Abstract
Background
This study examines whether adding nicotine replacement therapy (NRT) to cognitive behavioral therapy (CBT) for pregnant smokers increases rates of smoking cessation.
Methods
An open-label randomized trial (Baby Steps, n = 181) of CBT-only versus CBT+NRT (choice of patch, gum, or lozenge; 1:2 randomization) was used. Data were collected from 2003 through 2005; analyses were conducted in 2006 and 2007. Outcomes were biochemically validated self-reported smoking status at 7-weeks post-randomization, 38-weeks gestation, and 3-months postpartum.
Results
Women in the CBT+NRT arm were almost three times more likely than women in the CBT-only arm to have biochemically validated cessation at both pregnancy timepoints (after 7 weeks: 24% vs 8%, p = 0.02; at 38-weeks gestation: 18% vs 7%, p =0.04), but not at 3-months postpartum (20% vs 14%, p=0.55). Recruitment was suspended early by an independent Data and Safety Monitoring Board when an interim analysis found a greater rate of negative birth outcomes in the CBT+NRT arm than in the CBT arm. At the final analysis the difference between the arms in rate of negative birth outcomes was 0.09 (p=0.26), adjusted for prior history of preterm birth.
Conclusions
The addition of NRT to CBT promoted smoking cessation in pregnant women. This effect did not persist postpartum. More data are needed to determine the safety and to confirm the efficacy of NRT use during pregnancy.
INTRODUCTION
Smoking during pregnancy is associated with numerous adverse pregnancy outcomes, including low birthweight, miscarriage, stillbirth, placenta previa, placental abruption, and cognitive impairments.1–4 Despite awareness of the health consequences of smoking during pregnancy, at least half of women who smoke will continue to smoke throughout pregnancy.5
Cognitive behavioral therapy (CBT) interventions are effective during pregnancy6–24; pooled efficacy in one meta-analysis was 0.95 (95% confidence interval [CI]=0.92–0.97).25 Effectiveness is reduced among women who have a partner who smokes, are less educated, and are heavy smokers (smoke >10 cigarettes per day).11, 12, 26–29 Given the considerable risk of pregnancy complications related to smoking, more effective interventions are needed.
Nicotine replacement therapy (NRT) is highly effective in nonpregnant smokers30 and is widely available over the counter. Because evidence suggests that NRT use during pregnancy is not more harmful to the fetus than ad libitum smoking (in terms of maternal and fetal hemodynamics and nicotine exposure),31–33 there has been considerable interest in the use of NRT to improve cessation rates in pregnant smokers.34, 35
Previous randomized trials of NRT in pregnancy have shown reductions in smoking rates (5% absolute reduction) similar to other interventions.25, 36–38 In one trial,37 NRT was used as part of a multimodal intervention along with CBT, so direct estimation of the effect of NRT alone was not possible. In two other trials,36, 38 adherence to the use of nicotine patches was low.
To assess whether the addition of NRT to CBT resulted in improved smoking cessation rates, a randomized, open-label trial comparing CBT-only to CBT+NRT (choice of gum, lozenge, or patch) in pregnant smokers was conducted.
METHODS
Eligibility and Recruitment
The protocol was approved and monitored by the Institutional Review Boards of all participating institutions. Women were eligible if they were between 13- and 25-weeks pregnant, had smoked at least 100 cigarettes in their lifetime, currently smoked five or more cigarettes per day, were planning to continue prenatal care in one of the participating clinics, were aged at least 18 years, and spoke English. Recruitment occurred from May 2003 through August 2005. Analysis of data occurred in 2006 and 2007.
Participant eligibility was confirmed by an obstetrician reviewer. Women were excluded from the trial if they had: (1) evidence of cognitive or mental health problems (e.g., diagnosis of mental disorder in chart or interviewer or support specialist suspected problems); (2) evidence of possible drug or alcohol addiction; or (3) documented history of placental abruption, poorly controlled hypertension, cardiac arrhythmia, myocardial infarction within the past 6 months, previous pregnancy with congenital anomaly, or family history of congenital anomalies.
