cluster at k = 5). At k = 6, when 10 interneurons split off from the light gray to form the white cluster, two additional interneurons shift clusters—one interneuron moves from light to medium gray, and another interneuron moves from dark to light gray. With additional clusters (k = 7 and k = 8, not shown), new clusters continue to be split off from the existing clusters and only one interneuron changes clusters. In summary, the cluster configuration appearing at k = 4 is retained at k = 5 through k = 8, with the clusters being split but not exchanging members—only three interneurons change cluster membership.
Fig. 4.
Subgroup assignment of PV interneurons with k-means clustering. k-Means clustering was performed with the same data set as Ward's clustering. A: cluster assignments are depicted in a heat map in which each column represents 1 interneuron (n = 82) and each row contains the shaded k-means cluster assignment for k = 2 through k = 6. B: the silhouette index is plotted for each interneuron in each cluster generated by k-means clustering for k = 4. Interneurons are shaded black, dark gray, gray, and light gray, corresponding to the major clusters in A. As in Fig. 3B, interneurons are ordered by silhouette index within each cluster and do not correspond to the order of interneurons displayed in the heat map in A. C, top: the k-means cluster assignments for k = 4 (top) are depicted with the best cut cluster assignments from Ward's clustering (bottom). Cells clustered differently between the 2 methods are labeled. The 4 major k-means clusters are labeled with the corresponding subgroup identity; PV1a, PV1b, PV2, and PV3. C, bottom: the k-means cluster for k = 5 (top) is depicted with the cluster assignments from Ward's dendrogram cut at k = 5 (bottom), illustrating the further subdivision of PV3 into 2 subgroups.
Examining the k-means results for an overarching pattern, PV interneurons can be clustered into one consistent (black) cluster PV1a and three largely consistent clusters PV1b (dark gray), PV2 (medium gray), and PV3 (light gray). PV1a and PV1b are so named because in both Ward's and k-means clustering (see below), these clusters appear to be more closely associated with each other than PV2 or PV3.
Silhouette analysis was used to evaluate the k-means cluster results for k = 4 (Fig. 4B). For PV1a (average silhouette index 0.16), 10 of 11 interneurons have a positive silhouette index. All interneurons in PV1b (0.19) are positive. For PV2 (0.09), 28 of 31 and for PV3 (0.11) 16 of 18 interneurons are positive. In total, 76 of 82 interneurons have a positive silhouette index, with an average silhouette index of 0.13 ± 0.01. All interneurons with a negative silhouette index have cluster PV1b as a nearest neighbor.
The k-means cluster solution for k = 4 corresponds well to the 4 clusters identified with Ward's dendrogram (Fig. 4C): PV1a overlaps extensively with Ward's cluster 63, PV1b with cluster 76, PV2 with cluster 77, and PV3 with cluster 78. For k = 4, 72 of 82 or 88% of interneurons are assigned to the same clusters between the two methods. For seven of the interneurons that are differently assigned, the silhouette value in the Ward's clustering is negative and the nearest neighbor cluster is comparable to the cluster where k-means assigns it. Four of the ten interneurons that are differently assigned move between PV1a and PV1b, which are more closely related according to the Ward's dendrogram.
Based on the average cluster separation statistics (see materials and methods), k-means clusters the data comparably to or slightly better than Ward's. k-Means clusters have an average silhouette width (0.13) similar to Ward's (0.14) and a similar average between-cluster distance, or separation between clusters (k-means 5.95 vs. Ward's 5.94). However, k-means clusters outperform Ward's on other measures. For example, the average within-cluster distance (a measure of cluster tightness) for k-means is 4.61 compared with 4.68 for Ward's. Similarly, the Calinski-Harabasz index of between-/within-cluster distance is 13.44 for k-means and 12.60 for Ward's (a larger number indicates better clustering). On the basis of these indices and close examination of the 10 interneurons differing between the Ward's and k-means cluster assignments, we chose to use the k-means cluster assignments as the better clustering arrangement. Nevertheless, there is strong overall agreement between the Ward's and k-means cluster arrangements. In addition, the conclusions were comparable even if the Ward's cluster arrangement was used (data not shown).
