Abstract
Activation of receptors by serotonin (5-HT1) and norepinephrine (α2) on primary afferent terminals and excitatory interneurons reduces transmission in spinal sensory pathways. Loss or reduction of descending sources of serotonin and norepinephrine after spinal cord injury (SCI) and the subsequent reduction of 5-HT1/α2 receptor activity contributes, in part, to the emergence of excessive motoneuron activation from sensory afferent pathways and the uncontrolled triggering of persistent inward currents that depolarize motoneurons during muscle spasms. We tested in a double-blind, placebo-controlled study whether facilitating 5-HT1B/D receptors with the agonist zolmitriptan reduces the sensory activation of motoneurons during an H-reflex in both noninjured control and spinal cord-injured participants. In both groups zolmitriptan, but not placebo, reduced the size of the maximum soleus H-reflex with a peak decrease to 59% (noninjured) and 62% (SCI) of predrug values. In SCI participants we also examined the effects of zolmitriptan on the cutaneomuscular reflex evoked in tibialis anterior from stimulation to the medial arch of the foot. Zolmitriptan, but not placebo, reduced the long-latency, polysynaptic component of the cutaneomuscular reflex (first 200 ms of reflex) by ∼50%. This ultimately reduced the triggering of the long-lasting component of the reflex (500 ms poststimulation to end of reflex) known to be mediated by persistent inward currents in the motoneuron. These results demonstrate that facilitation of 5-HT1B/D receptors reduces sensory transmission in both monosynaptic and polysynaptic reflex pathways to ultimately reduce long-lasting reflexes (spasms) after SCI.
Keywords: serotonin, H-reflex, cutaneomuscular, spasticity, zolmitriptan
after a complete spinal cord injury (SCI), levels of serotonin and norepinephrine below the lesion decrease greatly because the major supply of these neuromodulators comes from descending pathways originating in the brain stem (Anden et al. 1964; Carlsson et al. 1963; Jacobs et al. 2002; Jordan et al. 2008; Rekling et al. 2000). Serotonin and norepinephrine normally inhibit transmission of ascending and segmental sensory pathways via the activation of Gi-coupled serotonin (5-HT1) and norepinephrine (α2) receptors located on sensory afferent terminals and excitatory interneurons (Clarke et al. 2002; Di Pasquale et al. 1997; Engberg et al. 1968; Jankowska et al. 1993; Jordan et al. 2008; Manuel et al. 1995; Millan 2002; Rekling et al. 2000; Singer et al. 1996; Yoshimura and Furue 2006). The reduction of 5-HT1 and α2 receptor activation (Murray et al. 2011; Rank et al. 2011) results in the enhanced transmission of low-threshold, cutaneomuscular afferent pathways to produce abnormally long (∼1 s) excitatory postsynaptic potentials (EPSPs) in response to brief sensory stimulation in both rat and human motoneurons (Li et al. 2004a; Norton et al. 2008). These long polysynaptic EPSPs contribute to spasticity after SCI by triggering slowly activating calcium-mediated persistent inward currents (CaPICs) that drive self-sustained motoneuron firing and involuntary muscle spasms (Bennett et al. 2001b; Gorassini et al. 2004; Heckman et al. 2008; Li et al. 2004a).
One strategy to reduce muscle spasticity after SCI has been to facilitate the activation of Gi-coupled pathways to reduce sensory transmission to motoneurons. The common antispastic medication baclofen, a GABAb receptor agonist, and clonidine/tizanidine, α2-receptor agonists, activate Gi-coupled pathways to reduce cAMP and Ca2+ entry into synaptic terminals (Curtis et al. 1997). This ultimately results in reducing the duration of polysynaptic EPSPs from about 1 s to less than 50 ms, and as a consequence the CaPIC is not activated and muscle spasms are reduced (Li et al. 2004b; Murray et al. 2011; Rank et al. 2011). However, both baclofen and tizanidine produce undesirable side effects such as tolerance, sedation, and hypotension (Davidoff 1985; Gracies et al. 1997; Krach 2001; Meleger 2006; Rosche 2002) and thus are not optimal oral antispastics. Recently, another strategy to activate Gi-coupled pathways via 5-HT1 receptors has been tried where both short- and long-latency polysynaptic EPSPs were reduced by either 5-HT1B or 5-HT1F receptor facilitation (Murray et al. 2011), leading to a reduction in CaPIC activation and muscle spasms. Application of the various 5-HT1B/F receptor agonists did not reduce the CaPIC itself, demonstrating that their antispastic effects were mediated by reducing the duration of sensory synaptic activation of the motoneuron so that the slowly activating CaPICs were not recruited.
One of the 5-HT1 receptor agonists tested in the Murray et al. (2011) study was zolmitriptan, a 5-HT1B/D receptor agonist that is approved for human use to treat migraines (Martin 1997; Martin et al. 1997; Peterlin and Rapoport 2007). Although zolmitriptan cannot be used on a daily basis due to adverse side effects, and thus cannot be used as an antispastic, we tested as a proof of principle whether facilitation of 5-HT1B/D receptors in humans also reduces segmental sensory transmission to motoneurons in both noninjured control and SCI participants. To examine the activation of the motoneuron by sensory inputs in isolation without CaPIC activation, we examined the effect of zolmitriptan on the soleus monosynaptic H-reflex. The EPSP produced during a monosynaptic reflex is very short, ∼5–10 ms in rat and cat motoneurons (Baker and Chandler 1987; Edwards et al. 1989; Jimenez et al. 1991; Li et al. 2004b), and is estimated to be ∼30 ms in humans on the basis of motor unit recordings (Miles et al. 1989). Thus depolarization of the motoneuron during an H-reflex is too brief (<50 ms) to activate a CaPIC, and any reduction in motoneuron output from zolmitriptan would likely be due to a reduction in its sensory synaptic activation. We also examined in SCI participants whether long-lasting polysynaptic reflexes activated by cutaneomuscular stimulation to the medial arch of the foot, as well as the subsequent long-lasting reflexes (spasms) they trigger, were also reduced by zolmitriptan to determine if facilitation of 5-HT1B/D receptors is a potential strategy to reduce spasticity after SCI. Some of these data have been published in abstract form (D'Amico and Gorassini 2012).
METHODS
Experiments were approved by the Health Research Ethics Board at the University of Alberta and conformed to the Declaration of Helsinki. The off-label use of the antimigraine drug zolmitriptan in noninjured and SCI participants was approved by Health Canada Clinical Trials. All participants gave written, informed consent before participating in the study. In total, six noninjured control (35 ± 13 yr, 2 female) and seven SCI participants with motor complete injuries (35 ± 10 yr, 2 female) took part in the study (Table 1). Three of the seven SCI participants (subjects 1M–3M in Table 1) also took part in the 5-HT2 receptor study of D'Amico et al. (2012). Drug screens were performed to rule out contraindications for zolmitriptan and to ensure participant safety. Five other SCI participants were excluded from the study due to drug contraindications from antidepressants, and one subject was excluded due to a blood clotting disorder that made it unsafe to administer zolmitriptan. An additional two SCI participants were excluded from the study (both motor complete, T11–T12 and T6–T7) because appreciable H-reflexes could not be evoked from either leg.
Table 1.
