Abstract
In animals, the recovery of motoneuron excitability in the months following a complete spinal cord injury is mediated, in part, by increases in constitutive serotonin (5-HT2) and norepinephrine (α1) receptor activity, which facilitates the reactivation of calcium-mediated persistent inward currents (CaPICs) without the ligands serotonin and norepinephrine below the injury. In this study we sought evidence for a similar role of constitutive monoamine receptor activity in the development of spasticity in human spinal cord injury. In chronically injured participants with partially preserved sensory and motor function, the serotonin reuptake inhibitor citalopram facilitated long-lasting reflex responses (spasms) previously shown to be mediated by CaPICs, suggesting that in incomplete spinal cord injury, functional descending sources of monoamines are present to activate monoamine receptors below the lesion. However, in participants with motor or motor/sensory complete injuries, the inverse agonist cyproheptadine, which blocks both ligand and constitutive 5-HT2/α1 receptor activity, decreased long-lasting reflexes, whereas the neutral antagonist chlorpromazine, which only blocks ligand activation of these receptors, had no effect. When tested in noninjured control participants having functional descending sources of monoamines, chlorpromazine was effective in reducing CaPIC-mediated motor unit activity. On the basis of these combined results, it appears that in severe spinal cord injury, facilitation of persistent inward currents and muscle spasms is mainly mediated by the activation of constitutive 5-HT2 and α1 receptor activity. Drugs that more selectively block these constitutively active monoamine receptors may provide better oral control of spasticity, especially in motor complete spinal cord injury where reducing motoneuron excitability is the primary goal.
Keywords: spasticity, motoneurons, serotonin, norepinephrine, inverse agonist
in rats, the recovery of motoneuron excitability in the months following a complete spinal cord injury is mediated, in part, by increases in constitutively active 5-HT2 and α1 receptors on the motoneuron (Murray et al. 2010; Rank et al. 2011). Continual activation of these monoaminergic receptors in the absence of the ligands serotonin and norepinephrine below the injury allows for continual facilitation of voltage-activated, persistent inward calcium currents (CaPICs) via downstream Gq-coupled pathways (Heckman et al. 2003; Hounsgaard et al. 1988; Mizuno and Itoh 2009; Perrier et al. 2002). Combined with the presence of prolonged, sensory-evoked excitatory postsynaptic potentials (EPSPs; Baker and Chandler 1987; Li et al. 2004a) and reduced inhibitory postsynaptic potentials (IPSPs; Boulenguez et al. 2010; Norton et al. 2008), the recovered CaPICs are more readily triggered and depolarize the motoneuron to produce unchecked and prolonged involuntary muscle spasms after spinal cord injury.
Evidence for constitutive receptor activation in animal models of complete spinal cord injury was provided by the combined finding that neutral antagonists, which only block ligand activation of the 5-HT2 (e.g., SB242084/methysergide) and α1 (REC15/2739) receptors, were ineffective in reducing the CaPIC, whereas inverse agonists (e.g., 5-HT2: SB206553/cyproheptadine; α1: WB4010/prazosin), which block both ligand and constitutive activation of these receptors, effectively reduced the CaPIC (Murray et al. 2010; Rank et al. 2011). In addition, mRNA analysis revealed that increases in the unedited and constitutively active isoform of the 5-HT2C receptor (INI) occurred below the injury site where levels of serotonin were greatly diminished (Murray et al. 2010). In this same study, we also provided evidence in human participants with spinal cord injury that cyproheptadine, an inverse agonist to 5-HT2 and α1 receptors, also decreased long-lasting reflexes (spasms), which are thought to be mediated by CaPIC activation (Gorassini et al. 2004). However, because these participants had preserved motor function, there existed the possibility that residual, descending sources of serotonin and norepinephrine were still present below the injury to provide ligand activation of the 5-HT2/α1 receptors. Therefore, this leaves the possibility that cyproheptadine could have acted solely by blocking the ligand activation of the 5-HT2/α1 receptors on the motoneuron, requiring further experiments to prove that constitutive monoaminergic receptor activity plays a role in the facilitation of involuntary motoneuron activity (muscle spasms) in humans with chronic spinal cord injury.
To this end, in this study we first tested if the participants with motor incomplete injuries from the Murray et al. (2010) study indeed had residual sources of serotonin that facilitate-CaPIC mediated long-lasting reflexes by examining whether a serotonin reuptake inhibitor (i.e., citalopram) could facilitate such reflexes. Because we observed evidence of such facilitation, we then compared the effects of the inverse agonist cyproheptadine and the neutral antagonist chlorpromazine in participants with motor or motor/sensory complete injuries where descending sources of serotonin and norepinephrine are likely greatly reduced or absent. Similar to the animal studies, we examined whether only the inverse agonist, and not the neutral antagonist, was effective in reducing CaPIC-mediated reflexes to provide more substantive evidence of the presence of constitutive monoaminergic receptor activation in humans after spinal cord injury. We also used paired motor unit recordings to provide a more direct estimate of the amplitude of CaPIC compared with the long-lasting reflexes (as per Udina et al. 2010) and explored the role of constitutive 5-HT2/α1 receptor activity on this CaPIC estimate.
METHODS
All experiments were approved by the Health Research Ethics Board at the University of Alberta and conformed to the Declaration of Helsinki, with off-label drug use approved by Health Canada Clinical Trials. All participants gave written, informed consent before participating in the study. In total, six uninjured control participants with no known neurological injury or impairment, nine incomplete spinal cord-injured (iSCI), and nine motor or motor/sensory complete SCI (cSCI) participants with traumatic injuries took part in this study (see Table 1 for details). In addition, drug screens were carried out and verified (by JDA, KMC, and MF) to rule out contraindications for the various drugs. One individual with SCI was excluded from the study because of warfarin use, which interacts with cyproheptadine.
Table 1.
Demographic and clinical description of SCI participants
| Preserved Sensation |
||||||||
|---|---|---|---|---|---|---|---|---|
| Subject Code | Age, yr | Age of Injury, yr | Injury Level | AIS | MAS | Penn Spasm Scale | Light touch | Pinprick |
| 1M | 61 | 6 | C3, C6 | C | 0 | 2 | √ | √ |
| 2M† | 54 | 12 | C5–C6 | C | 3 | 3 | √ | √ |
| 3F*† | 49 | 6 | T2–T4 | C | 0 | 3 | √ | √ |
| 4F*† | 23 | 2 | C6–C7 | C | 1 | 3 | √ | √ |
| 5M*† | 50 | 12 | C6–C7 | D | 3 | 2 | √ | √ |
| 6M* | 51 | 31 | C3–C4 | D | 1 | 2 | √ | √ |
| 7M* | 53 | 3 | C1–C3 | D | 1 | 2 | √ | √ |
| 8M | 61 | 2 | C3–C5 | C | NA | 1 | √ | √ |
| 9M* | 45 | 3 | C4–C5 | D | 1 | 3 | √ | √ |
| 10M‡ | 33 | 13 | C5–C6 | B | 1 | 3 | Left: √ | Left: √ |
| Right: √ | Right: √ | |||||||
| 11M‡ | 29 | 7 | C4–C5 | B | 1 | 3 | Left: none | Left: outer shin |
| Right: upper shin and foot | Right: √ | |||||||
| 12M‡ | 31 | 1.5 | T3–T4 | B | 0 | 3-4 | Left: √ except front of shin | Left: √ |
| Right: none | Right: inner side of leg | |||||||
| 13M‡ | 45 | 3 | T4–T5 | A | 1 | 2 | Left: none | Left: none |
| Right: none | Right: none | |||||||
| 14M‡ | 21 | 11 | C6–C7 | A | 1 | 2 | Left: none | Left: none |
| Right: none | Right: none | |||||||
| 15M‡ | 24 | 6 | C5–C6 | A | 2 | 1 | Left: none | Left: none |
| Right: none | Right: none | |||||||
| 16M‡ | 22 | 2 | T4 | A | 2 | 3 | Left: none | Left: none |
| Right: none | Right: none | |||||||
| 17M | 53 | 8 | C7–T1 | B | NA | NA | Left: none | Left: none |
| Right: none | Right: none | |||||||
| 18M | 23 | 2 | T4–T5 | A | 2 | 3 | Left: none | Left: none |
| Right: none | Right: none | |||||||
Description of participants including age of the participant and age of injury at the time of recording, level of injury, ASIA Impairment Scale (AIS), Modified Ashworth Score (MAS; 0–4), and Penn Spasm Frequency Scale (part 1: 0–4). NA, not available. Final columns characterize the preservation of light touch and pin prick sensation from the knee downward: check mark indicates preserved sensation. All participants were tested with cyproheptadine; symbols indicate participants in whom paired motor unit analysis was performed (*), participants tested with citalopram (†), and participants tested with chlorpromazine (‡). Shaded area indicates participants with preserved motor function in legs.