Procedure
Recruitment is reported in detail elsewhere.39 There were three phases of recruitment: (1) chart audit, (2) baseline survey, and (3) the first intervention session. Women were recruited from 14 clinical sites in Durham, Raleigh, and Fayetteville, North Carolina, including sites that provide prenatal care to active-duty soldiers and their dependents (Womack Army Medical Center at Fort Bragg). Obstetric nurses assessed smoking status for all new patients. Introductory letters and brochures describing the study were sent via United States Postal Service (USPS) 2-Day Priority Mail to these self-identified smokers. Women were instructed to call project staff using a toll-free number within 1 week if they did not want to be contacted.
Study staff performed a chart audit to determine a woman’s medical eligibility. A survey company contacted all medically eligible women to reassess smoking status and gestational age; surveyors obtained verbal consent and conducted the baseline survey. At the end of the survey, the women were asked their willingness to meet with a “support specialist” at their next prenatal appointment to discuss their smoking. If they agreed to set a quit date in the next 2–3 weeks and consider using NRT, support specialists randomized the women to the trial during the first intervention session.
Study Design and Intervention
A computerized random number generator was used to derive a list for randomization. Each support specialist had a handheld device that contained a randomization list. The randomization list was stratified by support specialist and gestational age (≤ 16 weeks versus >16 weeks). Women were randomized using a 1:2 allocation to CBT-only or CBT+NRT to allow comparisons between women who chose intermittent therapies (gum and lozenge) versus continuous therapy (patch).
The trial did not include a placebo NRT because the efficacy of NRT has been shown consistently among nonpregnant populations. Also, placebos for all of the forms of NRT were not available at the time of study initiation.
All women received six one-on-one counseling sessions (five face-to-face at prenatal visits and one via telephone) designed to enhance motivation and develop skills needed to quit smoking. The timing of the intervention contacts was designed to be relapse-sensitive (close in time to the woman’s quit date) and to coincide with prenatal visits.40,41 The first counseling session involved support specialists consenting, randomizing, and giving women a “quit kit”: a smoking cessation booklet designed for pregnant smokers (Make Yours a Fresh Start Family), water bottle, straws, hard candy, an exercise band, and a stress management tape. Support specialists helped the women devise an action plan. For women in the CBT+NRT arm, support specialists presented the advantages and disadvantages of the three types of NRT (patch, gum, and lozenge) and helped the women make an informed choice. Women received their first dose of NRT at the session (see below for dosing details). This session lasted a mean of 64.7 minutes (SD=15.8): CBT: M=61.7, SD=16.7; CBT+NRT: M=66.1, SD=15.7. After the first session, each woman was mailed a card detailing her action plan.
The second counseling session occurred over the phone about 48 hours after the quit date. The purpose of the call was to discuss alternatives if the quit attempt was not successful. The third session typically occurred 1 to 2 weeks after the phone session at the woman’s next prenatal visit. The fourth, fifth, and sixth sessions occurred between 2 and 4 weeks apart, depending on the timing of the prenatal appointments. In all sessions, support specialists attempted to increase the women’s motivation, self-efficacy, and skills. In addition to the smoking-specific content, support specialists covered a relevant content area (stress, rewards, social support, and relapse prevention). This gave support specialists topics to discuss when a woman was still smoking. Sessions 2–6 lasted a mean of 25.7 minutes (SD=14.1): CBT: M=23.6, SD=10.7; CBT+NRT: M=27.0, SD=13.9.
The counseling protocol was based on Motivational Interviewing,42 the Transtheoretical Model of Behavior Change,43 and Social Cognitive Theory.44 All support specialists attended 40 hours of training. Throughout the trial, they received supervision of their cases to assure adherence to Motivational Interviewing principles and the study protocol.