Demographics of SCI participants
| Preserved Sensation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject Code | Age, yr | Age of Injury, yr | Injury Level | AIS | Cause | MAS | Penn Spasm Scale | Light touch | Pinprick |
| 1M | 29 | 7 | C4–C5 | B | Trauma | 1 | 3 | Left: none | Left: outer shin only |
| Right: upper shin and foot only | Right: ✓ | ||||||||
| 2M | 31 | 1.5 | T3–T4 | B | Trauma | 0 | 3–4 | Left: ✓ except front of shin | Left: ✓ |
| Right: none | Right: inner side of leg only | ||||||||
| 3M | 33 | 13 | C5–C6 | B | Trauma | 1 | 3 | Left: ✓ | Left: ✓ |
| Right: ✓ | Right: ✓ | ||||||||
| 4F | 33 | 11 | C6–C7, L3 | B | Trauma | 0 | 3 | Left: ✓ | Left: ✓ |
| Right: ✓ | Right: ✓ | ||||||||
| 5M | 58 | 5 | C6–C7 | B | Trauma | 1+ | 2 | Left: ✓ | Left: ✓ |
| Right: ✓ | Right: ✓ | ||||||||
| 6M | 32 | 3 | C7 | A | Trauma | 1 | 2 | Left: none | Left: none |
| Right: none | Right: none | ||||||||
| 7F | 33 | 15 | T7 | B | Trauma | 4 | 2 | Left: ✓ | Left: ✓ |
| Right: ✓ | Right: ✓ | ||||||||
Description of spinal cord-injured (SCI) participants including age of participant and age of injury at the time of experiment, injury level, ASIA Impairment Scale (AIS), cause of injury, Modified Ashworth Score (MAS; 0–4), and Penn Spasm Frequency Scale (0–4). Final columns describe spared light touch and pinprick sensation of lower leg: check mark indicates preserved sensation from tested area of knee downward.
Drug administration.
All participants were required to come to the laboratory on two separate occasions (separated by at least 1 wk) to receive placebo or the drug zolmitriptan in random order. Drug and placebo were housed in a two-part telescoping capsule to conceal the identity of the drug. Noninjured control and SCI participants received a 10-mg dose of the 5-HTB/D agonist zolmitriptan (Proietti-Cecchini et al. 1997; Visser et al. 1996; Werhahn et al. 1998). Placebo was a sugar pill with similar weight to the zolmitriptan tablets. JDA, who performed the data analysis, was also blinded until data analysis was completed. Heart rate and blood pressure were measured before and every 60 min after drug intake. Participants were asked to report any physiological sensations experienced after taking zolmitriptan or placebo. Because plasma concentrations of zolmitriptan are detectable at 15 min after oral intake with peak concentrations occurring at 2–4 h (Peterlin and Rapoport 2007), reflex recordings were taken every 30 min for 2 h after drug intake. This allowed us to examine the onset of the drug effect and make measurements near the time of peak plasma concentrations. In pilot experiments, taking reflex recordings beyond 2 h after drug intake was too fatiguing for the participants.
H-reflex recordings.
H-reflex recordings were obtained in both noninjured and SCI participants. H-reflexes were evoked in the soleus muscle because they are readily elicited at rest, which was important for the SCI participants since they could not produce voluntary contractions. All noninjured participants, and SCI participants who were able to transfer safely, were placed in a supine position on a padded table. Two SCI participants were examined in a reclined position in their powered wheelchair (subjects 1M and 4F in Table 1). Two surface electrodes (2.2 × 3.3 cm; Kendall Soft-E, Chicopee, MA) were placed over the right soleus muscle to record electromyography (EMG) signals. The soleus H-reflex was evoked by stimulating the tibial nerve (DS7A constant-current stimulator NL703; Digitimer, Welwyn Garden City, UK) through a monopolar electrode once the best position was found with a probe electrode (1-ms pulse width, return electrode placed over patella). The surface EMG signal was amplified 200 or 1,000 times (depending on the size of the response) and filtered using a bandpass of 20–2,500 Hz (model 2024F; Intronix Technologies, Bolton, ON, Canada). All signals were digitized at a rate of 5 kHz using Axoscope hardware and software (Digidata 1440 Series; Molecular Devices, Sunnyvale, CA) and stored on a personal computer for off-line analysis.
H-reflex recruitment curves.
H-reflex responses were evoked at incrementing stimulus intensities to produce a recruitment curve before (2 baseline curves) and 30, 60, 90, and 120 min after drug/placebo intake. Before each H-reflex recruitment curve was produced, the motor threshold (MT) was determined online as the stimulation intensity required to elicit an M-wave of ∼100 μV. The stimulation intensity was expressed as a multiple of motor threshold (×MT) and was set from below H-reflex threshold (ranging from 0.5 to 0.7 ×MT) to when the H-reflex decreased after its peak (ranging from 1.2 to 1.6 ×MT) in steps of 0.05 ×MT. This ensured that a minimum of eight to nine points were collected along the steep portion of the H-reflex recruitment curve. Five reflexes were evoked at each stimulation intensity. Stimuli were delivered every 3 s, which allowed enough time to manually increase the stimulation intensity after every fifth trial. The maximal motor response (Mmax) was measured after each recruitment curve.
H- and M-wave amplitudes were measured as peak to peak using custom-written software in Matlab (The MathWorks, Natick, MA). The five H- and M-wave amplitudes, evoked at each stimulation intensity, were averaged together and normalized to Mmax. The amplitude of the normalized H- and M-waves was plotted at each stimulation intensity, with the latter expressed as a function of MT, to produce H- and M-wave recruitment curves. To standardize the measurement of MT across the different time points and participants, MT was recalculated off-line using the x-intercept method (as per Kerr and Vujnovich 2002; Lundbye-Jensen and Nielsen 2008). Briefly, the steep portion of the M-wave recruitment curve was fitted with a straight line, and its x-intercept was calculated as the new MT, producing better alignment of the M-wave recruitment curves. The H-reflex recruitment curve (up to its peak) was fitted with a three-parameter sigmoid function: H(s) = Hmax/[1 + em(S50 − s)] (Klimstra and Zehr 2008). The peak H-reflex (Hmax) and the stimulation intensity producing 50% of Hmax (S50) were measured off the fitted curve. The slope parameter m was too variable because it depended on the number of points along the recruitment curve, and therefore it was not analyzed (see also Klimstra and Zehr 2008). Typically, 98% of the variance in the H-reflex recruitment curve was accounted for by the sigmoidal fit with r2 values ranging from 0.92 to 0.99 (median = 0.99) in noninjured controls and from 0.87 to 0.99 (median = 0.98) in SCI participants. The threshold to evoke an H-reflex (Hthresh) was measured as the stimulus intensity required to elicit an H-reflex that was 5% of Hmax. Because there was large variability in the size of H-reflexes between SCI participants, the three parameters of the H-reflex recruitment curve (Hmax, S50, and Hthresh) at the 30-, 60-, 90-, and 120-min time points were expressed as a percentage of the predrug value and averaged across subjects.
Cutaneomuscular reflex recordings.
Cutaneomuscular reflexes were recorded in SCI participants only because long-lasting responses (>1 s) cannot be evoked in noninjured control participants. Cutaneomuscular reflexes were evoked in the tibialis anterior (TA) muscle because it has previously been shown that long-lasting responses, likely mediated by CaPIC activation, are readily produced in the TA after SCI (Norton et al. 2008). Cutaneomuscular afferents supplying the side and sole of the foot were stimulated with long pulse trains applied to the medial arch of the foot (300 Hz, 14 pulses, 0.5-ms pulse width; DS7A constant-current stimulator) at an intensity that was just below pain threshold (40 ± 15 mA on average). Surface EMG signals from the TA were amplified 1,000 times and filtered using a bandpass of 20–2,500 Hz (model 2024F; Intronix Technologies). Both limbs were tested, and the TA muscle that exhibited the longest reflex response predrug was used. In most SCI participants, the right TA had the longest responses, except for participants 2M and 5M. Stimulation was repeated 6 times every 6 s for each trial. Three to four predrug reflex responses were recorded until two consecutive responses fell within 10% of each other. These last two predrug reflex responses were averaged together to form the baseline reflex response. Cutaneomuscular reflex recordings were repeated at 30, 60, 90, and 120 min after drug intake and were performed immediately after each H/M recruitment curve.