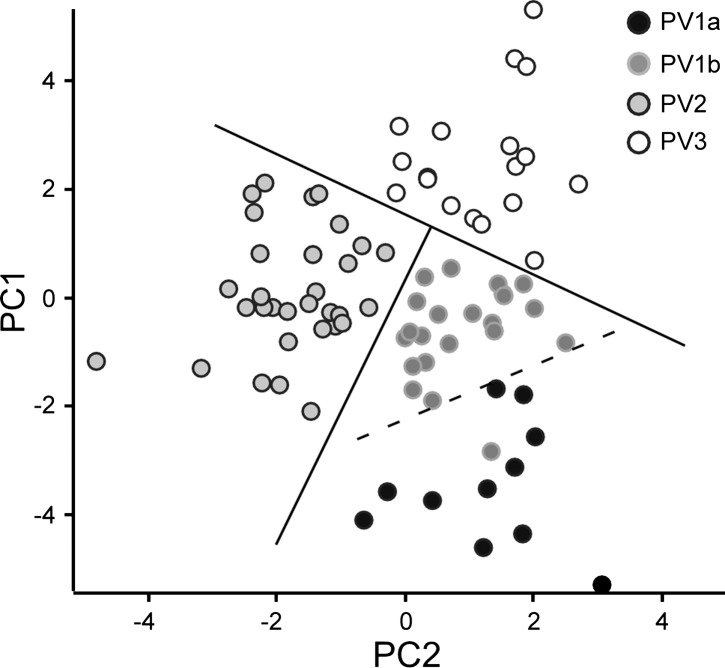
The Four Distinct Clusters Can Be Visualized with PCA
The first two PCs generated by PCA (PC1 and PC2) together account for 41% of the total variance in the data set. To further characterize the validity of the four clusters, we plotted PC1 against PC2 for each interneuron in a scatterplot (Fig. 5) coded according to the k-means cluster assignment. Three of the major subgroups (PV1b, PV2, and PV3) are clearly distinguishable from each other and can be completely separated by two straight lines drawn at cross angles to each other. PV2 is the best-separated cluster. PV3 and PV1b interneurons are also fairly well separated, with only one PV3 interneuron in close proximity to PV1b interneurons. No straight line can perfectly separate PV1a from PV1b in these dimensions; at least one PV1b is located far into the PV1a area, and PV1a is diffusely distributed with no discernible core cluster. None of the interneurons in the boundary between PV1a and PV1b switches cluster identity with different k in k-means clustering or between Ward's clustering and k-means clustering, and none has negative silhouette values. It is likely that additional separation between the two subgroups arises in the remaining variance (59%) not described by the first two PCs. Despite the limitations of using PCA to represent the data set, these results support the subgroups identified through k-means clustering.
Fig. 5.
Separation of subgroups in scatterplot of first 2 PCs. The first (PC1) and second (PC2) PC values derived for each interneuron are plotted against each other and shaded by k-means cluster (PV1a, PV1b, PV2, and PV3). Subgroups of interneurons are shaded black (PV1a), dark gray (PV1b), gray (PV2), and outlined light gray (PV3).
Membrane Properties of PV Interneuron Subgroups in Layers 2/3 of Visual Cortex
On the basis of k-means cluster analysis, we identify four major subgroups of PV interneurons in layers 2/3 of the visual cortex that we call PV1a, PV1b, PV2, and PV3 (Figs. 3, 4). PV1a and PV1b are more closely related to each other than to PV2 and PV3. The passive membrane properties, AP shape, and firing patterns of the different subgroups are illustrated in Fig. 6 and Fig. 7 and summarized in Table 2.
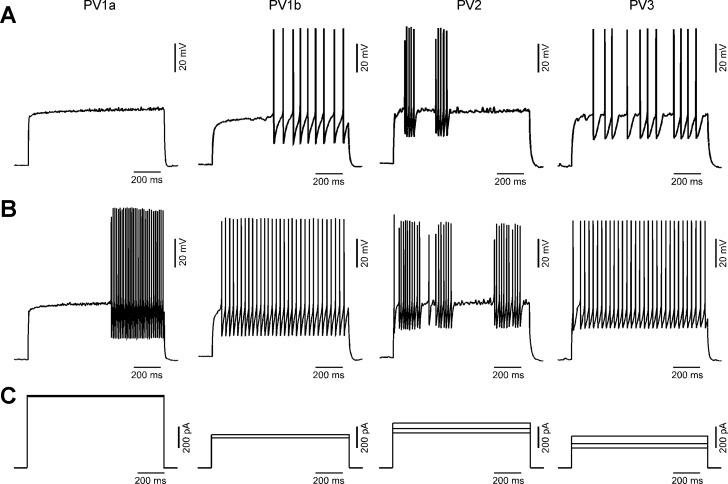
Fig. 6.
Membrane properties of PV interneuron subgroups in layers 2/3 of the visual cortex: AP firing patterns from representative interneurons from PV1a, PV1b, PV2, and PV3 subgroups (left to right). A: near-rheobase voltage traces contain ∼10 APs. PV1a interneurons typically jumped from no or a few APs to much higher frequencies (>25 Hz). The representative PV1a cell jumps from no to 30 APs, so the trace immediately before the 30 AP trace is shown. B: voltage traces (from the same cell shown in A) containing ∼30 APs. C: 1-s depolarizing currents injected into current-clamped PV interneurons generating the traces shown in A and B. The rheobase current is also shown for comparison if different from the stimulus trace for A and B.
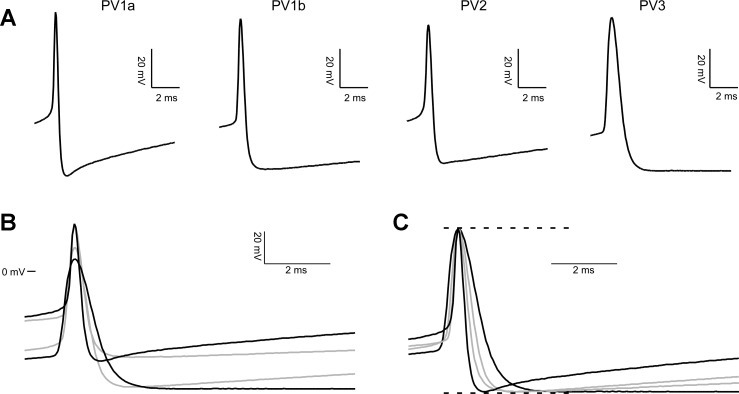
Fig. 7.