Dosing and Dispensing of NRT
Women in the CBT+NRT arm were allowed to choose the nicotine patch, gum, lozenge, or no NRT at all; they could also switch delivery modalities. Each woman’s NRT dose was based on current smoking level. For those who chose the patch, they were instructed to wear it for waking hours only (16 hours) as is recommended during pregnancy.34 The doses for the patch were: fewer than 10 cigarettes/day = 7 mg /day; 10–14 cigarettes/day = 14 mg /day; 15 or more cigarettes/day =21 mg /day. For those who chose the gum or lozenge, they were instructed to use one 2-mg piece for every cigarette they smoked per day. The women were given enough NRT to last until the next scheduled face-to-face session. Overall, they were encouraged to use NRT for 6 weeks to minimize nicotine exposure in the third trimester. However, if a woman felt that she would return to smoking, she was instructed to use NRT longer. Before the women left with their NRT, each was required to sign a contract promising not to smoke while using NRT.
Assessments
Telephone surveys were conducted with the women three times during pregnancy (at baseline, 7-weeks post-randomization, 38-weeks gestation) and once at 3-months postpartum. The primary outcome was self-reported 7-day point prevalence abstinence at the two pregnancy follow-up timepoints. Saliva samples for cotinine validation were collected at the intervention session that coincided with each telephone survey from all women regardless of smoking status. At the 3-month postpartum follow-up, saliva samples were collected via mail only from self-reported nonsmokers. Participants were paid $10 each time they provided a saliva sample and $10 in advance for each telephone survey ($70 total).
Safety Monitoring
To monitor safety, study staff conducted monthly chart audits to assess maternal blood pressure; urine dipstick (protein, blood, glucose, leukocyte esterase); maternal weight; fundal height as a measure of fetal growth; and examination for the presence of edema, threatened miscarriage, congenital anomalies, pelvic or abdominal surgical procedures, deep venous thrombosis, and malignancy. Study obstetricians reviewed these audits to determine whether a woman should be removed from NRT. Also, at any time, a woman’s personal obstetrician could request removal from NRT. In addition, if a woman reported wanting more NRT, her saliva was tested using a rapid assay assessment (NicAlert™, Craig Medical Distribution, Inc., Vista, CA) to ensure that her cotinine level did not exceed her baseline value, taken when she was smoking. Each woman also provided a breath sample to test for carbon monoxide (CO). Any woman with an exhaled CO >10 ppm and with a NicAlert greater than her baseline value was instructed to stop using the NRT. Finally, study staff audited the women’s delivery and neonatal records to assess pregnancy outcomes and maternal medical history.
Study staff identified potential serious adverse events (SAEs) that could be attributed to smoking or nicotine (preterm birth<37 weeks, low birthweight<2500 grams, pre-eclampsia, placental abruption, placental previa, neonatal ICU admission, fetal demise, and infant death). An independent Data and Safety Monitoring Board (DSMB) reviewed all SAE reports. Prior to the trial beginning, the DSMB decided that a statistically significant twofold increase in adverse birth outcomes would result in suspension of study enrollment. At a scheduled interim analysis with approximately half of participants enrolled, the DSMB found a twofold difference in SAEs between arms. Because of an a priori stopping rule, they recommended stopping enrollment. The DSMB did state, however, that they did not believe the SAEs were related to NRT use.
Analysis
The study was initially designed to enroll 300 women to have 91% power (two-sided α=0.025) to detect an arm difference in 7-week post-randomization quit rates of 0.20 versus 0.40. Three hundred patients also gave 80% power (two-sided α=0.025) to detect an arm difference in 38-week gestation quit rates of 0.13 versus 0.28.
For analyses of smoking cessation, an intent-to-treat analysis was used in which all women lost to follow-up were considered smokers. Smoking cessation at 7-weeks post-randomization and at 38-weeks gestation were the primary endpoints, and smoking cessation at 3-months postpartum was a secondary endpoint. Arm differences in cessation (yes/no), adverse events (yes/no), and other dichotomous endpoints were tested with the chi-square test and described with the risk difference (RD) and its 95% CI. Arm differences in birthweight, gestational age, and number of cigarettes smoked per day were tested with the Wilcoxon two-sample test and described with means ± standard deviation. The logistic regression model was used to test for arm differences in cessation rates controlling for number of completed sessions. The logistic regression model was also used to test for arm differences in adverse event rates, controlling for history of preterm birth. Because the study had two primary endpoints, a two-sided α of 0.025 was used to test for arm differences, while all other statistical tests used a two-sided α of 0.05. SAS version 9.1 was used to conduct all analyses.