Cutaneomuscular reflex analysis.
The cutaneomuscular reflex was divided into two components: a long-latency polysynaptic reflex (LPR) and a long-lasting reflex (LLR) as per Murray et al. (2010, 2011) and Rank et al. (2011). The LPR, which includes the start of the reflex response up until 300 ms after the first stimulation pulse, contains a mixture of both sensory-evoked EPSPs and CaPIC activation, because its amplitude is reduced to ∼50% by the Ca2+ channel blocker isradipine (Li et al. 2004a; Murray et al. 2011). The average latency of the LPR was 84 ± 14 ms, with an average duration of 215 ± 15 ms. The later, long-lasting reflex component (LLR) was defined as the time window from 500 ms after the first stimulation pulse to the end of the reflex response in the predrug trial, as per Murray et al. (2010, 2011) and Rank et al. (2011) (LLR duration: 400 ± 158 ms). Thus the LLR represents a period where most of the sensory synaptic drive to the motoneuron (i.e., EPSP) has subsided and is produced mainly by a depolarization from the CaPIC (Li et al. 2004a; Norton et al. 2008).
In Matlab, each EMG trace was first rectified and the mean EMG was calculated for the time windows of the two reflex components (LPR: start of reflex to 300 ms poststimulation; LLR: 500 ms poststimulation to end of predrug response). The mean rectified background noise, measured from 100 ms before the stimulation, was subtracted from the data. The mean EMG values for each of the six sweeps were averaged together to obtain LPR and LLR values for each time point. All values were expressed as a percentage of the predrug value and then averaged across subjects for each experimental session (zolmitriptan or placebo).
In vitro monosynaptic and polysynaptic reflex recordings.
To examine the effects of zolmitriptan applied directly to the spinal cord on monosynaptic reflexes that are similar to the H-reflexes recorded in our human participants, we used the in vitro sacral spinal cord preparation (Bennett et al. 2001a; Li and Bennett 2003). Under urethane anesthesia (1.8 g/kg), the whole spinal cord caudal to S2 (sacral) was removed from chronic spinal rats and immersed in oxygenated artificial cerebrospinal fluid (ACSF; flowing 8 ml/min); recordings were made starting 2.5 h later, as detailed previously (Bennett et al. 2001a; Li and Bennett 2003). Ventral (S4 and Co1, coccygeal) and dorsal (Co1) roots were mounted on silver wires above the ACSF and covered with Vaseline. The dorsal root was stimulated with a single pulse (0.1 ms, 0.02 mA: ∼3 times sensory afferent threshold; repeated 5 times every 10 s for 1 trial, with trials repeated every 12 min). With this stimulation, a monosynaptic reflex with a latency of 2 ms and lasting for ∼4 ms was evoked in the ventral roots. A 300 nM dose of zolmitriptan (AstraZeneca, Mississauga, ON, Canada) was used, a dose in the whole sacral spinal cord that is known to reduce polysynaptic EPSPs (Murray et. al. 2011).
Statistical analysis.
All statistical analysis was performed using SigmaPlot 11 software. Values are expressed in the text as means ± SD and in Figs. 2 and 3 as means ± SE. Normality for the parameters of the H-reflex recruitment curve (Hmax, S50, and Hthresh) and for the LPR and LLR components of the cutaneomuscular reflexes was first tested with the Shapiro-Wilk test. For each separate experiment (placebo or zolmitriptan), a one-way repeated-measures ANOVA for normally distributed data and a one-way repeated-measures ANOVA on ranks (χ2 test) for nonnormally distributed data were used to determine if there was an effect of the drug on the reflex parameters over the 30-, 60-, 90-, and 120-min time points. To compare between experiments and to determine whether placebo and zolmitriptan had different effects on the reflex parameters, a two-way repeated-measures ANOVA was used with the within-subject factors drug and time. A post hoc Holm-Sidak test, which corrects for multiple comparisons, was used to determine at which time points the zolmitriptan data differed from the placebo data. Significance was set to P < 0.05 in all cases.
Fig. 2.
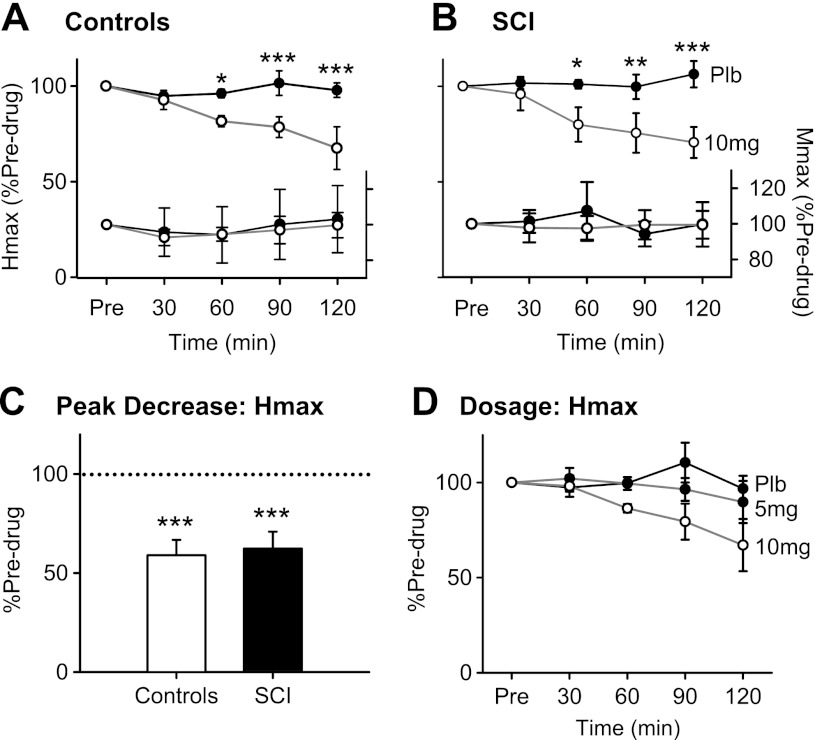
Group data: Hmax and Mmax after zolmitriptan. A, top: averaged Hmax, expressed as a percentage of predrug values, at 30, 60, 90, and 120 min after zolmitriptan (open circles) and placebo (Plb; filled circles) intake in 6 uninjured control participants. Bottom, average Mmax expressed as a percentage of predrug values at all time points after zolmitriptan (open circles) and placebo (filled circles). B: same as in A but for averaged data across the 7 SCI participants. C: peak decrease in Hmax, expressed as a percentage of predrug values, irrespective of time after zolmitriptan intake for both uninjured control (open bar) and SCI participants (filled bar). D: averaged Hmax, expressed as a percentage of predrug values, for 3 uninjured control participants receiving placebo (filled circles, black line), 5 mg of zolmitriptan (filled circles, gray line), and 10 mg of zolmitriptan (open circles, gray line), respectively. Error bars represent means ± SE. *P < 0.05; **P < 0.01; ***P < 0.005.
Fig. 3.