First AP evoked at rheobase in each PV subgroup. A: the first AP firing in response to rheobase current is shown magnified to 10 ms. Shading and line thickness are coded by subgroup, for use in interpreting B and C. Rheobase stimulus for the records shown is 677 (PV1a), 280 (PV1b), 330 (PV2), and 190 (PV3) pA. B: the traces shown in A are overlaid, with 0 mV used to align them vertically. C: the traces shown in A are scaled to the same Max and Min, then overlaid.
Table 2.
Passive and active membrane properties of PV interneuron subgroups
| PV1a | PV1b | PV2 | PV3 | Tukey Test | |
|---|---|---|---|---|---|
| Passive | |||||
| Vm, mV | −72 ± 2 | −77 ± 1 | −77 ± 2 | −83 ± 1 | 1a, 3 2, 3 |
| Cm, pF | 20.4 ± 1.2 | 20.4 ± 1.1 | 19.4 ± 0.6 | 18.4 ± 0.8 | N.D. |
| Rm, MΩ | 84 ± 8 | 129 ± 13 | 104 ± 5 | 213 ± 20 | 1a, 3 1b, 3 2, 3 |
| Rheobase, pA | 570 ± 60 | 320 ± 30 | 400 ± 20 | 200 ± 20 | 1a, 1b 1a, 2 1a, 3 1b, 3 2, 3 |
| AP shape | |||||
| AP threshold, mV | −25.8 ± 2.4 | −30.4 ± 1.9 | −39.5 ± 1.3 | −42.3 ± 1.4 | 1a, 2 1a, 3 1b, 2 1b, 3 |
| AP peak, mV | 21.1 ± 2.8 | 23.2 ± 2.1 | 16.0 ± 2.0 | 12.2 ± 2.8 | 1b, 3 |
| AP 1/2-width, ms | 0.40 ± 0.03 | 0.48 ± 0.03 | 0.48 ± 0.02 | 0.73 ± 0.03 | 1a, 3 1b, 3 2, 3 |
| AHP peak, mV | −26.9 ± 1.2 | −27.0 ± 0.6 | −23.4 ± 0.5 | −23.9 ± 0.7 | 1a, 2 1b, 2 1b, 3 |
| AHP latency, ms | 1.4 ± 0.1 | 2.1 ± 0.2 | 1.7 ± 0.1 | 3.7 ± 0.4 | 1a, 3 1b, 3 2, 3 |
| AHP area, V·s | 170 ± 20 | 270 ± 10 | 180 ± 10 | 390 ± 40 | 1a, 1b 1a, 3 1b, 2 1b, 3 2, 3 |
| Firing pattern | |||||
| Delay 1st AP, ms | 360 ± 130 | 590 ± 60 | 490 ± 60 | 460 ± 60 | N.D. |
| Δ amplitude, mV | 5.3 ± 1.7 | −2.2 ± 0.8 | 2.2 ± 0.8 | 0.7 ± 0.8 | 1a, 1b 1a, 3 1b, 2 |
| ISI average, ms | 12.9 ± 1.0 | 26.8 ± 0.8 | 29.5 ± 0.7 | 30.5 ± 0.9 | 1a, 1b 1a, 2 1a, 3 1b, 3 |
| Frequency adapt. | 0.98 ± 0.12 | 0.96 ± 0.06 | 1.22 ± 0.38 | 0.89 ± 0.11 | N.D. |
| Max. inst. freq., Hz | 95.9 ± 6.0 | 45.6 ± 1.6 | 66.7 ± 4.4 | 43.2 ± 2.0 | 1a, 1b 1a, 2 1a, 3 1b, 2 2, 3 |
| Min. inst. freq., Hz | 59.5 ± 7.1 | 29.0 ± 1.9 | 8.6 ± 1.1 | 20.4 ± 2.6 | 1a, 1b 1a, 2 1a, 3 1b, 2 2, 3 |
| Max/min inst. | 1.8 ± 0.3 | 2.0 ± 0.5 | 13.8 ± 2.6 | 4.5 ± 1.5 | 1a, 2 1b, 2 2, 3 |
| CV frequency | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.27 ± 0.01 | 0.13 ± 0.01 | 1a, 2 1b, 2 2, 3 |
| I/F slope | 0.6 ± 0.2 | 1.5 ± 0.2 | 2.4 ± 0.3 | 2.1 ± 0.2 | 1a, 2 1a, 3 1b, 2 |
| Age, dpn | 18.1 ± 0.2 | 17.3 ± 0.3 | 16.2 ± 0.2 | 16.4 ± 0.3 | 1a, 2 1a, 3 1b, 2 |
| No. of interneurons | 11 | 22 | 31 | 18 | |
| B13 | 0 | 3 | 5 | 1 | |
| PV-tdTomato | 11 | 19 | 26 | 17 |
Values are means ± SE. PV subgroups identified by k-means cluster analysis (k = 4) are characterized by passive membrane properties, action potential (AP) shape, and firing pattern (see materials and methods). Listed are the 17 properties used for clustering plus 2 additional parameters: maximum instantaneous frequency and the maximum-to-minimum instantaneous frequency ratio (Max/Min inst.). Vm, membrane potential; Cm, membrane capacitance; Rm, membrane resistance; AHP, afterhyperpolarization; ISI, interspike interval; CV, coefficient of variation; dpn, postnatal day. ANOVA followed by Tukey test (P < 0.05) was used to identify significant differences between subgroup parameters. N.D., none of the subgroups was significantly different.