RESULTS
Recruitment and Enrollment
Figure 1 illustrates recruitment, enrollment, and retention. The CBT-only and CBT+NRT arms did not differ statistically in their follow-up rates at 7-weeks post-randomization, 38-weeks gestation, or 3-months postpartum: 63% vs 76% (p=0.08), 49% vs 60% (p=0.20), and 66% vs 62% (p=0.74), respectively. Compared to women who completed the trial, women lost to follow-up had a greater probability of having had a previous pregnancy loss (64% vs 42%, p=0.01) and having made a 24-hour quit attempt during a prior pregnancy (66% vs 44%, p=0.007). They also smoked more cigarettes per day (means of 14 (±7) vs 10 (±5), p<0.001).
Figure 1. Flow of participants through the study.
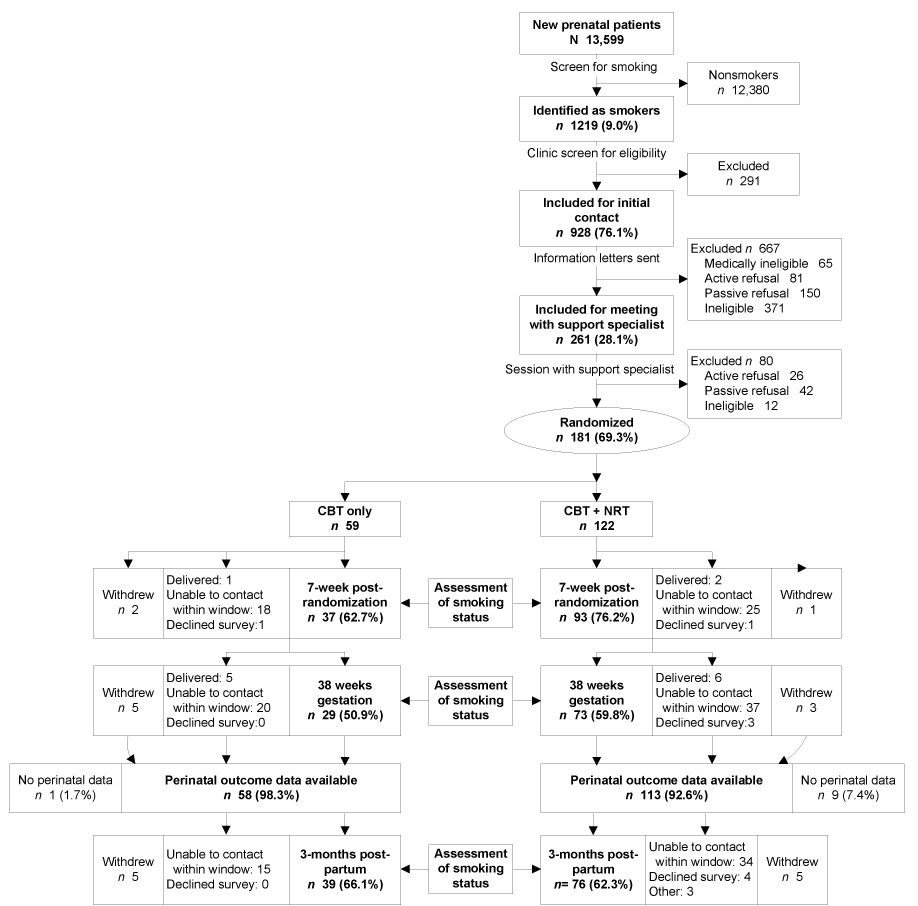
“Active refusal” indicates potential participants who were contacted but declined to participate. “Passive refusal” indicates potential participants with whom contact was not possible. Once randomized, self-reported smoking status was assessed by telephone interview 7 weeks after randomization, at 38-weeks gestational age, and at 3-months postpartum. At each timepoint, “delivered” indicates participants who delivered prior to the time of assessment; “unable to contact within window” indicates participants who were not reachable by phone within the 3-week window allowed for contact; “withdrew” indicates participants who refused further participation in surveys; “declined survey” indicates participants who declined to respond to the phone interview at that point in time but indicated willingness to be contacted in the future; and “withdrew” indicates participants who both refused to participate and did not wish to be contacted at future timepoints.