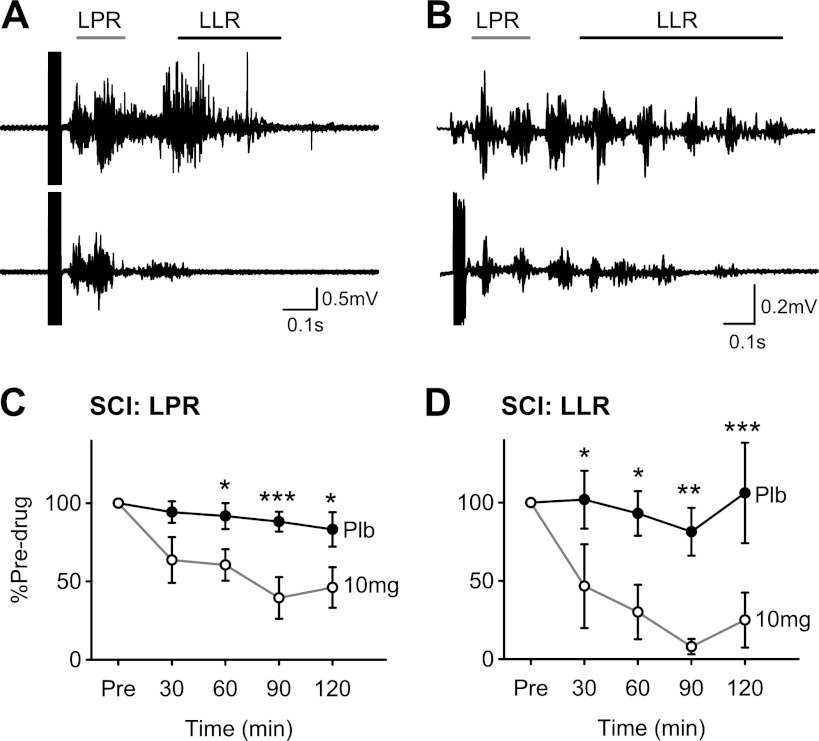
Zolmitriptan and CMR in SCI participants. A: overlay of 6 unrectified tibialis anterior (TA) EMG traces before (top traces) and after 10 mg of zolmitriptan (bottom traces) in a single C6–C7 SCI participant (subject 5M in Table 1). Gray bar denotes the window calculated for the long polysynaptic reflex (LPR; start of reflex to 300 ms), and black bar denotes the long-lasting reflex (LLR; 500 ms poststimulation to end of reflex). B: similar to A but for C6–C7/L3 SCI participant (subject 4F in Table 1). C: averaged LPR, expressed as a percentage of predrug values, at 30, 60, 90, and 120 min after 10 mg of zolmitriptan (open circles) and placebo (filled circles) in 6 of the 7 SCI participants (subjects 1M–6M in Table 1). D: same as in C but for the LLR in 5 of the 7 SCI participants (subjects 1M–5M in Table 1). Error bars represent means ± SE. *P < 0.05; **P < 0.01; ***P < 0.005.
RESULTS
Effects of zolmitriptan on the H-reflex recruitment curve.
A 10-mg oral dose of the 5-HT1B/D receptor agonist zolmitriptan reduced the amplitude of the maximum H-reflex (Hmax) in both noninjured and SCI participants. The peak-to-peak amplitude of Hmax was reduced 120 min after zolmitriptan intake at similar stimulation intensities to those predrug as reflected in the matched M-wave before (black trace) and after (gray trace) drug intake for both noninjured control (Fig. 1A) and SCI participants (Fig. 1C). As shown for the participants in Fig. 1, the average nonnormalized Hmax measured before zolmitriptan intake was significantly larger in controls (2.95 ± 1.36 mV) compared with SCI participants (1.35 ± 1.31 mV, P = 0.05). Likewise, Mmax was larger in controls (6.00 ± 2.50 mV) compared with SCI participants (3.10 ± 1.58 mV, P = 0.03), resulting in Hmax-to-Mmax ratios being similar between the two groups (controls: 0.51 ± 0.17; SCI: 0.42 ± 0.25, P = 0.46).
Fig. 1.
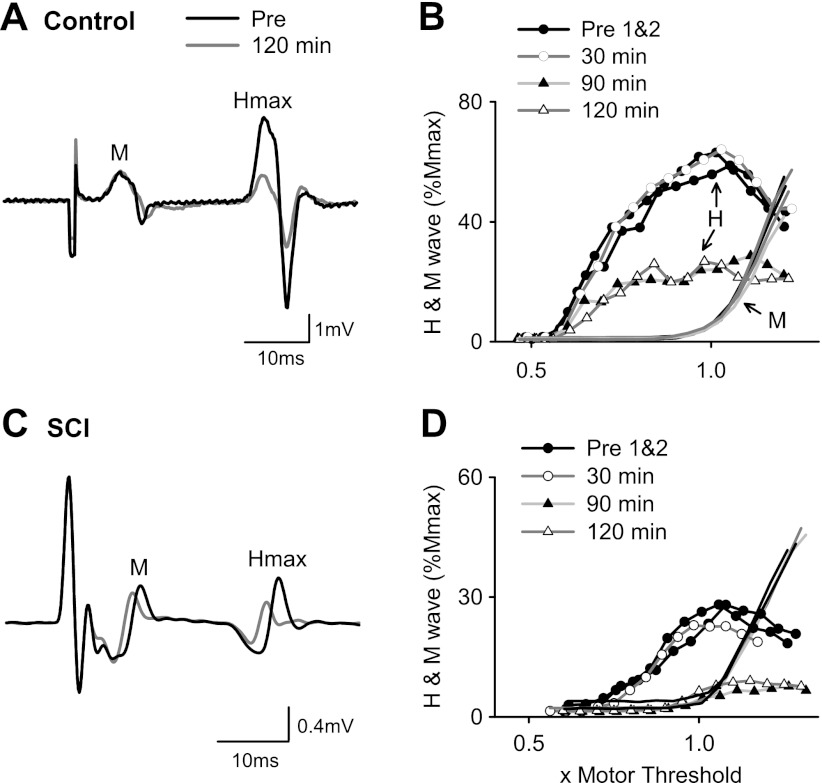
H-reflexes after zolmitriptan in uninjured control and spinal cord-injured (SCI) participants. A: M-wave (M) and maximum H-reflex (Hmax) recorded in a single uninjured control participant before (black trace) and 120 min after (gray trace) 10 mg of zolmitriptan. B: corresponding H-reflex and M-wave recruitment curve from same participant in A plotted peak to peak and normalized to Mmax. Stimulation intensity is expressed as a multiple of motor threshold. The 2 predrug H-wave recruitment curves (Pre1&2) are represented by black lines and solid circles, the 30-min curve by a dark gray line and open circles, the 90-min curve by a light gray line and solid triangles, and the 120 min curve by a dark gray line and open triangles (data at 60 min not shown for clarity). M-wave recruitment curves have similar line colors but with no symbols for clarity. C and D: similar to A and B but for a T3–T4 SCI participant (subject 2M in Table 1).
As shown from the corresponding H-reflex recruitment curves from the these two participants (Fig. 1, B and D), zolmitriptan mainly affected the amplitude of the H-reflex and not its overall excitability, because there were no lateral shifts in the recruitment curve plotted as a function of MT. The reduction in H-reflex size occurred even though the M-wave recruitment curves remained unchanged, signifying a reduction in the transmission of Ia afferent pathways to the soleus motoneuron pool. As in most subjects, the decrease in H-reflex amplitude was most pronounced at 90 and 120 min after drug intake (triangles). In four of six noninjured controls and in five of seven SCI participants, the H-reflex was suppressed at all stimulation intensities, as shown for the two participants in Fig. 1, B and D. In the remaining participants, H-reflexes began to decrease near S50, the stimulation intensity producing half of Hmax. H-reflex recruitment curves before zolmitriptan intake (Pre1&2: solid circles, black lines) and at 30 min postdrug (open circles, dark gray line) were reproducible, suggesting that the H-reflex did not spontaneously decrease over time, similar to the recruitment curves at all time points after placebo intake (data not shown).
Group data: H-reflex recruitment curve.