Drug administration.
All 5-HT2/α1 receptor drugs were administered orally and were housed in a two-part telescoping capsule to blind participants as to which drug they were taking. For example, iSCI participants took either cyproheptadine (8 mg), a serotonin 5-HT2/α1 receptor inverse agonist in spinal motoneurons (Murray et al. 2010), or citalopram (20 mg), a selective serotonin reuptake inhibitor (SSRI; Hyttel 1982). Increases in PIC-mediated responses (described in Reflex analysis and Estimation of PIC amplitude) by citalopram in iSCI participants indicated the presence of spared functional sources of serotonin. We were only able to test citalopram in four of the nine iSCI participants (subjects 2M–5M in Table 1) because four of the others were already taking an SSRI and one out-of-town participant was not able to return for the second experiment. cSCI participants took either cyproheptadine (12 mg) or an equivalent dose of chlorpromazine (12.5 mg), a 5-HT2/α1 receptor neutral antagonist (see discussion and Herrick-Davis et al. 2000; Rauser et al. 2001; Richelson and Nelson 1984). Evidence for the presence of constitutive 5-HT2/α1 receptor activity was considered if only the inverse agonist (cyproheptadine), which blocks both constitutive and ligand activation of the receptor, and not the neutral antagonist (chlorpromazine), which only blocks ligand activation of the receptor, was effective in reducing the PIC-mediated responses. If SCI participants were on oral baclofen, they were asked to skip their morning pill before the experiment. Noninjured (NI) control participants were given the same oral dose of citalopram and chlorpromazine. JDA, who performed the data analysis, was also blinded to the drug given. Heart rate and blood pressure were measured before and every 30 min after drug intake. Participants were also asked to report any changes in physiological sensations from the drug.
Long-duration reflexes.
Reflex recordings were done in SCI participants only, who were seated in their wheelchairs with limbs unconstrained. Two surface electrodes (2.2 × 3.3 cm; Kendall Soft-E, Chicopee, MA) were placed over the tibialis anterior and soleus muscles to record electromyography (EMG) signals. The surface EMG was amplified 1,000 times, filtered using a bandpass of 10–1,000 Hz (Octopus; Bortec Technologies, Calgary, AB, Canada) or 20–2,500 Hz (model 2024F; Intronix Technologies, Bolton, ON, Canada). The EMG signals were digitized using Axoscope hardware and software at a rate of 5 kHz (Digidata 1440 Series; Molecular Devices, Sunnyvale, CA) and stored on a personal computer for off-line analysis. To evoke long-duration (>1 s) reflex responses in the tibialis anterior, which we have previously demonstrated to be largely mediated by CaPICs (Gorassini et al. 2004; Li et al. 2004a), we stimulated cutaneomuscular afferents supplying the side and sole of the foot with long pulse trains. These many-second-long reflexes (or spasms) were evoked at rest by electrical stimulation to the medial arch of the foot (300 Hz, 14 pulses, 0.5-ms pulse width) using a DS7A constant-current stimulator (NL703; Digitimer, Welwyn Garden City, UK). The intensity of stimulation was chosen to maximize the duration of the evoked reflex without being too painful for the subject. Higher stimulation intensities were needed in the motor complete SCI participants (75.0 ± 22.0 mA) compared with the incomplete SCI participants (27.6 ± 11.8 mA; P < 0.001, Mann-Whitney). Stimulation was repeated 6 times at 5- to 10-s intervals for each trial. Two to three trials were taken before drug administration to establish a stable baseline. Reflex measures were repeated every 30 min after drug intake for 2 h. Although peak plasma levels of the various drugs occur ≈2–4 h after oral intake (Bezchlibnyk-Butler et al. 2000; Mendes et al. 2012; Yeung et al. 1993), we only tested motoneuron excitability until 2 h postdrug because in pilot studies, maintaining a similar seated posture for more than 3 h (+1 h predrug measures) was too fatiguing for the NI control participants who are not accustomed to sitting for long, uninterrupted periods. As with other drug studies (Ziemann 2004), we were able to observe effects of the various drugs on neuronal pathways within 30 to 120 min after drug intake, well before their estimated peak plasma times.
Reflex analysis.
Three components of the long-duration reflex response were measured using custom-written Matlab software (The MathWorks, Natick, MA). The early-latency (onset at 50–70 ms after first stimulation pulse), short-lasting polysynaptic reflex (SPR) was defined as a distinct large-amplitude response that occurred in the first 50 ms of the reflex, as per Murray et al. (2010, 2011a) and Rank et al. (2011). The SPR, which was only evoked in the iSCI participants (see also Roby-Brami and Bussel 1987), is useful to examine sensory-evoked EPSPs in isolation because they are not inhibited by a block of CaPICs with isradipine (Murray et al. 2011a). The more polyphasic and longer lasting polysynaptic reflex (LPR), which included the start of the reflex response up until 300 ms after the first stimulation pulse, contains a mixture of both sensory-evoked EPSPs and CaPIC activation because its amplitude is reduced to around 50% by isradipine (Li et al. 2004a; Murray et al. 2011a). The later long-lasting reflex component (LLR) was defined as the time window from 500 ms after the first stimulation pulse to the end of the reflex response in the predrug trial, as per Murray et al. (2010, 2011a, 2011b) and Rank et al. (2011) (LLR duration: 1,445 ± 704 ms in iSCI and 645 ± 454 ms in cSCI participants). Thus the LLR represents a period when most of the sensory synaptic drive to the motoneuron (EPSP) has subsided and is produced mainly by a depolarization from the CaPIC (Li et al. 2004a; Norton et al. 2008).
To measure the amplitude of the various reflex components using Matlab software, each EMG trace was first rectified and the mean EMG was value calculated for the SPR, LPR, and LLR time windows defined above. The mean rectified background noise, measured from 100 ms before the stimulation, was subtracted from the data. The mean EMG values for each of the six stimulation trials were averaged together to obtain SPR, LPR, and LLR values for each time point. All SPR, LPR, and LLR values postdrug (at 30, 60, 90, and 120 min) were then expressed as a percentage of the predrug value (postdrug/predrug × 100%). Because there was no statistical difference between the two SPR, LPR, or LLR values measured immediately before the drug intake, the two baseline values were averaged together for each reflex component. Percent values at each time point were averaged across participants for each of the different drug experiments.
Motor unit recordings.
Motor unit recordings in iSCI participants and NI control participants were used to examine the effects of the various monoamine drugs on the estimation of PIC amplitude using paired motor unit analysis (as per Udina et al. 2010). Motor unit recordings were taken during the same experimental session as the reflex recordings in the iSCI participants. Participants were seated in their wheelchairs or in a comfortable chair with the foot strapped onto a metal plate that was coupled to a force transducer. Participants were tasked with tracking a triangle drawn on a transparency placed over the computer screen using either the dorsiflexion torque or integrated surface EMG signal, whichever they preferred. The y-axis of the torque or EMG signal was adjusted so that only two to three motor units were recruited during the ascending phase of the contraction, and the x-axis was adjusted so that the total contraction lasted at least 15 s. Intramuscular electrodes used for recording single-motor unit action potentials were inserted into the tibialis anterior muscle using a 25-gauge needle (for electrode details see Udina et al. 2010). Intramuscular EMG was amplified 5,000 times, high-pass filtered at 200 Hz using an isolated, high-impedance amplifier (model 2024F; Intronix Technologies), with a DC frequency response to 10 kHz, containing an imbedded Butterworth filter with a 12-dB/octave cutoff. All data were digitized using a Power 1401 analog-to-digital converter and Spike2 (version 6) software (Cambridge Electronics Design, Cambridge, UK) with a sampling rate of 25 kHz for the intramuscular EMG, 5 kHz for surface EMG, and 100 Hz for the torque signal.
Estimation of PIC amplitude.
Data were analyzed offline using commercial spike discrimination software (Spike2; Cambridge Electronic Design). Single-motor unit action potentials were first selected by setting a horizontal threshold and inspecting each waveform visually to identify a given motor unit on the basis of waveform shape. When possible, the same motor unit pair (control and test) was followed both before and after drug administration. However, due to electrode movement during the long experiment (typically 3–4 h), one or both of the motor unit pairs could be lost, and new unit pairs were sampled after drug intake. In the cyproheptadine experiments, 11 of the 15 motor unit pairs recorded in the iSCI participants were the same both pre- and postdrug, whereas in the chlorpromazine experiments, 5 of the 7 motor unit pairs recorded in the NI control participants were the same both pre- and postdrug.