Firing properties.
The most distinctive differences between the subgroups are firing patterns. In general, interneurons in the PV1 subgroups fire regularly and continuously at all current injections above rheobase (Fig. 6, A and B, left), with PV1a interneurons firing at a higher frequency than PV1b. Both PV2 and PV3 interneurons fire with an irregular stuttering pattern near rheobase (Fig. 6A, right). At a higher stimulus evoking firing in the 30-Hz range, PV2 firing remains irregular while PV3 firing becomes regular (Fig. 6B, right). With stronger stimuli and higher-frequency firing, the majority of PV2 interneurons (19/31) ultimately reach a regular continuous firing pattern (data not shown). Thus PV2 interneurons show irregular firing over a significantly wider range of stimuli and frequencies than PV3 interneurons. In general, PV1a and PV1b interneurons can be classified as “continuous firing,” PV2 as “strongly stuttering,” and PV3 as “weakly stuttering” interneurons.
Quantitatively, the difference in firing patterns is most discernible in the Max/Min and the CV frequency (Table 2). The Max/Min for continuous-firing PV1a (1.8 ± 0.3, mean ± SE) and PV1b (2.0 ± 0.05) is significantly less than strongly stuttering PV2 (8.6 ± 1.1). Similarly, the CV frequency is less variable in PV1a (0.08 ± 0.01) and PV1b (0.09 ± 0.01) than in PV2 (0.27 ± 0.01). The weakly stuttering PV3 displays intermediate values of Max/Min (4.5 ± 1.5) and CV frequency (0.13 ± 0.01), which are still significantly lower than PV2.
The major difference between PV1a and PV1b is that PV1a interneurons fire at a higher frequency, so the PV1a interneurons have a statistically lower ISI average (12.9 ± 1.0 ms) than PV1b (26.8 ± 0.08 ms) and higher maximum (95.9 ± 6.0 Hz) and minimum (59.5 ± 7.1 Hz) instantaneous firing frequency than PV1b (45.6 ± 1.6 Hz, 29.0 ± 1.9 Hz). PV1a interneurons show a significant decrease in peak AP amplitude (positive Δamplitude) with repeated firing (5.3 ± 1.7 mV), while PV1b interneurons show a slight increase (−2.2 ± 0.8 mV).
Passive membrane properties.
PV3 is significantly different from all other subgroups in passive membrane properties related to membrane resistance (Rm and rheobase). PV3 interneurons have higher Rm (213 ± 20 MΩ) compared with PV1a (84 ± 8 MΩ), PV1b (129 ± 13 MΩ), and PV2 (104 ± 5 MΩ) (Table 2). PV3 interneurons also require less current to depolarize to threshold (rheobase) (200 ± 20 pA) compared with PV1a (570 ± 60 pA), PV1b (320 ± 30 pA), or PV2 (400 ± 20 pA) (Table 2, Fig. 6C). PV1a has a significantly higher rheobase than PV1b.
Action potential shape.
PV3 also differs from all other subgroups in features of AP shape dependent on the membrane time constant (AP half-width, AHP latency, and AHP area) (Fig. 7). The AP threshold of both PV1a (−25.8 ± 2.4 mV) and PV1b (−30.4 ± 01.9 mV) is significantly more depolarized than either PV2 (−39.5 ± 1.3 mV) or PV3 (−42.3 ± 1.4 mV) (Fig. 7B). The AP half-width of PV3 interneurons is wider (0.73 ± 0.03 ms) than that of PV1a (0.40 ± 0.03 ms), PV1b (0.48 ± 0.03 ms), or PV2 (0.40 ± 0.02 ms) (Fig. 7C). PV3 also has slower, larger AHPs—PV3 AHP latency (3.7 ± 0.4 ms) and area (390 ± 40 V·s) are significantly greater than PV1a (1.4 ± 0.1 ms, 170 ± 20 V·s), PV1b (2.1 ± 0.2 ms, 270 ± 10 V·s), and PV2 (1.7 ± 0.1 ms, 180 ± 10 V·s). PV1a has a significantly smaller AHP area than PV1b.