When the normalized Hmax value was plotted as a percentage of the predrug value across the different time points, Hmax after zolmitriptan intake (solid circles) deviated from placebo values (open circles) at 60 min and onward, with Hmax being reduced to 67.5 ± 0.27% and 70.6 ± 0.21% of predrug values at 120 min in noninjured controls (Fig. 2A) and SCI participants (Fig. 2B), respectively. There was a significant reduction in Hmax over time after zolmitriptan intake compared with predrug (controls: F = 4.77, P = 0.007; SCI: F = 3.318, P = 0.027) but not after placebo (controls: F = 0.62, P = 0.65; SCI: F =0.42, P = 0.79). Two-way ANOVA revealed a significant drug × time interaction (F =3.507, P = 0.025), with post hoc tests showing Hmax after zolmitriptan intake was significantly smaller compared with that after placebo at the 60-, 90-, and 120-min time points (all P < 0.05). There were no significant increases in Mmax over time (expressed as a percentage of predrug value, Fig. 2, A and B, bottom) after either placebo or zolmitriptan in both controls and SCI participants (all F and χ2 > 0.55 and 1.86, respectively, all P > 0.33), indicating that the observed decreases in the normalized Hmax did not result from dividing Hmax by a steadily increasing Mmax.
In some subjects, the peak reduction in Hmax did not occur at 120 min after zolmitriptan intake but earlier at 90 (n = 3 control, n = 1 SCI) or 60 min (n = 1 control, n = 1 SCI). Thus, when the peak decrease in Hmax occurring at either of these time points (60, 90 or 120 min) was plotted, the Hmax as a percentage of the predrug value was even lower at 58.9 ± 0.1% for controls and 62.3 ± 0.23% for SCI participants (Fig. 2C), with the peak decrease in Hmax similar between the two groups (P = 0.78). In three preliminary control participants, the dosage of zolmitriptan needed to be at least 10 mg to show a reduction in Hmax given that 5 mg, like placebo, did not produce a decrease in Hmax (Fig. 2D). Finally, as reflected in the recruitment curves of Fig. 1, there were no changes in Hthresh or S50 for noninjured control and SCI participants after zolmitriptan or placebo (all F > 0.16, all P > 0.12).
Cutaneomuscular reflex in SCI.
Long-duration reflex responses (spasms) were evoked in the TA muscle in response to a train of pulses (300 Hz, 14 pulses, 0.5-ms pulse width) applied to the medial arch of the foot as shown for the two participants represented in Fig. 3, A and B. Both the long polysynaptic component of the reflex (LPR, marked by gray bar), which is mediated by both sensory-evoked EPSPs and PICs, and the long-lasting reflex component (LLR, marked by black bar), which is mainly mediated by PICs (see methods, Cutaneomuscular reflex analysis for rationale) were reduced by zolmitriptan. It was possible to evoke long-duration reflexes in six of the seven motor complete SCI participants. Similar to the H-reflex, zolmitriptan reduced the size of the LPR over time (Fig. 3C), decreasing it to 46.1 ± 0.32% of predrug values at 120 min (F = 8.92, P < 0.001). In comparison, there was no decrease of the LPR after placebo intake (χ2 = 7.07, P = 0.13). A two-way ANOVA revealed a significant drug × time interaction (F = 5.325, P = 0.004), with the LPR after zolmitriptan significantly smaller compared with placebo at the 60-, 90-, and 120-min time points (P < 0.05). The reduced LPR consequently resulted in a reduced or nearly abolished LLR (spasm) after zolmitriptan (F = 7.26, P = 0.002) but not placebo (χ2 = 3.68, P = 0.45), with the LLR being reduced to 25.0 ± 0.39% of its predrug value at 120 min. Two-way ANOVA revealed a significant drug × time interaction (F = 3.67, P = 0.026), with the LLR significantly smaller after zolmitriptan compared with placebo at the 30-, 60-, 90-, and 120-min time points (P < 0.05).
Monosynaptic reflexes after direct application of zolmitriptan to rat spinal cord.
Because zolmitriptan was given orally in the control and SCI participants, this leaves open the possibility that the reduction in H-reflexes could have been due, in part, to systemic actions of the drug on 5-HT1B/D receptors located on blood vessels in the spinal cord, a distinct possibility given that the main clinical use of zolmitriptan is to reduce vasodilatation during migraines (Martin 1997; Peterlin and Rapoport 2007). Therefore, we examined the effects of applying zolmitriptan directly to the spinal cord on monosynaptic reflexes evoked from Co1 dorsal root stimulation in an in vitro sacral spinal cord preparation (see methods). When 300 nM zolmitriptan was applied directly to the spinal cord, the amplitude of the monosynaptic reflex was reduced (Fig. 4A), similar to that seen for the H-reflex in human participants (Fig. 1). Zolmitriptan reduced the size of the monosynaptic reflex by 40% or more in five of five rats tested at 15 min after bath application of the drug.
Fig. 4.
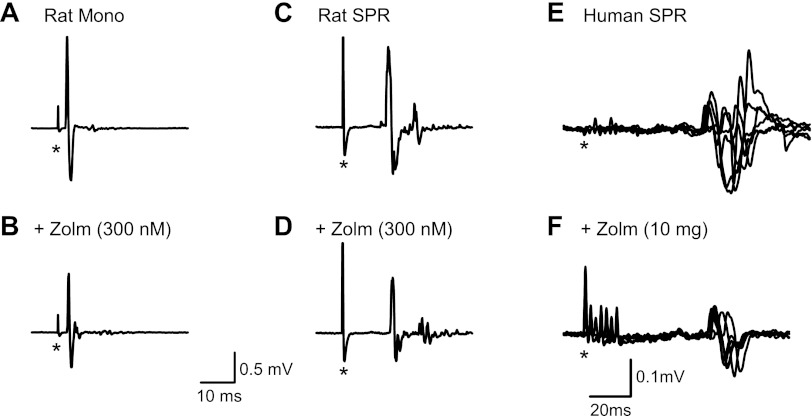
Effects of zolmitriptan verified in a rat model of SCI. A: monosynaptic reflex (Mono) recorded from a ventral root of a chronically spinalized rat that did not display a polysynaptic reflex response. B: monosynaptic reflex from A following 300 nM bath application of zolmitriptan (+Zolm). C: short-latency polysynaptic reflex (SPR) recorded from a ventral root of a different chronically injured rat (no monosynaptic response was evoked). D: polysynaptic reflex from C after 300 nM application of zolmitriptan (modified from Fig. 5 in Murray et al. 2011). E and F: overlays of 6 SPRs recorded from TA in a SCI participant (subject 3M in Table 1) before (E) and 120 min after (F) 10 mg of oral zolmitriptan. In A–F, asterisks mark time of single pulse, or start of multiple pulse, stimulation.
Short-latency polysynaptic reflexes: rat and human.
In some rats, rather than a monosynaptic reflex, an SPR was evoked in the ventral root that lasted from 10 to 40 ms poststimulation (Fig. 4C) and that was also reduced by zolmitriptan (Fig. 4D; data from Murray et al. 2011). A similar distinct SPR was also evoked in three of the SCI participants during the cutaneomuscular reflex recordings (see also D'Amico et al. 2012). The SPR had a latency of ∼70 ms and a duration of 50 ms (Fig. 4E). In all three SCI participants, zolmitriptan reduced the SPR to 56% of its predrug value 120 min after drug intake, as shown for the SCI participant in Fig. 4F (subject 6M, Table 1).
DISCUSSION
We have demonstrated that facilitation of 5-HT1B/D receptors with zolmitriptan, but not placebo, reduced sensory transmission to motoneurons as evidenced by the suppression of H-reflexes in noninjured and SCI participants. Likewise, in participants with SCI, zolmitriptan reduced long-latency polysynaptic reflexes evoked by cutaneomuscular stimulation to ultimately reduce the triggering and overall amplitude of long-lasting reflexes (spasms). Although zolmitriptan cannot be taken orally on a daily basis, these results open the possibility that other 5-HT1B/D receptor agonists may be useful to control sensory transmission and reduce the triggering of muscle spasms after SCI.