Participant Characteristics
Table 1 describes participant characteristics. By chance alone, an imbalance between the arms was observed in the proportion of women with a prior preterm birth (12% in CBT-only vs 32% in CBT+NRT).
Table 1.
Participant characteristics
| Characteristics | Total (N=181) Mean (±)/% | CBT-only (N=59) Mean (±)/% | CBT+NRT (N=122) Mean (±)/% |
|---|---|---|---|
| Demographics | |||
| Age (M, SD) | 27 (6) | 26 (5) | 27 (6) |
| N (%) | N (%) | N (%) | |
| Partnered (%) | 67 | 69 | 66 |
| Education (%) | |||
| Less than high school | 28 | 31 | 27 |
| High school/ GED | 31 | 33 | 31 |
| Vocational school | 7 | 10 | 6 |
| Some college | 28 | 17 | 33 |
| College graduate or higher | 5 | 9 | 3 |
| Race (%) | |||
| White | 69 | 73 | 67 |
| Black | 24 | 19 | 26 |
| Other | 8 | 8 | 7 |
| Employment (%) | |||
| Full-time | 30 | 31 | 30 |
| Part-time | 17 | 9 | 21 |
| Not employed | 53 | 60 | 49 |
| Site (%) | |||
| Durham | 41 | 39 | 42 |
| Fayetteville | 59 | 61 | 58 |
| First pregnancy (%) | 16 | 12 | 18 |
| Weeks of pregnancy at enrollment (M, SD) | 15 (3) | 15 (3) | 14 (3) |
| Weeks of pregnancy at randomization (M, SD) | 18 (3) | 18 (4) | 17 (3) |
| Smoking history prior to pregnancy | |||
| Cigs smoked daily 30d before pregnant (M, SD) | 19 (9) | 20 (8) | 19 (9) |
| Ever had 24-hr quit attempt (%) | 58 | 61 | 57 |
| Among those who made 24 hour quit | |||
| Times made a 24 hour quit attempt (M, SD) | 3 (3) | 2 (2) | 3 (3) |
| Longest quit in days (M, SD) | 93 (158) | 79 (133) | 100 (171) |
| Smoking history in previous pregnancies | |||
| Ever had 24-hr quit attempt (%) | 54 | 62 | 50 |
| Longest quit in days (M, SD) | 87 (105) | 63 (90) | 102 (111) |
| Current smoking history | |||
| Cigarettes smoked daily (M, SD) | 11 (5) | 12 (5) | 11 (5) |
| Quit at least 24 hours during this pregnancy (%) | 28 | 29 | 28 |
| Desire to quit (M, SD) | 6 (1) | 6 (1) | 6 (1) |
| Nicotine dependence (M, SD) | 3 (1) | 3 (1) | 3 (1) |
| Stage of readiness (%) | |||
| Precontemplation | 1 | 4 | 0 |
| Contemplation | 7 | 10 | 6 |
| Preparation | 92 | 86 | 94 |
| Total (N=152) Median | CBT-only (N=52) Median | CBT+NRT (N=100) | |
| (IQR)/% | (IQR)/% | Median (IQR)/% | |
| Prior pregnancy history (n=152) | |||
| Number of prior pregnancies median (IQR) | 2 (1,4) | 2 (1,3) | 2 (1,4) |
| Number of live births median (IQR) | 1 (1,2) | 1 (1,2) | 1 (1,2) |
| Any preterm births (%) | 25 | 12 | 32 |
| Full term low birthweight (LBW) (%) | 5 | 5 | 5 |
| Premature rupture of membranes (%) | 7 | 10 | 5 |
| High blood pressure (%) | 19 | 21 | 18 |
| Prior history of miscarriage, ectopic pregnancy, or stillbirth (%) | 50 | 40 | 55 |
CBT, cognitive-behavioral therapy; NRT, nicotine replacement therapy
Intervention Outcomes
Biochemical confirmation of self-reported abstinence
The proportion who returned saliva samples differed significantly between the CBT+NRT and CBT-only arms with more women in the CBT+NRT arm providing samples at the 7-week post-randomization (65% vs 47%, respectively, p=0.03), but not at the 38-week gestation follow-up (47% vs 39%, p= 0.33) or 3-month follow-up (42% vs 25%, p=0.68). The nondisclosure rate was 14% in the CBT arm and 15% in the CBT+NRT arm.