Excitatory Synaptic Inputs Differ Between Some PV Interneuron Subgroups
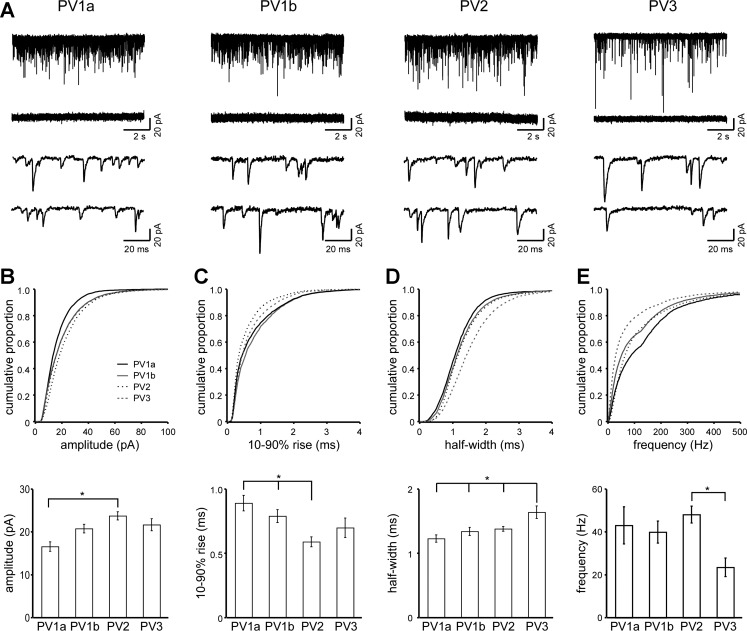
To find out whether the identified subgroups also differed in excitatory inputs, we recorded AMPAR-mediated mEPSCs in interneurons with stable, low noise resting membrane currents (52 of the 82 interneurons were recorded: PV1a, 7; PV1b, 15; PV2, 18; PV3, 12). Figure 8 illustrates 10-s and 100-ms current traces from representative interneurons in each subgroup (Fig. 8A) and cumulative histograms (top) and ANOVA of means (bottom) for mEPSC amplitude (Fig. 8B), 10–90% rise (Fig. 8C), half-width (Fig. 8D), and frequency (Fig. 8E). The KS test finds significant differences between each of the subgroups in amplitude, 10–90% rise, half-width, and frequency as well as area (data not shown) (P < 0.05). A comparison of mean values, using ANOVA (see materials and methods), highlights the largest differences visible in the cumulative histograms. PV1a has the smallest mEPSC amplitudes (16.5 ± 1.1 pA), significantly smaller than PV2 (23.8 ± 0.9 pA) (Fig. 8B), and the smallest mEPSC area (19.6 ± 1.4 pA·ms), significantly smaller than PV2 (27.9 ± 1.0 pA·ms) and PV3 (29.3 ± 1.6 pA·ms) (data not shown). PV2 has the fastest 10–90% rise times (0.59 ± 0.04 ms), significantly faster than PV1a and PV1b (PV1a 0.89 ± 0.06, PV1b 0.79 ± 0.05 ms) (Fig. 8C). PV3 half-widths (1.64 ± 0.10 ms) are significantly wider than those of PV1a (1.23 ± 0.06 ms), PV1b (1.34 ± 0.06 ms), and PV2 (1.34 ± 0.04 ms) (Fig. 8C). PV3 interneurons also show less frequent mEPSCs (23.5 ± 4.3 Hz) than PV2 (48.1 ± 4.0 Hz), but not less than PV1a or PV1b (Fig. 8E).
Fig. 8.
Miniature excitatory postsynaptic current (mEPSC) characteristics of PV interneuron subgroups. A: representative current traces from each subgroup (10 s total time shown) in whole cell voltage clamp (holding potential −70 mV) in the presence of Mg2+, tetrodotoxin (TTX), and bicuculline (top traces) or in the same solution but with added 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, middle traces). The bottom 2 traces (100 ms each) are expanded from the top trace to show details. B–E: cumulative histograms (top) and mean values (bottom) of mEPSC amplitudes (B), 10–90% rise time (C), half-width (D), and frequency (E) for each subgroup. See materials and methods for details. For cumulative histograms, the Komogorov-Smirnov test finds significant differences between subgroups for each parameter (P < 0.05). *Significantly different values (Tukey, P < 0.05). Error bars are ±SE.
DISCUSSION
To identify subgroups of fast-spiking PV interneurons in layers 2/3 of the visual cortex, we used two different clustering algorithms: Ward's and k-means. We initially used the Ward's dendrogram to visualize the relationships between clusters of interneurons and used this information to aid in interpreting k-means clustering (which requires a defined number of clusters). The two clustering algorithms largely converged on the same four subgroups of PV interneurons (Figs. 3 and 4), supporting that our results yielded good cluster assignments. Furthermore, the four subgroups probably do not contain major representations of chandelier (Woodruff et al. 2009) or multipolar bursting (Blatow et al. 2003) cells, which we largely excluded on the basis of anatomical location and membrane properties (see materials and methods).
Using multidimensional cluster analysis of passive membrane properties, AP shape, and firing patterns, we identified four major subgroups of PV interneurons in layers 2/3 of the juvenile mouse visual cortex. Most interneurons in all four subgroups, if injected with high levels of current, fired APs in a regular, continuous fashion. However, at stimulations closer to rheobase, the four subgroups showed notable differences (Table 2): PV1a and PV1b fired regularly and continuously at all current injections, with PV1a firing at higher rates (Fig. 6, A and B). In contrast, PV2 exhibited stuttering firing patterns over a wide range of current injections above rheobase (Fig. 6B). PV3 was somewhat more complex but typically showed stuttering firing patterns at stimulations just above rheobase and then transitioned to continuous firing upon slightly higher stimulation levels (Fig. 6C). PV3 was also notable for its slow passive membrane properties and broad AP shape (Fig. 7).