Mechanism of action of zolmitriptan on soleus H-reflexes.
In most control and SCI participants (8/13), the H-reflex was reduced at all stimulation intensities, indicating that all reflex pathways, including those with the lowest thresholds, were affected by 5-HT1B/D receptor facilitation. In the remainder of participants (5/13), only H-reflexes activated at stimulation intensities >S50 were reduced by zolmitriptan, indicating that only the higher threshold sensory pathways were affected in these participants. In all participants, there was a consistent decrease in Hmax at matched amplitudes of M-wave activation, the latter an indirect indication that the number of sensory afferents activated pre- and postdrug was similar (Misiaszek 2003; Zehr 2002). The decrease in Hmax and the absence of any change in Hthresh or S50 suggest that facilitation of 5-HT1B/D receptors by zolmitriptan specifically reduced the transmission of sensory-activated inputs in the H-reflex pathway without reducing the excitability of motoneurons (Misiaszek 2003). This finding in the human is in agreement with animal studies where zolmitriptan specifically reduced sensory-evoked EPSPs but did not influence motoneuron properties such as input resistance, resting membrane potential, and spike threshold (Murray et al. 2011). Taken together, this suggests that the reduction in H-reflexes was due to decreases in transmission of sensory pathways to the motoneuron, likely via increases in presynaptic inhibition on terminals of sensory afferents or excitatory interneurons or from postsynaptic inhibition of excitatory interneurons, as a result of 5-HTB/D receptor facilitation. In addition, because H-reflexes were evoked at a rate of 0.33 Hz, a frequency at which “rate-dependent” or “homosynaptic” depression occurs (Crone and Nielsen 1989), zolmitriptan may have reduced the amplitude of the H-reflexes by facilitating a rate-dependent, inhibitory mechanism.
Mechanism of action of zolmitriptan on cutaneomuscular reflexes.
Similar to the Ia-mediated H-reflex pathway, zolmitriptan also reduced short- and long-latency polysynaptic reflexes (SPR and LPR) evoked from cutaneomuscular afferent stimulation in participants with SCI. Reduction of the polysynaptic reflexes was also associated with a reduction in long-lasting reflexes (LLR or spasms). As shown from animal studies with cutaneous reflexes very similar to those of humans, the LPR can last for 500–1,000 ms and is an ∼50% mixture of EPSP and PIC activation, whereas the LLR, which lasts for many seconds, is mainly mediated by PIC activation (Murray et al. 2011). It is the long EPSP during the LPR that provides sufficient depolarization of the motoneuron to activate the CaPIC, which then drives self-sustained firing of the motoneuron during a muscle spasm. Because zolmitriptan only reduces sensory activation of the motoneuron and not the PIC (Murray et al. 2011), the reduction in LLR (spasm) activity was likely mediated by the inability of the reduced LPR to trigger a CaPIC and self-sustained firing of the motoneuron. Although we did not estimate the effects of zolmitriptan on PIC activation in this study (e.g., with paired motor unit analysis), we believe a similar mechanism occurred in the human participants with SCI. For instance, results from the H-reflex experiments suggest that sensory transmission, but not motoneuron excitability, was affected by 5-HT1B/D receptor facilitation.
Effect of zolmitriptan on sensory transmission likely via 5-HT1B/D receptors.
Zolmitriptan is a commonly prescribed antimigraine medication that is able to cross the blood-brain barrier, although only at relatively high doses (Proietti-Cecchini et al. 1997; Visser et al. 1996; Werhahn et al. 1998). It displays high affinity to 5-HT1B (Ki = 5.01 nM) and 5-HT1D (Ki = 0.63 nM) receptors, with modest affinity to the 5-HT1F receptor (Ki = 63.09 nM) (Martin et al. 1997). Zolmitriptan likely exerts at least some of its effects on the transmission of cutaneomuscular afferent pathways via activation of the 5-HT1B receptor given that in animal studies, the potency of various 5-HT1 receptor agonists in reducing cutaneous polysynaptic reflexes and sensory-evoked EPSPs was correlated to the published binding affinity of only 5-HT1B and 5-HT1F receptor agonists and not to other 5-HT receptor agonists (Murray et. al. 2011). Likewise, only 5-HT1B receptor antagonists reversed the effects of zolmitriptan on long-latency polysynaptic reflexes in animals. Thus the activation of 5-HT1B, and not 5-HT1D, receptors, by zolmitriptan likely produced the reduction of cutaneomuscular polysynaptic reflexes evoked in the SCI participants in this study. It remains to be determined in animal studies whether the action of zolmitriptan in reducing the Ia-mediated monosynaptic (H) reflex is also mediated via the 5-HT1B receptor or if there is also involvement of the 5-HT1D receptor to which zolmitriptan has a high binding affinity towards (Honda et al. 2003).
It is interesting that zolmitriptan produced similar decreases in H-reflexes in participants with and without SCI at the 10-mg dose, even though reduced levels of endogenous 5-HT were likely present below the lesion in the SCI participants (Murray et al. 2010). This suggests that after SCI, 5-HT1B/D receptors do not develop supersensitivity to applied agonists, similar to findings in rats where intravenous administration of sumatriptan, a similar 5-HT1B/D receptor agonist, depressed the monosynaptic reflex to the same degree in noninjured and SCI rats (Honda et al. 2006). It would be interesting to examine if other 5-HT receptors, such as 5-HT1A, 5-HT2A, and 5-HT7 receptors, which have been shown to help facilitate locomotion after SCI (Antri et al. 2005; Vinay et al. 2012), develop supersensitivity to 5-HT receptor agonists in the presence of reduced levels of endogenous 5-HT.
Clinical implications.
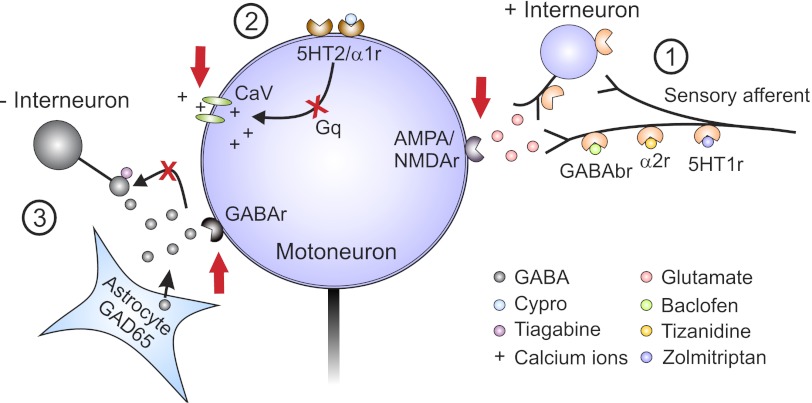
Following spinal cord injury, the activation of Gq-coupled pathways in the motoneuron via constitutive 5-HT2/α1 receptors facilities the activation of CaPICs that mediate, in part, the self-sustained activation of motoneurons during involuntary muscle spasms evoked by brief sensory afferent inputs. This is illustrated in the schematic of Fig. 5, which summarizes the animal and human data from Murray et al. (2010, 2011), Rank et al. (2011) and the previous (D'Amico et al. 2012) and current articles. One strategy to reduce muscle spasms is to reduce 5-HT2/α1 receptor activity via inverse agonists such as cyproheptadine (site 2, Fig. 5), resulting in a direct reduction of motoneuron excitability, rather than reducing sensory inputs to the motoneuron like many antispastic drugs used currently (discussed below). Thus the suppression of 5-HT2/α1 receptors, specifically 5-HT2B/C and α1A receptors, has the potential to reduce excessive muscle activation regardless of the etiology of the spasticity given that the final common pathway, the motoneuron, is directly affected. This strategy would be useful for patients in whom reducing muscle spasticity is a more important goal than preserving residual motor function, such as for patients with motor complete spinal cord injuries or severe brain damage where functional motor movements are lost and spasticity produces painful contractures and joint deformities. These studies highlight the need to develop inverse agonists to 5-HT2B/C and α1A receptors that are more specific than cyproheptadine, which has undesirable side effects of drowsiness, histamine receptor activation, and appetite stimulation (Gracies et al. 1997).