The amplitude of PIC activation was estimated utilizing the paired motor unit analysis technique (Bennett et al. 2001; Gorassini et al. 2002, 2004). The times of occurrences for the spikes obtained using Spike2 were exported via a text file to a custom-written Matlab program. The instantaneous firing rates of the units were then calculated as the reciprocal of each interspike interval. The firing rate profile of a lower threshold control motor unit was used as a measure of the synaptic input to the motoneuron pool, specifically to a relatively higher threshold motor unit, termed the test unit. To calculate the firing rate of the control unit at recruitment and derecruitment of the test unit, a fifth-order polynomial was used to smooth the firing rate profiles. The smoothed firing rate of the control unit at recruitment and derecruitment of the test unit was determined automatically, and the difference in smoothed firing rate of the control unit when the test unit was derecruited, compared with when it was recruited, was computed: ΔF = Fderecruitment − Frecruitment. This ΔF, therefore, corresponds to the reduction in synaptic input needed to counteract the depolarization from the PIC and provides an indirect estimate of PIC amplitude (Bennett et al. 2001; Gorassini et al. 2004). The accuracy of the ΔF measure relies on how well the firing rate of the control unit reflects the synaptic drive to the test unit, which requires that both units receive a common synaptic drive. To estimate this, the smoothed firing rate of the control unit was plotted against the smoothed firing rate of the test unit, and the coefficient of variation (r2) was calculated (as per Gorassini et al. 2002; Udina et al. 2010).
Five of a possible 8–10 contraction trials were chosen to calculate an average ΔF value for each subject at each time point measured (predrug and 30, 60, 90, and 120 min postdrug). Contractions were chosen on the basis of the recruitment profiles of the motor units, i.e., if the recruitment of the control and test motor units was separated by more than 2 s, if the test unit was activated for 2 s or more during the ascending phase of the contraction to ensure full CaPIC recruitment, and if the rate of increase in firing rate of the control unit during the ascending phase of the contraction was similar to the rate of decrease in firing rate during the descending phase of the contraction (Udina et al. 2010). All participants, including the iSCI, were able to produce triangular firing rate profiles of the control motor unit that lasted for at least 10–15 s. These profiles were produced to best emulate the firing rate profiles used to calculate PIC amplitude from intracellular recordings in rat motoneurons (Gorassini et al. 2004). Postdrug ΔF values were expressed as a percentage of the predrug value and averaged across participants.
In vitro ventral root recordings of long-duration reflexes in rats.
To verify that the human-approved drug chlorpromazine works as a neutral antagonist on 5-HT2/α1 receptors in spinal motoneurons, i.e., only blocking ligand activation of the receptor, we used the in vitro sacral spinal cord preparation, a rat model that has been used to distinguish inverse agonists from neutral antagonists (Murray et al. 2010). Under urethane anesthesia (1.8 g/kg), the whole spinal cord caudal to the S2 injury was removed from chronic (>2 mo) spinal rats and immersed in oxygenated artificial cerebrospinal fluid (ACSF; flowing 8 ml/min); recordings were made starting 2.5 h later, as detailed previously (Bennett et al. 2001; Li and Bennett 2003). Ventral (S4 and Co1) and dorsal (Co1, coccygeal) roots were mounted on silver wires above the ACSF and covered with Vaseline. The dorsal root was stimulated with a single pulse (0.1 ms, 0.02 mA, 3 times reflex threshold; repeated 5 times at 10-s intervals for 1 trial, with trials repeated every 12 min), and the LLR response was recorded on the ventral roots and then analyzed as for the surface EMG in humans. The LLR was quantified by averaging the rectified ventral root activity over a time window 500–4,000 ms poststimulus, a period previously shown to reflect the motoneuron CaPIC activity in isolation (Murray et al. 2010; Rank et al. 2011). A 5 μM dose of chlorpromazine (Sigma-Aldrich) was used, a dose in the whole sacral spinal cord that is known to be effective for other neutral antagonists to the 5-HT2C receptor, such as SB242084, that have similar binding affinities to that of chlorpromazine (Ki < 20 nM; see Table 4 in Murray et al. 2011b). The LLR was quantified at 30 min postdrug application because the effect of 5-HT2/α1 receptor antagonists applied in vitro peak at this time (Murray et al. 2010). A lack of effect of chlorpromazine on the LLR was taken as evidence that it acted as a neutral antagonist given that in chronically injured animals, there are little to no functional sources of serotonin for it to interfere with (Murray et al. 2010). Furthermore, to verify that the 5 μM dose of chlorpromazine was effective, the EC50 of the 5-HT2 receptor agonist α-methyl-5-HT (Murray et al. 2011b) was measured in response to a prior application of 5 μM chlorpromazine. At 15-min intervals 10, 100, 1,000, and 10,000 nM doses of α-methyl-5-HT were applied cumulatively starting at 15 min after chlorpromazine administration. An increase in the EC50, i.e., the concentration of α-methyl-5-HT needed to produce 50% of its maximal facilitation of the LLR, indicated that a 5 μM dose of chlorpromazine was effective in blocking a ligand if present and thus acted as a neutral antagonist to the 5-HT2 receptor at this dose.
Statistics.
All statistics were performed using SigmaPlot 11 software (Systat Software). All values presented in the text are means ± SD, and data presented in Figs. 1–5 are means ± SE. Normality was tested with the Shapiro-Wilk test for SPR, LPR, LLR, and ΔF values. One-factor repeated-measures ANOVA for normally distributed data was used to determine the effect of the various drugs over time on the SPR, LPR, LLR, and ΔF values, and repeated-measures ANOVA on ranks was used for nonnormally distributed data. Post hoc Bonferroni-corrected t-tests were used to isolate which postdrug response at the 30-, 60-, 90-, or 120-min time point differed from the predrug response. Significance was set to P ≤ 0.05.
Fig. 1.
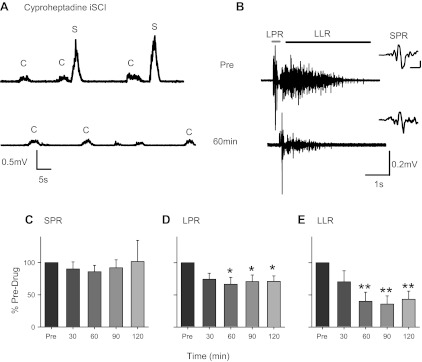
Cyproheptadine in incomplete spinal cord injury (iSCI). A: rectified surface electromyography (EMG) from tibialis anterior (TA) during dorsiflexion contractions (C) that trigger involuntary muscle spasms (S) in a T2–T4 iSCI participant before (Pre) and 60 min after an 8-mg dose of cyproheptadine (subject 3F, Table 1). B: for same participant in A, unrectified surface EMG during long-duration reflex evoked in the TA from medial arch stimulation (22 mA, 300 Hz, 14 pulses, 0.5 ms wide) before (top) and ∼60 min after (bottom) cyproheptadine. The long polysynaptic reflex (LPR; from start of reflex to 300 ms poststimulation) is marked by the gray bar, and the long-lasting reflex (LLR; from 500 ms after stimulation to 3,500 ms) is indicated by the black bar. Insets at right show short polysynaptic reflex (SPR) on expanded time scale; scale bars, 20 ms/0.2 mV. Bottom trace shifted to the right to reveal SPR. Averaged SPR (C), LPR (D), and LLR (E) are expressed as a percentage of predrug values at different time points across the 8 iSCI participants tested (subjects 1M–8M, Table 1). Some of the data from 6 of the 8 iSCI participants have been published previously (Murray et al. 2010). Error bars represent means ± SE. *P < 0.05; **P < 0.005.
Fig. 2.