Comparison to Previously Published Results
Continuous and stuttering firing patterns have previously been observed in PV interneurons (Goldberg et al. 2008; Miyoshi et al. 2007; Povysheva et al. 2008), and, as shown here, stuttering converted to continuous firing with strong stimulus (Povysheva et al. 2008). Earlier papers have not specifically explored variations in PV basket cell stuttering patterns and the variation in transition from stuttering to continuous firing.
A delay from depolarization to the appearance of the first AP is common in PV interneurons, particularly in layer 2 (Goldberg et al. 2008; Miyoshi et al. 2007). The delay is correlated with a more depolarized threshold mediated by Kv1.1, and stronger depolarizing stimuli convert the firing to continuous fast spiking. All subtypes described here contain a majority of interneurons with delayed firing, and in all subgroups stronger depolarizing stimuli reduces the delay, suggesting that this feature does not contribute to the subgroups identified here.
Another commonly noted feature of PV interneurons is an abrupt onset of firing, in which the rheobase response fires multiple spikes (averaging 16 Hz) (Goldberg et al. 2008; Kawaguchi 1995). In our data set, the majority of interneurons in all groups fired fewer than 10 spikes (10 Hz) in response to rheobase stimulus. PV1b interneurons had the largest number (6 of 22) interneurons that showed an abrupt onset (10 or more Hz), while PV2 had the least (1 of 31 fired at 10 Hz). PV1a interneurons jumped abruptly from firing none or a few APs to frequencies over 30 Hz, while the other subgroups saw a more gradual response. Thus the classical abrupt onset is not present as described in most PV interneurons and is absent in the strongly stuttering (PV2) subgroup.
Many fast-spiking interneurons show a slight spike frequency adaptation in the first few APs (Cauli et al. 1997). PV2, the largest subgroup, is the only subgroup with a frequency adaptation ratio over 1 (Table 2), and the degree of adaptation (1.22) appears similar to the degree of adaptation identified previously.
Subthreshold membrane oscillations have been observed in fast-spiking interneurons, possibly mediated by TASK K2P channels (Goldberg et al. 2011). A qualitative comparison of membrane voltage traces between subgroups suggests that PV2 may have more oscillations than other subgroups (see PV2 trace, Fig. 6A). This is consistent with the lower Rm in this subgroup, also associated with TASK channels. Nevertheless, future experiments will be required to define the role of TASK channels and subthreshold oscillations in the stuttering firing patterns seen in PV2 interneurons.
Most immunochemicals expressed in other interneurons are absent in PV interneurons (Gonchar et al. 2007). However, the mRNAs of neuropeptide Y (NPY) and calbindin (CB) have been detected (Cauli et al. 1997; Karagiannis et al. 2009), raising the possibility of immunochemistry-based PV subgroups. NPY mRNA expression is correlated with a delay to AP onset (Karagiannis et al. 2009). However, as noted above, there is no significant difference in delay between subgroups, so the subgroups described here likely do not align with NPY expression. CB mRNA is present in a significant percentage (44%) of PV cells (Cauli et al. 1997), but the electrophysiological features of CB-expressing PV interneurons have not been characterized.
PV is found in large basket cells (LBCs), nest basket cells (NBCs), and small basket cells (SBCs) (Wang et al. 2002) as well as chandelier (Kawaguchi and Kubota 1998), wide arbor (Kawaguchi and Kondo 2002), and local arbor (Miyoshi et al. 2007) cells. However, these morphologies are not unique to PV expression; other biochemical markers including somatostatin and vasoactive intestinal peptide are expressed in interneurons with the same morphologies (Kawaguchi and Kondo 2002; Wang et al. 2002). The literature on firing patterns associated with different PV morphologies is incomplete and contradictory. One report suggests the delay to onset is restricted to fast-spiking interneurons with a local arbor (Miyoshi et al. 2007), while another reports finding delays to onset in all basket cell morphological subtypes (Gupta et al. 2000). Interestingly, stuttering may be one of several electrophysiological profiles expressed by NBCs (Gupta et al. 2000), but it is unknown how this relates to the subset of PV-expressing NBCs.
Chandelier cells are the most extensively characterized as a distinct morphological PV subtype. Anatomically, in layers 2/3 chandelier cells are not evenly distributed but are principally located at the border of layer 1 (Kawaguchi 1995; Taniguchi et al. 2013; Woodruff et al. 2009). Chandelier cells also display membrane properties different from basket cells (Woodruff et al. 2009). We took advantage of these properties to try to minimize the contribution of chandelier cells to our data set (see materials and methods). Nevertheless, in the absence of axonal morphology we cannot rule out that some chandelier cells are present. Indeed, the PV3 subgroup shares some features with chandelier cells (higher membrane resistance, lower rheobase, wider half-width). However, future experiments will be needed to define whether any of the subgroups described here align strongly with a particular morphology.