Fig. 5.
Target sites for antispastic drugs. Presynaptic (1), motoneuron (2), and GABAergic (3) sites of action for antispastic drugs are depicted. Site 1: GABAb, α2, and 5-HT1 receptors (r) located on presynaptic sensory terminals or on pre- or postsynaptic sites on interposed excitatory interneurons activated by baclofen, tizanidine, and zolmitriptan, respectively, to reduce glutamate release and activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors on motoneuron. Site 2: 5-HT2/α1 receptors on motoneuron with constitutive or ligand activation, which facilitates downstream voltage-gated calcium channels (CaV) mediating PICs via Gq protein-coupled pathways. Inverse agonists switch the 5-HT2/α1 receptors into their inactive states to reduce activity in the Gq pathway, lessen facilitation of CaV receptors, and reduce PICs and, consequently, muscle spasms. Site 3: spinal injection of the HIV1-CMV-GAD65 lentivirus leads to an increase in GAD65 gene expression and GABA release from astrocytes. Combined systemic administration of tiagabine, a GABA reuptake inhibitor, increases levels of GABA to a sufficient level to activate pre- and postsynaptic GABA receptors to reduce spasticity. Cypro, cyproheptadine.
In patients with residual motor function, severely reducing motoneuron excitability to alleviate muscle spasticity may not be the best strategy, because this would also reduce activation of the motoneuron by preserved descending inputs. Another strategy to reduce spasticity in this population would be to restore the balance between excitatory and inhibitory activation of the motoneuron by sensory afferent and interneuronal inputs via the activation of Gi-coupled pathways (site 1, Fig. 5) with GABAb (baclofen: Curtis et al. 1997; Li et al. 2004b), α2 (tizanidine: Krach 2011; Meleger 2006), and on the basis of the current study, 5-HT1B/D (zolmitriptan) receptor activation. The main antispastic effect of these drugs is to reduce the sensory-evoked EPSP from direct afferent and interposed interneuronal inputs, allowing an unmasking of an inhibitory postsynaptic potential (IPSP) to ultimately reduce the unchecked activation of CaPICs in the motoneuron (Li et al. 2004b; Murray et al. 2011; Rank et al. 2011). However, all of these Gi-coupled drugs taken orally have unwanted side effects, such as drowsiness and drug tolerance (Krach 2001; Meleger 2006; Nielsen et al. 2002; Rosche 2002). In addition, zolmitriptan cannot be taken daily due to the risk of harmful by-product production and, ironically, induction of headaches (Martin 1997; Peterlin and Rapoport 2007). Again, this study highlights the need to develop better 5-HT1B/D, and possibly 5-HT1F, receptor agonists (Murray et al. 2011) with fewer side effects than baclofen or tizanidine. Alternatively, a moderate suppression of motoneuron activity by cyproheptadine (Wainberg et al. 1990), combined with suppression of sensory inputs, may also strike a proper balance between spasticity control and preservation of residual movements in patients with incomplete injuries.
Although the antispastic drugs shown in Fig. 5 can be problematic when taken orally, results from the studies summarized here open new possibilities for spinally directed approaches in controlling spasticity, such as the use of intrathecal drug delivery. First, cyproheptadine may provide better control of spasticity than baclofen because it works directly on the motoneuron, thereby preventing aberrant descending inputs from the cortex and brainstem from activating the motoneuron that baclofen does not affect. This approach may be useful for many causes of spasticity such as amyotrophic lateral sclerosis (ALS), cerebral palsy, and brain trauma/injury in addition to spinal cord injury. Second, intrathecal baclofen can have potentially fatal side effects if suddenly withdrawn, as occurs during sudden blockage of the catheter (Awaad et al. 2012; Lazorthes et al. 1990; Meythaler et al. 2003; Mohammed and Hussain 2004; Stempien and Tsai 2000). Thus a potential strategy would be to give a combination of GABAb, α2, and 5-HT1B/D/F receptor agonists, which all converge to activate Gi-coupled pathways, at individually lower doses to potentially reduce severe side effects after sudden drug withdrawal.
Another spinally targeted strategy has recently been proposed by Marsala's group (site 3, Fig. 5) whereby in a rat model of ischemic spinal cord injury, increases in GABA release and reduction of spastic stretch reflexes were produced by the combined upregulation of GAD65 gene expression in lumbar astrocytes (from spinal injections of lentivirus) and the systemic administration of tiagabine, a GABA uptake inhibitor (Kakinohana et al. 2012). With spinally targeted interventions (intrathecal or spinal transfections), spasticity may be better controlled without the unwanted side effects of sedation, tolerance, and appetite stimulation. The combined use of these strategies, including activating 5-HT1B/D/F receptors and suppressing 5-HT2/α1 receptor activity, provides new avenues for antispastic treatment.
GRANTS
This work was supported by Canadian Institute of Health Research Grants MOP-106549 (to M. A. Gorassini) and MOP-14697 (to D. J. Bennett) and National Institute of Neurological Disorders and Stroke Grant R01 NS047567 (to D. J. Bennett). Salary support was provided by Alberta Innovates: Health Solutions (to M. A. Gorassini, D. J. Bennett, and J. D'Amico) and the Alberta Paraplegic Foundation (to J. D'Amico).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D. and M.A.G. conception and design of research; J.D., Y.L., D.J.B., and M.A.G. performed experiments; J.D., Y.L., and D.J.B. analyzed data; J.D. and M.A.G. interpreted results of experiments; J.D., D.J.B., and M.A.G. prepared figures; J.D. drafted manuscript; J.D. and M.A.G. edited and revised manuscript; J.D., Y.L., D.J.B., and M.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for their large time commitment and effort in this study. We are grateful to Drs. Richard Stein and Francois Roy for helpful comments on the manuscript. We appreciate the technical assistance of Leo Sanelli and Marilee Stephens and extend thanks to Jennifer Nevett-Duchcherer for helping out with the experiments.