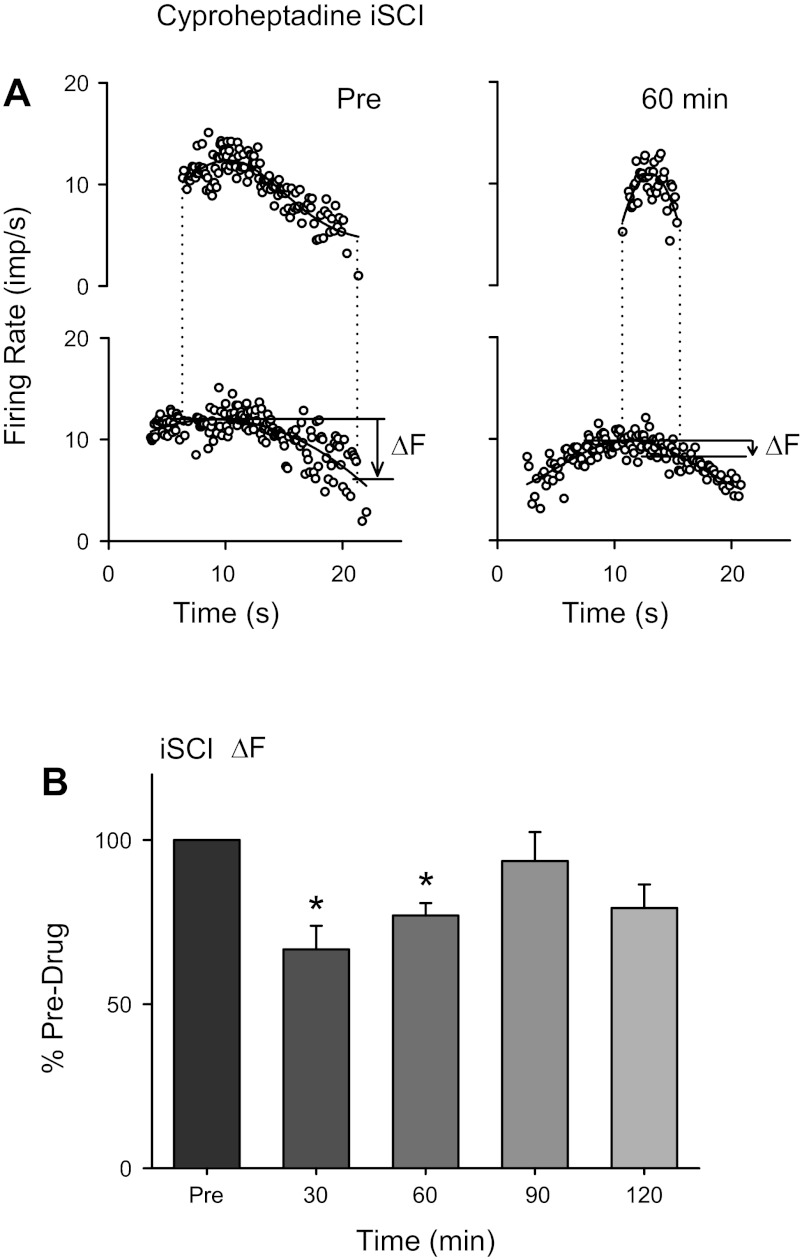
Effects of cyproheptadine on estimated persistent inward current (PIC). A: instantaneous firing rate of lower threshold control (bottom) and higher threshold test motor units (top) during isometric dorsiflexion before (left) and 60 min after (right) oral intake of 8 mg of cyproheptadine in an iSCI participant (imp/s, impulses per second). The thick black line represents a 5th-order polynomial fit through the firing rate points. Dashed vertical lines mark the time of occurrence of recruitment and derecruitment of the higher threshold test unit. Solid horizontal lines indicate the firing frequency of the control unit when the test unit was recruited and derecruited, with the magnitude of difference in the firing rates between the 2 time points (ΔF) marked by the arrow. B: average ΔF expressed as a percentage of the predrug values at different time points across the 6 iSCI participants tested (subjects 3F–7M and 9M, Table 1). Error bars represent means ± SE. *P < 0.05.
Fig. 3.
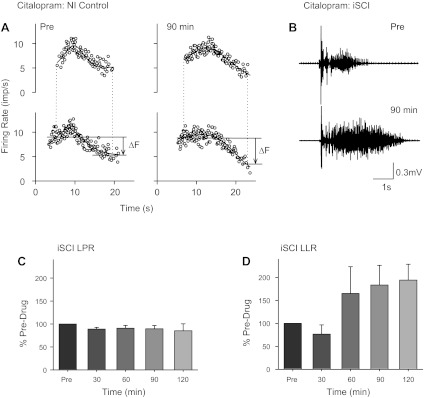
Citalopram in noninjured (NI) controls and iSCI. A: paired motor unit recordings as shown in Fig. 2A before (left) and 90 min after 20-mg citalopram administration (right) in a single NI control participant. B: long-duration reflex recorded in TA (medial arch stimulation: 15.7 mA, 300 Hz, 14 pulses, 0.5-ms pulse width) in a single iSCI participant (subject 3F, Table 1) before and ∼90 min after 20 mg of citalopram. Averaged LPR (C) and LLR (D) are expressed as a percentage of predrug values at different time points across the 4 iSCI participants tested (subjects 2M–5M, Table 1). Error bars represent means ± SE.
Fig. 4.
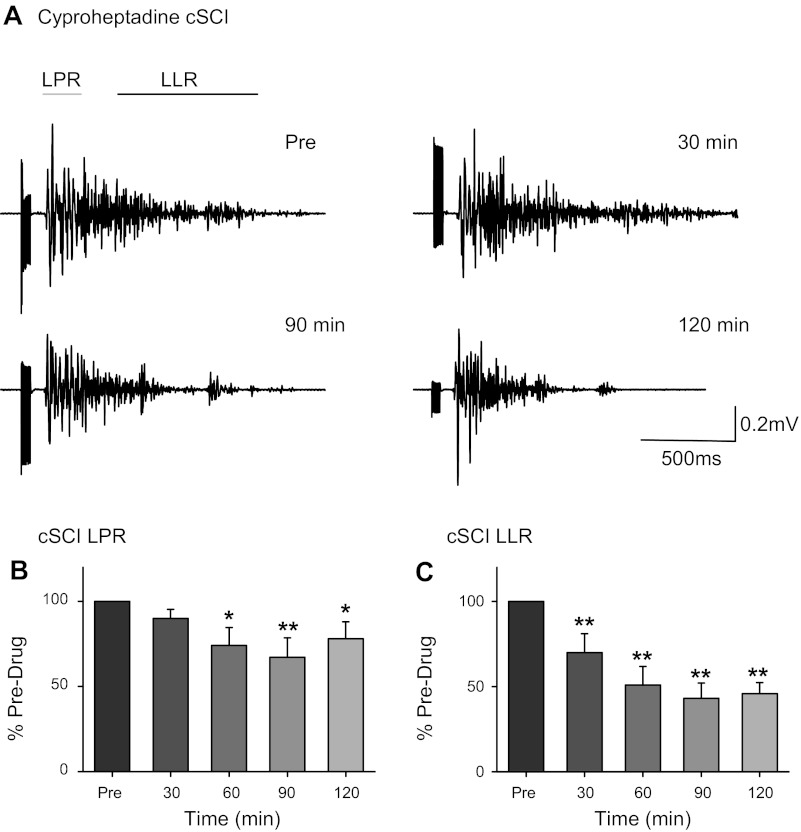
Cyproheptadine in complete SCI (cSCI). A: long-duration reflexes recorded in TA (medial arch stimulation: 60 mA, 300 Hz, 14 pulses, 0.5-ms pulse width) in a single cSCI participant (subject 16M, Table 1) before and 30, 90, and 120 min after 12 mg of cyproheptadine. Averaged LPR (B) and LLR (C) expressed as a percentage of predrug values at different time points across the 9 cSCI participants tested (subjects 10M–18M, Table 1). Error bars represent means ± SE. *P < 0.05; **P < 0.005.
Fig. 5.
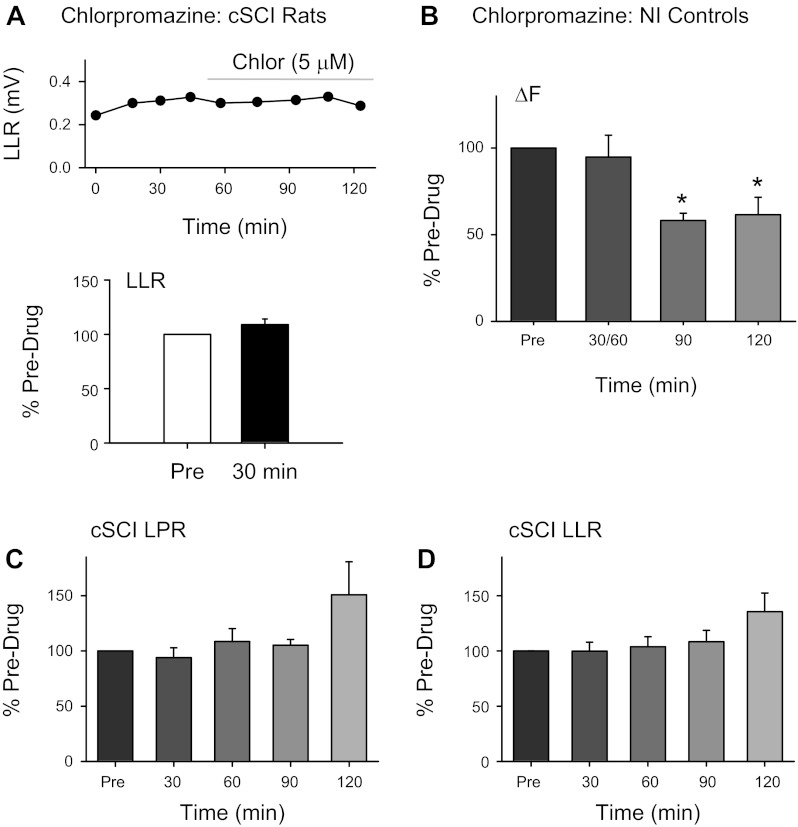
Chlorpromazine in rat cSCI and human NI controls and cSCI. A, top: amplitude of LLR measured in ventral root evoked from single-pulse dorsal root stimulation (0.1 ms, 0.02 mA, 3 times reflex threshold) from a single completely spinalized rat before and after 5 μM chlorpromazine (time of drug application marked by gray horizontal line). Bottom, average of LLR expressed as a percentage of predrug value (open bar) at 30 min after (filled bar) application of chlorpromazine to bath solution (n = 6 roots). B: average ΔF expressed as a percentage of predrug values at different time points across 4 NI control participants. Averaged LPR (C) and LLR (D) are expressed as a percentage of predrug values at different time points across 7 of the 9 cSCI participants tested (subjects 10M–16M, Table 1). Error bars represent means ± SE. *P < 0.05.