Developmental Changes in Membrane Properties and Relevance to Subgroups
The precise window of developmental changes depends on cortical area. In layers 2/3 of barrel cortex some of these developmental changes occur between P14 and P18 (Goldberg et al. 2011), while in layers 2/3 of visual cortex changes may not be fully underway before P22 (Desai et al. 2002; Goldberg et al. 2011; Lazarus and Huang 2011; Okaty et al. 2009). When quantified as a group, only the I/F slope varied from P15 to P19 (Fig. 1). However, some of the differences observed between subgroups could be due to developmental changes in PV interneuron properties. Specifically, PV1a differs from other groups in having a more mature profile in a number of developmental characteristics (Rm, rheobase, half-width, AHP latency, AHP peak, and firing frequency; Goldberg et al. 2011; Lazarus and Huang 2011; Okaty et al. 2009). On the other hand, the slower passive and active membrane properties seen in PV3 are associated with the developmentally immature PV interneuron.
Basis for Differences in Firing Patterns
Our experiments do not directly address the molecular mechanisms underlying the different firing patterns. Nevertheless, previous experiments have identified some key factors contributing to differences in membrane properties, AP shape, and firing patterns. Kv3-type ion channels are necessary and sufficient for fast-spiking behavior in PV interneurons (Erisir et al. 1999), and expression density is correlated with firing frequency (Gu et al. 2011; Toledo-Rodriguez et al. 2004). The Kv3.1b splice variant enables neurons to fire at a higher rate, similar to firing observed in PV1a (Gu et al. 2011). The other subgroups presumably lack this feature. Kv1 is a slowly inactivating channel that is responsible both for the delay to firing observed around threshold (Goldberg et al. 2008) and for the stuttering quality of near-threshold firing patterns (Povysheva et al. 2008; Toledo-Rodriguez et al. 2004) like those observed in PV2 and PV3. However, since PV1b showed significant delay but little to no stuttering, there may be variations in channel subunit composition. Furthermore, Kv1 subunits associate with β-subunits (Rhodes et al. 1997), which are modulated by second messengers (Pan et al. 2011) and can form heteromultimers with other K channel subtypes, any of which could interact with Kv1 to generate the stuttering behavior in PV2 and PV3 but not PV1b. Blocking HCN channels also creates an interrupted firing pattern (Toledo-Rodriguez et al. 2004), providing another possible factor underlying stuttering. The slower passive membrane properties and AP shape described in PV3 are seen with a stronger potassium leak current from Kir2.2, Kir2.3, K2p1.1, and K2p9.1 (Goldberg et al. 2011; Okaty et al. 2009). These same features are associated with a lower Kv3 expression, along with a lower overall firing frequency. These slower features may enable this subgroup to convert to continuous firing at a lower firing frequency (for example, by altering recovery from inactivation). Further experiments are needed to define how specific K as well as Na channels combine to generate the diverse PV fast-spiking firing patterns described here.
Differences in Excitatory Input to PV Interneuron Subgroup
The different subgroups showed differences in excitatory input as assayed by AMPAR-mediated mEPSCs (Fig. 8), which provides an independent verification of the subgroups. The larger PV3 mEPSC half-width could in theory be due to mEPSC amplitude, distance from soma, glutamate receptor subtype, or membrane resistance. The average mEPSC amplitude and area are not significantly larger in PV3 interneurons. The mEPSC 10–90% rise is not significantly larger in PV3 interneurons (not shown), suggesting that the difference in half-width is not due to distance from soma or membrane resistance (if slow membrane kinetics or filtering were the sole contributor to the slow half-width, the 10–90% rise time would be slower as well). This suggests that there is a real difference in the quality of excitatory input onto PV3 interneurons, possibly through different glutamate receptors. GluA1, GluA2, and GluA4 subunits can be expressed in PV interneurons (Chang et al. 2010; Geiger et al. 1995), although GluA2 may be absent in a majority of PV interneurons (Kondo et al. 1997). The slower kinetics of PV3 mEPSCs could be explained by a higher ratio of GluA1- to GluA4-containing synapses. Alternatively, the kinetics could be slower because of a preponderance of flip splice variants in the AMPARs (Lambolez et al. 1996) or the presence of transmembrane AMPAR regulatory proteins (TARPs) (Milstein and Nicoll 2008). Additional experiments will be required to discover the unique combination of AMPAR subunits contributing to the difference in excitatory input between subgroups.
The slightly lower mEPSC frequency seen in PV3 interneurons could be due to fewer synapses contacting PV3 dendrites, to a lower probability of release at some or all of the synapses, or to some combination of the above. If there are indeed fewer synapses overall, this could be due to either a smaller dendritic tree or lower synaptic density on some or all dendrites. Future work might confirm or eliminate some of these possibilities. Finally, the higher membrane resistance observed in the PV3 subgroup could be a result of the relatively lower numbers of synaptic events (Pare et al. 1998).
Since the role of a neuron in a circuit is dependent on its passive and active membrane properties, the different PV subgroups described here would behave differently in the context of a cortical circuit. For example, PV1a interneurons would not participate in the circuit response to a weak stimulus but in response to a sufficiently strong stimulus would inhibit its postsynaptic targets and with other PV1a interneurons would impose a rhythmic oscillation in the gamma range that scales up with stimulus intensity. In contrast, PV3 interneurons would participate in feedforward and feedback inhibition in response to weak inhibition. In groups, their irregular firing at low frequencies would inhibit synchronized firing in the sub-gamma range.