REFERENCES
- Anden NE, Haeggendal J, Magnusson T, Rosengren E. The time course of the disappearance of noradrenaline and 5-hydroxytryptamine in the spinal cord after transection. Acta Physiol Scand 62: 115–118, 1964 [DOI] [PubMed] [Google Scholar]
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett 384: 162–7, 2005 [DOI] [PubMed] [Google Scholar]
- Awaad Y, Rizk T, Siddiqui I, Roosen N, Mcintosh K, Waines GM. Complications of intrathecal baclofen pump: prevention and cure. ISRN Neurol 2012: 575168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001b [DOI] [PubMed] [Google Scholar]
- Carlsson A, Magnusson T, Ronsengren E. 5-Hydroxytryptamine of the spinal cord normally and after transection. Experientia 19: 359, 1963 [DOI] [PubMed] [Google Scholar]
- Clarke RW, Eves S, Harris J, Peachey JE, Stuart E. Interactions between cutaneous afferent inputs to a withdrawal reflex in the decerebrated rabbit and their control by descending and segmental systems. Neuroscience 112: 555–571, 2002 [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen JB. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res 78: 28–32, 1989 [DOI] [PubMed] [Google Scholar]
- Curtis DR, Gynther BD, Lacey G, Beattie DT. Baclofen: reduction of presynaptic calcium influx in the cat spinal cord in vivo. Exp Brain Res 113: 520–533, 1997 [DOI] [PubMed] [Google Scholar]
- D'Amico JM, Gorassini MA. Sensory afferent transmission to the motoneuron is reduced by 5HT1B/D receptor activation in uninjured and spinal cord injured subjects. Soc Neurosci Abstr 552.02, 2012 [Google Scholar]
- D'Amico JM, Li C, Bennett DJ, Gorassini MA. Constitutively active 5HT2/α1 receptors facilitate muscle spasms in human spinal cord injury. J Neurophysiol (December 5, 2012). doi:10.1152/jn.00821.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff RA. Antispasticity drugs: mechanisms of action. Ann Neurol 17: 107–116, 1985 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman J, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett 230: 29–32, 1997 [DOI] [PubMed] [Google Scholar]
- Edwards FR, Harrison PJ, Jack JJ, Kullmann DM. Reduction by baclofen of monosynaptic EPSPs in lumbosacral motoneurones of the anaesthetized cat. J Physiol 416: 539–556, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, Ryall RW. Reticulospinal inhibition of transmission in reflex pathways. J Physiol 194: 201–223, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasticity after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl 6: S92–S120, 1997 [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Tanabe M, Ono H. Spinal cord injury-specific depression of monosynaptic spinal reflex transmission by l-5-hydroxytryptophan results from loss of the 5-HT uptake system and not 5-HT receptor supersensitivity. Exp Neurol 202: 258–261, 2006 [DOI] [PubMed] [Google Scholar]
- Honda M, Tanabe M, Ono H. Serotonergic depression of spinal monosynaptic transmission is mediated by 5-HT1B receptors. Eur J Pharmacol 482: 155–161, 2003 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev 40: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Skoog B, Noga BR. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol 461: 705–722, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez I, Rudomin P, Enriquez M. Differential effects of (−)-baclofen on Ia and descending monosynaptic EPSPs. Exp Brain Res 85: 103–113, 1991 [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008 [DOI] [PubMed] [Google Scholar]
- Kakinohana O, Hefferan MP, Miyanohara A, Nejime T, Marsala S, Juhas S, Juhasova J, Motlik J, Kucharova K, Strnadel J, Platoshyn O, Lazar P, Galik J, Vinay L, Marsala M. Combinational spinal GAD65 gene delivery and systemic GABA-mimetic treatment for modulation of spasticity. PLoS One 7: 1–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JM, Vujnovich LB. The intercept method: a novel method for establishing consistency of M-wave recruitment curves. Electromyogr Clin Neurophysiol 42: 423–432, 2002 [PubMed] [Google Scholar]
- Klimstra M, Zehr EP. A sigmoid function is the best fit for the ascending limb of the Hoffman reflex recruitment curve. Exp Brain Res 186: 93–105, 2008 [DOI] [PubMed] [Google Scholar]
- Krach LE. Pharmacotherapy of spasticity: oral medications and intrathecal baclofen. J Child Neurol 16: 31–36, 2001 [DOI] [PubMed] [Google Scholar]
- Lazorthes Y, Sallerin-Caute B, Verdie JC, Bastide R, Carillo JP. Chronic intrathecal baclofen administration for control of severe spasticity. J Neurosurg 72: 393–402, 1990 [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004b [DOI] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following 1 wk of wrist and hand immobilization. J Appl Physiol 105: 139–151, 2008 [DOI] [PubMed] [Google Scholar]
- Manuel NA, Wallis DI, Crick H. Ketanserin-sensitive depressant actions of 5-HT receptor agonists in the neonatal rat spinal cord. Br J Pharmacol 116: 2647–2654, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Pre-clinical pharmacology of zolmitriptan (Zomig; formerly 311C90), a centrally and peripherally acting 5HT1B/1D agonist for migraine. Cephalalgia 17, Suppl 18: 4–14, 1997 [DOI] [PubMed] [Google Scholar]
- Martin GR, Robertson AD, MacLennan SJ, Prentice DJ, Barrett VJ, Buckingham J, Honey AC, Giles H, Moncada S. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5HT1B/D receptor partial agonist, 311C90 (zolmitriptan). Br J Pharmacol 121: 157–164, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleger AL. Muscle relaxants and antispasticity agents. Phys Med Rehabil Clin N Am 17: 401–413, 2006 [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Roper JF, Brunner RC. Cyproheptadine for intrathecal baclofen withdrawal. Arch Phys Med Rehabil 84: 638–642, 2003 [DOI] [PubMed] [Google Scholar]
- Miles TS, Turker KS, Le TH. Ia reflexes and EPSPs in human soleus motor neurones. Exp Brain Res 77: 628–636, 1989 [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol 66: 355–474, 2002 [DOI] [PubMed] [Google Scholar]
- Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28: 144–160, 2003 [DOI] [PubMed] [Google Scholar]
- Mohammed I, Hussain A. Intrathecal baclofen withdrawal syndrome-a life threatening complication of baclofen pump: a case report. BMC Clin Pharmacol 4: 6, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico JM, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5HT2C receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D'Amico J, Gorassini MA, Bennett DJ. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106: 925–943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JF, Hansen HJ, Sunde N, Christensen JJ. Evidence of tolerance to baclofen in treatment of severe spasticity with intrathecal baclofen. Clin Neurol Neurosurg 104: 142–145, 2002 [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BL, Rapoport AM. Clinical pharmacology of the serotonin receptor agonist, zolmitriptan. Expert Opin Drug Metab Toxicol 3: 899–911, 2007 [DOI] [PubMed] [Google Scholar]
- Proietti-Cecchini A, Afra J, Schoenen J. Intensity dependence of the cortical auditory evoked potentials as a surrogate marker of central nervous system serotonin transmission in man: demonstration of a central effect for the 5HT1B/D agonist zolmitriptan (311C90, Zomig). Cephalalgia 17: 849–854, 1997 [DOI] [PubMed] [Google Scholar]
- Rank MM, Murray KC, Stephens MJ, D'Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol 105: 410–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosche J. Treatment of spasticity. Spinal Cord 40: 261–262, 2002 [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol 76: 799–807, 1996 [DOI] [PubMed] [Google Scholar]
- Stempien L, Tsai T. Intrathecal baclofen pump use for spasticity: a clinical survey. Am J Phys Med Rehabil 79: 536–541, 2000 [DOI] [PubMed] [Google Scholar]
- Vinay LR, Bos R, Boulenguez P, Bras H, Brocard C, Buttigieg G, Haase S, Liabeuf K, Sadlaoud K. Activation of 5-HT2A receptors reduces spasticity after spinal cord injury via PKC-dependent up-regulation of KCC2 function. Soc Neurosci Abstr 182.07, 2012 [Google Scholar]
- Visser WH, Klein KB, Cox RC, Jones D, Ferrari MD. 311C90, a new central and peripherally acting 5-HT1D receptor agonist in the acute oral treatment of migraine: a double-blind, placebo-controlled, dose-range finding study. Neurology 46: 522–526, 1996 [DOI] [PubMed] [Google Scholar]
- Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 53: 754–763, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Forderreuther S, Straube A. Effects of the serotonin 5HT1B/1D receptor agonist zolmitriptan in motor cortical excitability in humans. Neurology 51: 896–898, 1998 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101: 107–117, 2006 [DOI] [PubMed] [Google Scholar]
- Zehr EP. Considerations for the use of the Hoffman reflex in exercise studies. Eur J Appl Physiol 86: 455–468, 2002 [DOI] [PubMed] [Google Scholar]