RESULTS
Cyproheptadine: incomplete SCI.
Involuntary muscle spasms in the tibialis anterior muscle were sometimes triggered after a voluntary dorsiflexion contraction as shown in Fig. 1A, top trace, for a participant with a T2–T4 incomplete SCI (subject 3F in Table 1). The incidence of these contraction-induced spasms decreased 60 min following the oral administration of the inverse agonist cyproheptadine (8 mg; Fig. 1A, bottom trace). In this same participant, cyproheptadine also reduced the long-duration reflexes (i.e., muscle spasms) in the tibialis anterior triggered by electrical stimulation to the medial arch of the foot (Fig. 1B). Both the EPSP/PIC-mediated LPR and the PIC-mediated LLR (see methods for definitions) were reduced. In the eight iSCI participants tested (subjects 1M–8M, Table 1), cyproheptadine decreased both the LPR and LLR components of the long-duration reflex (Fig. 1, D and E; 1-way ANOVA, LPR: F = 3.66, P < 0.05; LLR: F = 7.73, P < 0.001). Post hoc testing revealed a significant decrease in both the LPR and LLR at 60, 90, and 120 min compared with the predrug value (Bonferroni paired t-test, all P < 0.05). In contrast, the pure EPSP-mediated component of the reflex, the SPR (see Fig. 1B, inset), was not affected by cyproheptadine (Fig. 1C; F = 0.27, P = 0.89).
In six iSCI participants (subjects 3F–7M and 9M, Table 1), we were able examine the effect of cyproheptadine on the estimated amplitude of the motoneuron PIC using paired motor unit analysis (see methods, Estimation of PIC amplitude). Briefly, the firing rate of a lower threshold control motor unit recorded from the tibialis anterior (Fig. 2A, bottom) was used as a measure of synaptic drive to a higher threshold test motor unit (Fig. 2A, top) during a triangular voluntary dorsiflexion. The difference in the smoothed firing rate of the control motor unit when the test motor unit was derecruited compared with when it was recruited (ΔF) was used as a measure of the reduction in synaptic input needed to counteract the depolarization from the PIC and provides an indirect estimate of PIC amplitude. As shown for the single subject represented in Fig. 2A, the higher threshold test motor unit was derecruited at a higher firing rate of the control motor unit at 60 min after cyproheptadine (right) compared with before (left). This produced a decrease in the ΔF measurement (arrows in Fig. 2A) to signify a reduction in the estimated PIC. In the six iSCI participants in which two motor units of different recruitment thresholds could be distinguished for the 2 h after drug intake, there was a significant decrease in the ΔF value after cyproheptadine (Fig. 2B; F = 4.85, P = 0.007), with ΔF significantly smaller by 29% (on average) at 30 and 60 min postdrug (P < 0.05).
Although the test unit was derecruited earlier, the firing rate profile of the control motor unit remained similar after cyproheptadine with a mean firing rate throughout the contraction of 8.51 ± 0.35 Hz postdrug compared with 8.8 ± 1.1 Hz before drug intake (P = 0.63), signifying that the voluntary synaptic drive was similar before and after drug intake. Likewise, the test motor units were activated for a similar duration of time during the ascending phase of the contraction before cyproheptadine intake (median 3.53 s) compared with after (median 3.21 s, P = 0.62), ensuring that enough time was given for full activation of the CaPIC. Finally, a large percentage of the firing rate profile of the test unit could be accounted for by the firing rate profile of the control unit, both before and after cyproheptadine administration (median r2 = 0.80 predrug, 0.73 postdrug at 60 min), indicating that the control unit provided similar information about the synaptic drive to the test unit both before and after drug intake.
Citalopram: incomplete SCI.
Because of the incompleteness of the injury, the iSCI participants described above could have had residual sources of serotonin or norepinephrine below the injury from spared descending axons. Thus the observed decreases in the PIC-mediated responses (LLR and ΔF values) from the inverse agonist cyproheptadine could have arisen not only from suppression of constitutive 5-HT2/α1 receptor activation but also by blocking ligand activation of the receptor. To test for this possibility, in four of the iSCI participants tested, a 20-mg dose of citalopram, an SSRI, was also given in a separate experiment to examine if the PIC-mediated LLR could be facilitated by increases in the release of serotonin from spared descending axons. We first examined in NI control participants, who have intact descending 5-HT axons, if a 20-mg dose of citalopram was effective in increasing the PIC-mediated ΔF. As shown for a single control subject in Fig. 3A, at 90 min postdrug, the test motor unit continued to fire at much lower rates of the control motor unit just before derecruitment compared with before the drug, thereby producing a larger ΔF value (see arrow). An average increase in the ΔF value of ∼80% was obtained in three NI control participants (79 ± 11% increase averaged across all time points; data not shown), verifying that a 20-mg dose of citalopram should be effective in increasing the PIC-mediated LLR if iSCI participants have some residual descending monoaminergic axons. As shown for the same iSCI participant represented in Fig. 1, a 20-mg dose of citalopram prolonged the long-duration reflex evoked from stimulation of the medial arch of the foot (Fig. 3B). The average LLR increased by 152 ± 67% in the 4 iSCI participants tested with citalopram (Fig. 3D; F = 3.56, P = 0.04); however, none of the individual values postdrug reached statistical significance compared with predrug (P > 0.05). In contrast, the mixed sensory/PIC-mediated component of the response (LPR) remained unchanged over time (F = 0.56, P = 0.70).
On the basis of these results, it is likely that many of the iSCI participants tested had spared descending sources of monoamines. Thus we cannot determine how much the reduction of PIC-mediated responses by the inverse agonist cyproheptadine was due to a suppression of constitutive 5-HT2/α1 receptor activity or a reduction of ligand activation of the receptor. As a result, we repeated these experiments in SCI participants with motor or motor/sensory complete injuries (cSCI) where there should be little to no residual sources of serotonin and norepinephrine below the lesion (Murray et al. 2010; Rank et al. 2011).
Cyproheptadine: motor and motor/sensory complete SCI.
Figure 4A shows the gradual decrease in the long-duration reflex, especially the LLR component, from a motor/sensory complete SCI participant (subject 16M in Table 1) after a 12-mg dose of cyproheptadine. The dose of cyproheptadine was increased to 12 mg because an 8-mg dose was minimally effective in 2 pilot participants with motor complete SCI (data not shown). The data from 9 cSCI participants (subjects 10M–18M, Table 1) showed similar effects with a 48 ± 12% reduction in the LLR averaged over 30 to 120 min after drug intake (Fig. 4C; F = 13.93, P < 0.001) and a smaller 22 ± 10% reduction in the LPR over the same time period (Fig. 4B; F = 4.75, P = 0.005). Post hoc analysis revealed that many time points were significantly smaller postdrug (see Fig. 4, B and C; all P < 0.05). A distinct, early-latency SPR was recorded in only two of the nine cSCI participants and thus was not analyzed further.
Chlorpromazine: motor and motor/sensory complete SCI.