GRANTS
This work was supported by National Eye Institute RO1 Grant EY-O1697905 (L. P. Wollmuth).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H. and L.P.W. conception and design of research; J.H. and G.A. performed experiments; J.H. and G.A. analyzed data; J.H. and L.P.W. interpreted results of experiments; J.H. prepared figures; J.H. and L.P.W. drafted manuscript; J.H., G.A., and L.P.W. edited and revised manuscript; J.H., G.A., and L.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rashek Kazi for helpful discussions and comments on the manuscript, Dr. Josh Huang and the rest of his laboratory for assistance with mice, and Drs. Arianna Maffei and Brian Fix for technical assistance.
REFERENCES
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73: 159–170, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38: 805–817, 2003 [DOI] [PubMed] [Google Scholar]
- Burkhalter A. Many specialists for suppressing cortical excitation. Front Neurosci 2: 155–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17: 3894–3906, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA 97: 6144–6149, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13: 1090–1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789, 2002 [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Cossart R, Huang J, Yuste R. Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cereb Cortex 17: 81–91, 2007 [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999 [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science 303: 1681–1683, 2004 [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15: 193–204, 1995 [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Single Channel Recording, edited by Neher E, Sakmann B. New York: Plenum, 1995, p. 155–198 [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58: 387–400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Jeong HY, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb Cortex 21: 666–682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7: 347–358, 1997 [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat 1: 1–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274–3286, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Barry J, McDougel R, Terman D, Gu C. Alternative splicing regulates kv3.1 polarized targeting to adjust maximal spiking frequency. J Biol Chem 287: 1755–1769, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L, McGarry LM, Robles V, Bielza C, Larrañaga P, Yuste R. Comparison between supervised and unsupervised classifications of neuronal cell types: a case study. Dev Neurobiol 71: 71–82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30: 15134–15145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 287: 273–278, 2000 [DOI] [PubMed] [Google Scholar]
- Halabisky B, Shen F, Huguenard JR, Prince DA. Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96: 834–845, 2006 [DOI] [PubMed] [Google Scholar]
- Hennig C. fpc: Flexible procedures for clustering, 2010 [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3: e159, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755, 1999 [DOI] [PubMed] [Google Scholar]
- Jagadeesh B, Gray CM, Ferster D. Visually evoked oscillations of membrane potential in cells of cat visual cortex. Science 257: 552–554, 1992 [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci 27: 30–40, 2004 [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci 29: 3642–3659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube F, Kubota Y, Kawaguchi Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci 24: 2853–2865, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci 15: 2638–2655, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31: 277–287, 2002 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70: 387–396, 1993 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically identified GABAergic cells in the rat frontal cortex. Neuroscience 85: 677–701, 1998 [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF. GABAergic networks of basket cells in the visual cortex. Prog Brain Res 90: 385–405, 1992 [DOI] [PubMed] [Google Scholar]
- Kondo M, Sumino R, Okado H. Combinations of AMPA receptor subunit expression in individual cortical neurons correlate with expression of specific calcium-binding proteins. J Neurosci 17: 1570–1581, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol 94: 3009–3022, 2005 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci 20: 375–386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS One 3: e2005, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B, Ropert N, Perrais D, Rossier J, Hestrin S. Correlation between kinetics and RNA splicing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci USA 93: 1797–1802, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Huang ZJ. Distinct maturation profiles of perisomatic and dendritic targeting GABAergic interneurons in the mouse primary visual cortex during the critical period of ocular dominance plasticity. J Neurophysiol 106: 775–787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30: 16796–16808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488: 379–383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35: 57–67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WP, Liu BH, Li YT, Huang ZJ, Zhang LI, Tao HW. Visual representations by cortical somatostatin inhibitory neurons—selective but with weak and delayed responses. J Neurosci 30: 14371–14379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26: 5069–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci 30: 343–349, 2007 [DOI] [PubMed] [Google Scholar]
- Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13: 107–120, 2012 [DOI] [PubMed] [Google Scholar]
- McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits 4: 12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci 29: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci 27: 7786–7798, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh F, Heck A. Multivariate Data Analysis. Dordrecht, The Netherlands: Kluwer Academic, 1987 [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol 89: 1541–1566, 2003 [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci 29: 7040–7052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Weng J, Levin EJ, Zhou M. Oxidation of NADPH on Kvbeta1 inhibits ball-and-chain type inactivation by restraining the chain. Proc Natl Acad Sci USA 108: 5885–5890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons In vivo. J Neurophysiol 79: 1450–1460, 1998 [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol 100: 2348–2360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2005 [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci 17: 8246–8258, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20: 53–65, 1987 [Google Scholar]
- Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron 67: 847–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P. A specific “axo-axonal” interneuron in the visual cortex of the rat. Brain Res 136: 345–350, 1977 [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16: 392–417, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H. pvclust: Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling, 2009 [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006 [DOI] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci 18: 4255–4270, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339: 70–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, Markram H. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex 14: 1310–1327, 2004 [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex 12: 395–410, 2002 [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488: 343–348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits 3: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518: 389–404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]