To further exclude the possibility that the effects of cyproheptadine on the cSCI long-duration reflex were due to a block of endogenous serotonin or norepinephrine activating the receptors (and therefore could only result from a decrease in constitutive receptor activity), we also examined whether the neutral antagonist chlorpromazine, which only blocks ligand activation of the 5-HT2/α1 receptor in transfected cells (Herrick-Davis et al. 2000; Rauser et al. 2001), had no effect on the LPR and LLR. We first needed to determine if chlorpromazine acted as a neutral antagonist on 5-HT2/α1 receptors in spinal motoneurons. In chronically spinalized rats with no functional sources of serotonin or norepinephrine (Murray et al. 2010; Rank et al. 2011), chlorpromazine was ineffective in reducing the PIC-mediated LLR (Fig. 5A, top) at a dose (5 μM) comparable to that used for other 5-HT2/α1 receptor neutral antagonists. The average amplitude of the LLR was 109.1 ± 12.7% of the predrug value at 30 min after drug application (Fig. 5A, bottom; P = 0.14), confirming that it was not acting as an inverse agonist in spinal motoneurons but only as a neutral antagonist. Moreover, a prior application of 5 μM chlorpromazine significantly increased the EC50 of α-methyl-5-HT, a 5-HT2 receptor agonist, to 1,670 ± 1,120 nM (n = 10) compared with an EC50 of 62.84 ± 26.51 nM (n = 14; Murray et al. 2011b) when α-methyl-5-HT was applied alone (data not shown; P < 0.001). The ∼30-fold increase in the EC50 of α-methyl-5-HT by chlorpromazine demonstrated that a 5 μM dose was effective in blocking a ligand if one was present, and thus chlorpromazine acted as a neutral antagonist at this dose.
When we examined the effects of chlorpromazine on the ΔF in NI control participants having full sources of serotonin and/or norepinephrine, chlorpromazine was effective in reducing the ΔF at 90 and 120 min after drug intake (F = 6.72, P = 0.011; 90 and 120 min, P < 0.05). Contractions were similar before and after drug intake as reflected by similar mean firing rates of the control motor units (predrug: 10.76 ± 1.27 Hz vs. postdrug: 10.35 ± 1.94 Hz, P = 0.50) and median durations of test motor unit activation during the ascending phase of the contraction (predrug: 5.5 s vs. postdrug: 5.1 s, P = 0.26). Similar to the cyproheptadine data, a large percentage of the firing rate profile of the test unit could be accounted for by the firing rate profile of the control unit, both before and after chlorpromazine (median r2 = 0.78 predrug, 0.82 postdrug at 90 min).
In contrast to the NI controls, chlorpromazine had no effect on either the LPR (Fig. 5C) or LLR (Fig. 5D) in the cSCI participants with likely little to no residual serotonin or norepinephrine below the injury (LPR: χ2 = 1.44, P = 0.84; LLR: F = 1.16, P = 0.36). When the effects of chlorpromazine were analyzed separately in the three SCI participants with some sensory preservation (subjects 10M–12M, Table 1), the LPR and LLR across all postdrug time points were still unchanged (5 ± 4% and 0 ± 10% change, respectively), suggesting that although there was some sparing in ascending sensory pathways, there was unlikely any functional descending sources of the ligand serotonin or norepinephrine to the motoneuron in these participants. Taken together, these results suggest that chlorpromazine acts as a neutral antagonist on spinal motoneurons, decreasing 5-HT2/α1 receptor activation only if there are residual sources of the ligand present (i.e., as in NI controls) and does not reduce constitutive 5-HT2/α1 receptor activity (i.e., in chronic spinal rats and cSCI participants) that is reduced by the inverse agonist cyproheptadine.
DISCUSSION
Motoneuron PICs are facilitated by the activation of 5-HT2 and α1 receptors (Harvey et al. 2006a, 2006b; Hounsgaard et al. 1988; Hsiao et al. 2005; Perrier and Cotel 2008), specifically the 5-HT2B/C (Murray et al. 2011b) and α1A (Rank et al. 2011) receptor subtypes. In this study cyproheptadine, an inverse agonist that reduces both constitutive and ligand activation of 5-HT2/α1 receptors, reduced the PIC-mediated LLR in iSCI participants. However, citalopram, an SSRI, also increased the LLR in all of the iSCI participants tested, indicating that functional sources of serotonin are likely present in incomplete spinal cord injury (see also Thompson et al. 2011). Thus we cannot determine if the reduction of the LLR by cyproheptadine in the iSCI group was produced solely by a reduction in constitutive 5-HT2/α1 receptor activity, given that there was likely ligand activation of the receptor, as well (cf. Murray et al. 2010). When tested in motor or motor/sensory complete SCI participants with likely little to no functional sources of descending monoamines from spared or sprouted axons (see Spared descending monoaminergic tracts after motor complete SCI), cyproheptadine still reduced the LLR substantially. Moreover, the neutral antagonist chlorpromazine, which only blocks ligand activation of the 5-HT2/α1 receptor, had no effect on the LLR in the cSCI participants at oral doses that were very effective in reducing ΔF (PIC) measures in uninjured controls having descending sources of 5-HT (as shown by citalopram). The combined results showing that the inverse agonist reduced the LLR (which blocks both ligand and constitutive receptor activation) but the neutral antagonist (which only blocks ligand activation of the receptor) had no effect suggests that after motor complete SCI, PIC-mediated long-lasting reflexes (i.e., spasms) are facilitated solely by the presence of constitutive 5-HT2/α1 receptor activation on motoneurons, as previously shown in animal models of complete SCI (Murray et al. 2010; Rank et al. 2011).
Selectivity of cyproheptadine in reducing the motoneuron PIC.
Reduction of the LLR by cyproheptadine in SCI participants could have arisen by reducing the transmission of sensory afferent inputs onto the motoneuron, which then subsequently activate the motoneuron PIC to trigger a LLR or spasm (Li et al. 2004a; Norton et al. 2008). However, as shown for the iSCI participants, the SPR occurring in the first 50 ms of the reflex was not affected by cyproheptadine. This short-latency, short-lasting polysynaptic response has been shown in rats to be solely mediated by a short-lasting, sensory-evoked EPSP because it is not reduced by blocking the CaPIC with isradipine (Murray et al. 2011a) and is not affected by blocking of 5-HT2/α1 receptors (Murray et al. 2010). Moreover, because the CaPIC takes 50 ms or longer to activate (Li et al. 2004a; Murray et al. 2011a), it is unlikely that it contributes substantially to motoneuron activation during this early time. The longer latency and longer lasting (∼200 ms) LPR, on the other hand, is mediated by both a long EPSP and the CaPIC, as revealed by blocking PICs with either cell hyperpolarization or isradipine (Murray et al. 2011a). In contrast, the majority of the LLR (85–90%) is mediated by CaPIC activation. It is interesting that in the SCI participants, cyproheptadine was proportionally more effective in reducing reflex components that contained proportionally larger amounts of CaPIC activation as determined from the rat studies. For example, in iSCI participants, the SPR, containing little to no CaPIC, was reduced by 5% on average; the LPR, which is partly mediated by the CaPIC, was reduced by 30%; and the LLR, which is mainly mediated by the CaPIC, was reduced by 60%. This suggests that cyproheptadine selectively reduced the CaPIC via its action on the 5-HT2/α1 receptors.
In agreement with the PIC-mediated LLR, the more direct estimation of PIC amplitude via the ΔF measures was also reduced by cyproheptadine. Thus the ΔF measurement is sensitive to drug effects, as was also shown for the chlorpromazine data in this article and previously for amphetamine administration in uninjured control participants (Udina et al. 2010). We were able to follow the same units during voluntary contractions both before and after drug intake for the majority of the motor unit pairs (see methods, Estimation of PIC amplitude), which helped to reduce the influence of sampling bias on the ΔF measurements. Moreover, the firing rate relationship between the control and test motor units and the mean rates of the control units were not affected by the drugs, suggesting that the synaptic drive to the tibialis anterior motoneuron pool did not change after drug intake to potentially influence the ΔF measurements.
Selectivity of cyproheptadine in blocking 5-HT2/α1 receptors.
Cyproheptadine is a broad-spectrum antagonist, binding with high affinity (Ki values <30 nM) to 5-HT2 (A, B and C subtypes: Boess and Martin 1994; Bonhaus et al. 1997; Martin 1997), norepinephrine (α1: Yoshio et al. 2001), histamine (H1: Moguilevsky et al. 1994), muscarine (M1–M5: Stanton et al. 1993), and dopamine (D3: Toll et al. 1998) receptors. However, the reduction of the motoneuron PIC by cyproheptadine is mainly mediated by blocking the 5-HT2B/C and α1 receptors, because specific antagonists to these receptors completely mimic the action of cyproheptadine on the CaPIC (Harvey et al. 2006a, 2006b). Moreover, blocking the other receptors that cyproheptadine binds to, such as H1 (Harvey PJ and Bennett DJ, unpublished observations), D3 (Dai et al. 2009), and M2 (Miles et al. 2007), does not affect the motoneuron PIC. Therefore, although cyproheptadine can strongly affect dopamine, muscarine, and histamine receptors, its effects on the motoneuron, as measured by the LLR and ΔF, are likely restricted to affecting the 5-HT2 and α1 receptors.
Chlorpromazine as neutral antagonist on 5-HT2/α1 receptors.
Similar to cyproheptadine, chlorpromazine also has high affinity (Ki values <20 nM) to 5-HT2 (A and C subtypes: Boess and Martin 1994; Herrick-Davis et al. 2000; Rauser et al. 2001; Toll et al. 1998), 5-HT6 (Kroeze et al. 2003; Roth et al. 1994), norepinephrine (α1: Kroeze et al. 2003; Richelson 1988; Richelson and Nelson 1984), dopamine (D2, D3, and D4: Kroeze et al. 2003; Richelson 1988; Seeman et al. 1997; Stormann et al. 1990; Toll et al. 1998), and histamine (H1: Kroeze et al. 2003; Richelson 1988) receptors. However, as stated above, dopamine, histamine, and 5-HT6 receptors do not modulate motoneuron PIC activation (Murray et al. 2011b), so chlorpromazine's actions on these receptors would likely not affect the LLR and ΔF measurements in this study.
There is strong supportive evidence that chlorpromazine, at the doses used in this study, acted as a neutral antagonist to 5-HT2/α1 receptors on motoneurons. For example, 5 μM chlorpromazine was ineffective in suppressing the 5-HT2/α1 receptor-mediated facilitation of the LLR in chronically injured animals, where there is little to no functional source of the ligand 5-HT (Murray et al. 2010). However, this same dose was effective in decreasing the efficacy of the 5-HT2 receptor agonist, α-methyl-5-HT, in facilitating the LLR (by 30-fold), providing strong evidence that the 5 μM dose used was effective in blocking ligand activation of the 5-HT2 receptor when present. In support of this, in COS-7 cells transfected with the highly constitutive isoform of the human 5-HT2C (INI) receptor, chlorpromazine does not modulate 5-HT2C receptor activity when applied in isolation, but only suppresses the facilitation of 5-HT2C receptors after the application of the ligand 5-HT (Herrick-Davis et al. 2000). In the lumbar spinal cord, Barbeau et al. (1981) also demonstrated in chronically spinalized rats that spontaneous leg muscle activity induced by 5-hydroxytryptophan, which is known to be mediated by 5-HT2/α1 receptor facilitation of the motoneuron PIC, is suppressed by chlorpromazine (Barbeau et al. 1981). Likewise, suppression of PIC-mediated polysynaptic reflexes by chlorpromazine only occurs in acutely injured animals (Ito et al. 1982), where there are abundant stores of 5-HT present (Murrray et al. 2010), but not in chronically injured animals, where levels of the ligand 5-HT are nearly abolished. The high affinity of chlorpromazine to 5-HT2C (6.1 nM) and α1 (2.6 nM) receptors and its strong suppression of motoneuron PIC activation only when a ligand is present strongly support our claim that chlorpromazine is acting as a neutral antagonist, failing to reduce the LLR in rat spinal (and likely human) cords devoid of 5-HT but reducing the ΔF in NI controls with descending sources of 5-HT.
Constitutive 5-HT2/α1 receptor activity in iSCI.
Although we cannot prove the existence of constitutive 5-HT2/α1 receptor activity in the motor incomplete spinal cord participants using pharmacology, this does not mean that constitutive receptor activity is absent in these participants, or in the NI control participants for that matter. For instance, in unlesioned control spinal cords, ∼10% of the 5-HT2C receptors in the sacral spinal cord are of the unedited INI and weakly edited VSI isoforms (Murray et al. 2010) that display higher degrees of constitutive receptor activity compared with the more edited isoforms of the receptor (e.g., VSV). The presence of some degree of constitutive activity likely ensures a basal level of motoneuron PIC facilitation from 5-HT2/α1 receptors given that the number of brain stem serotonergic neurons with descending projections to the spinal cord is relatively low (∼3,000) in rodents compared with other descending pathways (Jacobs et al. 2002). Thus with incomplete SCI there may be an increase in the proportion of monoaminergic receptors with constitutive activity to compensate for the partial loss of serotonin and norepinephrine below the injury.
Spared descending monoaminergic tracts after motor complete SCI.
Persons with motor/sensory (AIS A) or motor (AIS B) complete injuries typically have little white matter sparing as based on tensor diffusion imaging studies (Ellingson et al. 2008; Petersen et al. 2012). In addition, patients with bilateral surgical lesions with sparse (approximately <10%) white matter sparing to the posterior half of the spinal cord, where descending monoaminergic fibers to the ventral horn are located (Holstege and Kuypers 1987; Martin et al. 1978), demonstrate remarkable recovery of leg movements and even walking function in the months following surgery (Nathan 1994). Thus persons with permanent motor or motor/sensory complete deficits, such as the participants studied presently, likely have extensive white matter damage in the posterior half of the spinal cord that contains descending monoaminergic axons. The lack of effect of the ligand-blocking neutral antagonist chlorpromazine in completely injured rats and motor complete SCI participants also supports this assumption. Future experiments demonstrating a lack of effect of serotonin/norepinephrine reuptake inhibitors on PIC-mediated reflexes evoked from spinal segments below the injury would also support this claim.
Clinical implications.
Because functional sources of monoamines are present below the level of injury following incomplete spinal cord injury, as shown from the citalopram results, motoneuron excitability is likely facilitated by both ligand and constitutive activation of monoaminergic receptors. A contribution from ligand activation of monoaminergic receptors to spasticity in incomplete spinal cord injury is evidenced by the findings that oral administration of the neutral antagonist chlorpromazine, in a controlled study, reduced muscle tone in patients with multiple sclerosis where spasticity was primarily the result of spinal cord involvement (Cohan et al. 1980; see also Basmajian and Szatmari 1955). In contrast, in motor complete SCI, it appears that motoneuron excitability is solely maintained via the activation of constitutive monoaminergic receptor activation, because we found that blocking ligand activation of monoaminergic receptors with neutral antagonists was ineffective. The slow recovery of motoneuron excitability in the first few months after SCI, especially in severe injuries, is likely due to the slow increase of constitutive 5-HT2/α1 receptors, which then reactivate the motoneuron PIC. Moreover, inhibition of excitatory sensory inputs onto the motoneuron is also reduced after SCI (Boulenguez et al. 2010; Murray et al. 2011a; Norton et al. 2008), creating a perfect storm to trigger the unchecked activation of PICs and associated involuntary muscle spasms.
The findings in this study have potentially important therapeutic implications because they point to a new target to pharmacologically reduce the unwanted activation of motoneurons producing involuntary muscle spasms. In contrast to the most commonly prescribed antispastic medications, baclofen (Lioresal) and tizanidine (Zanaflex), which act to decrease sensory afferent transmission (see Fig. 5 in discussion of accompanying article, D'Amico et al. 2012), inverse agonists to 5-HT2/α1 receptors (cyproheptadine, SB206553) exert their effects directly on the motoneuron by suppressing the activity of both ligand and constitutively active receptors to reduce the facilitation of voltage-dependent CaPICs via Gq-coupled pathways. Although severely reducing PIC activation would not be desirable in patients with incomplete SCI because it would also weaken activation of the motoneuron from spared descending inputs, this is not a concern in motor complete SCI.
Cyproheptadine has been shown to be as effective in reducing excessive muscle activation compared with clonidine and baclofen in spastic SCI patients (Nance 1994), and at a dose of 12 mg/day it was well tolerated during a 2-mo trial (Wainberg et al. 1990). Associated with cyproheptadine's antihistamine and anticholinergic properties were side effects such as drowsiness, dry mouth, appetite stimulation, and thus the potential for weight gain, although the latter has not been quantified in a closed, placebo-controlled trial in patients with SCI (see Gracies et al. 1997 for review; Tóth and Szönyi 1976). The development of more specific inverse agonists to 5-HT2/α1 receptors, with fewer side effects than cyproheptadine or SB206553 (carcinogenic by-products), may lead to better oral control of involuntary muscle spasms in SCI, especially in motor complete patients where suppression of unwanted muscle activity is the primary goal. However, moderate suppression of motoneuron excitability leading to a decrease in clonus and involuntary spasms by cyproheptadine can produce improvements in residual walking function in participants with incomplete SCI (Wainberg et al., 1990). Based on our better understanding of the mechanism of action of cyproheptadine, perhaps closed, placebo-controlled trials and long-term studies are warranted to determine the potential cost/benefits of cyproheptadine in both complete and incomplete SCI. In addition, in severe cases of spasticity where intrathecal delivery of baclofen is used (Lazorthes et al. 1990; Nielsen et al. 2002; Stempien and Tsai 2000), it may be worthwhile to test whether cyproheptadine, which acts directly on motoneurons and thus can suppress all spared aberrant inputs (descending and peripheral), is more effective compared with baclofen, which acts only to suppress sensory transmission to the motoneuron (Curtis et al. 1997; Li et al. 2004b).
GRANTS
This work was supported by the Canadian Institute of Health Research Grants MOP-106549 (to M. A. Gorassini) and MOP 14697 (to D. J. Bennett) and by National Institute of Neurological Disorders and Stroke Grants R01 NS048170 (to M. A. Gorassini) and R01 NS047567 (to D. J. Bennett). Salary support was provided by Alberta Innovates: Health Solutions (to M. A. Gorassini, D. J. Bennett, and J. M. D'Amico) and the Alberta Paraplegic Foundation (to J. M. D'Amico).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.D. and M.A.G. conception and design of research; J.M.D., K.C.M., Y.L., D.J.B., and M.A.G. performed experiments; J.M.D., K.C.M., and D.J.B. analyzed data; J.M.D., K.M.C., M.G.F., D.J.B., and M.A.G. interpreted results of experiments; J.M.D., D.J.B., and M.A.G. prepared figures; J.M.D. drafted manuscript; J.M.D., K.M.C., D.J.B., and M.A.G. edited and revised manuscript; J.M.D., K.C.M., Y.L., K.M.C., M.G.F., D.J.B., and M.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for their large time commitment and effort in this study, as well as Jennifer Nevett-Duchcherer and Leo Sanelli for excellent technical assistance.
REFERENCES
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 [DOI] [PubMed] [Google Scholar]
- Barbeau H, Filion M, Bedard P. Effects of agonists and antagonists of serotonin on spontaneous hindlimb EMG activity in chronic spinal rats. Neuropharmacology 20: 99–107, 1981 [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Szatmari A. Effect of Largactil (chlorpromazine) on human spasticity and electromyogram. Arch Neurol Psychiatry 73A: 224–231, 1955 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001 [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk-Butler K, Aleksic I, Kennedy SH. Citalopram-a review of pharmacological and clinical effects. J Psychiatry Neurosci 25: 241–254, 2000 [PMC free article] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology 33: 275–317, 1994 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective 5-HT2C receptor antagonist. Neuropharmacology 36: 621–629, 1997 [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010 [DOI] [PubMed] [Google Scholar]
- Cohan SL, Raines A, Panagakos J, Arimitage P. Phenytoin and chlorpromazine in the treatment of spasticity. Arch Neurol 37: 360–364, 1980 [DOI] [PubMed] [Google Scholar]
- Curtis DR, Gynther BD, Lacey G, Beattie DT. Baclofen: reduction of presynaptic calcium influx in the cat spinal cord in vivo. Exp Brain Res 113: 520–533, 1997 [DOI] [PubMed] [Google Scholar]
- D'Amico J, Li Y, Bennett DJ, Gorassini MA. Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans. J Neurophysiol (December 5, 2012). doi:10.1152/jn.00822.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Carlin KP, Li Z, McMahon DG, Brownstone RM, Jordan LM. Electrophysiological and pharmacological properties of locomotor activity-related neurons in cfos-EGFP mice. J Neurophysiol 102: 3365–3383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol 29: 1976–1982, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasticity after spinal cord injury. Brain 127: 2247–58, 2004 [DOI] [PubMed] [Google Scholar]
- Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl 6: S92–S120, 1997 [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behaviour. Trends Neurosci 26: 688–695, 2003 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 295: 226–232, 2000 [PubMed] [Google Scholar]
- Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience 23: 809–821, 1987 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurons in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Wu N, Chandler SH. Voltage-dependent calcium currents in trigeminal motoneurons of early postnatal rats: modulation by 5-HT receptors. J Neurophysiol 94: 2063–2072, 2005 [DOI] [PubMed] [Google Scholar]
- Hyttel J. Citalopram—pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry 6: 277–295, 1982 [DOI] [PubMed] [Google Scholar]
- Ito T, Furukawa K, Karasawa T, Kadokawa T, Shimizu M. Effects of chlorpromazine, imipramine and baclofen on the spinal polysynaptic reflex in acute, chronic and 6-hydroxydopamine-treated spinal rats. Jpn J Pharmacol 32: 1125–1133, 1982 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev 40: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28: 519–526, 2003 [DOI] [PubMed] [Google Scholar]
- Lazorthes Y, Sallerin-Caute B, Verdie JC, Bastide R, Carillo JP. Chronic intrathecal baclofen administration for control of severe spasticity. J Neurosurg 72: 393–402, 1990 [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004b [DOI] [PubMed] [Google Scholar]
- Martin GR. Pre-clinical pharmacology of zolmitriptan (Zomig; formerly 311C90), a centrally and peripherally acting 5HT1B/1D agonist for migraine. Cephalalgia 17, Suppl 18: 4–14, 1997 [DOI] [PubMed] [Google Scholar]
- Martin RF, Jordan LM, Willis WD. Differential projections of cat medullary raphe neurons demonstrated by retrograde labelling following spinal cord lesions. J Comp Neurol 182: 77–88, 1978 [DOI] [PubMed] [Google Scholar]
- Mendes GD, Arruda A, Chen LS, de Almeida Magalhaes JC, Alkharfy KM, De Nucci M. Quantification of cyproheptadine in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry in a bioequivalence study. Biomed Chromatogr 26: 129–136, 2012 [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals 17: 42–54, 2009 [DOI] [PubMed] [Google Scholar]
- Moguilevsky N, Varsalona F, Noyer M, Gillard M, Guillaume JP, Garcia L, Szpirer C, Szpirer J, Bollen A. Stable expression of human H1-histamine-receptor cDNA in Chinese hamster ovary cells: pharmacological characterisation of the protein, tissue distribution of messenger RNA and chromosomal localisation of the gene. Eur J Biochem 224: 489–495, 1994 [DOI] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D'Amico J, Gorassini MA, Bennett DJ. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106: 925–943, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105: 731–748, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico JM, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance PW. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 17: 150–156, 1994 [DOI] [PubMed] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain 117: 337–346, 1994 [DOI] [PubMed] [Google Scholar]
- Nielsen JF, Hansen HJ, Sunde N, Christensen JJ. Evidence of tolerance to baclofen in treatment of severe spasticity with intrathecal baclofen. Clin Neurol Neurosurg 104: 142–145, 2002 [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca channels. Brain Res Rev 40: 223–229, 2002 [DOI] [PubMed] [Google Scholar]
- Perrier JF, Cotel F. Serotonin differentially modulates the intrinsic properties of spinal motoneurons from the adult turtle. J Physiol 586: 1233–1238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 29: 1556–1566, 2012 [DOI] [PubMed] [Google Scholar]
- Rank MM, Murray KC, Stephens MJ, D'Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol 105: 410–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5HT2C receptor. J Pharmacol Exp Ther 299: 83–89, 2001 [PubMed] [Google Scholar]
- Richelson E. Neuroleptic binding to human brain receptors: relation to clinical effects. Ann NY Acad Sci 537: 435–442, 1988 [DOI] [PubMed] [Google Scholar]
- Richelson E, Nelson A. Antagonism by neuroleptics of neurotransmitter receptors of normal human brain in vitro. Eur J Pharmacol 103: 197–204, 1984 [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long-latency spinal reflex in main after flexor reflex afferent stimulation. Brain 110: 707–725, 1987 [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther 268: 1403–1410, 1994 [PubMed] [Google Scholar]
- Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology 16: 93–110, 1997 [DOI] [PubMed] [Google Scholar]
- Stanton T, Bolden-Watson C, Cusack B, Richelson E. Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol 45: 2352–2354, 1993 [DOI] [PubMed] [Google Scholar]
- Stempien L, Tsai T. Intrathecal baclofen pump use for spasticity: a clinical survey. Am J Phys Med Rehabil 79: 536–541, 2000 [DOI] [PubMed] [Google Scholar]
- Stormann TM, Gdula DC, Weiner DM, Brann MR. Molecular cloning and expression of a dopamine D2 receptor from human retina. Mol Pharmacol 37: 1–6, 1990 [PubMed] [Google Scholar]
- Thompson CK, Jayaraman A, Kinnaird C, Hornby TG. Methods to quantify pharmacologically induced alterations in motor function in human incomplete SCI. J Vis Exp 50: 2148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedey JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178: 440–466, 1998 [PubMed] [Google Scholar]
- Tóth K, Szönyi A. The appetite stimulating and weight gain promoting effect of Peritol (cyproheptadine) examined on a great number of outpatients. Ther Hung 24: 24–32, 1976 [PubMed] [Google Scholar]
- Udina E, D'Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor unit activity. J Neurophysiol 103: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 53: 754–763, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung PKF, Hubbard JW, Korchinski ED, Midha KK. Pharmacokinetics of chlorpromazine and key metabolites. Eur J Clin Pharmacol 45: 563–569, 1993 [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol 86: 189–195, 2001 [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol 115: 1717–1729, 2004 [DOI] [PubMed] [Google Scholar]