Adherence to NRT
Of the 122 women in the CBT+NRT arm, 72 selected the patch, 32 selected the gum, 12 selected the lozenge, and six opted to use no NRT. Study staff dispensed a mean of 40 patches to the women (this would last 5.7 weeks); however, women reported using a mean of only 23.4 patches (lasting 3.3 weeks). Study staff dispensed enough gum to last the women 18 days; they reported using gum for 8 days. Staff dispensed lozenges to last 19 days; the women reported using lozenges for a mean of 4 days. Overall, 76% of the women in the CBT+NRT arm reported using some form of NRT. Nineteen percent of those who were unable to quit with one formulation chose another. Only four women in the CBT arm reported NRT. Twelve women of 112 who completed the 3-month survey reported using NRT; two were in the CBT arm. Only one woman had a CO reading greater than 10 ppm, indicating that she was smoking. Five women had NicAlert readings higher when using NRT than when they reported smoking. These women were given lower doses of NRT to reduce their nicotine exposure. Thus, there did not seem to be much indication that the women were concomitantly smoking and using NRT.
Dose of counseling intervention
A greater proportion of the women in the CBT+NRT arm completed four or more sessions (four was median number of sessions) than did women in the CBT-only arm (70% vs 53%, p=0.02, RD=0.17, 95% CI=0.10–0.24).
Prevalent and sustained abstinence
In intent-to-treat analyses, there were significant arm differences in the proportion of the women who reported not smoking any cigarettes in the prior 7 days at both pregnancy follow-ups. Women in the CBT+NRT arm were more likely to report being abstinent than the women in the CBT-only arm at 7-weeks post-randomization (RD=0.19, 95% CI=0.08–0.30, p=0.005) and at 38-weeks gestation (RD=0.16, 95% CI=0.06–0.26, p=0.008) (Table 2). No substantial change in these RDs were found after controlling for number of counseling sessions; specifically, at 7-weeks post-randomization, the adjusted RD was 0.16 (p=0.02) and at 38-weeks gestation the adjusted RD was 0.13 (p=0.03). The arm differences remained when comparing biochemically validated smoking status: 7-weeks (24% vs 8%, p=0.02); 38-weeks gestation (18% vs 7%, p=0.04) (Table 3). The results did not change when those assigned to the CBT+NRT arm who did not use NRT were removed from the analyses. No statistically significant arm differences were found for 3-month postpartum and sustained abstinence (reported 7-day point prevalence abstinence at all three timepoints) (Table 2). No site differences in abstinence rates were found.
Table 2.
Unadjusted and adjusted percentages of women’s self-report of 7-day point prevalent abstinence
| Arm | p value | |||
|---|---|---|---|---|
| CBT-only | CBT+NRTa | Unadj. | Adjb | |
| Time-point | N=59 | N=122 | ||
| Unadj (Adj.) | Unadj (Adj.) | |||
| 7-weeks post-randomization | 10% (10%) | 29% (26%) | 0.005 | 0.02 |
| 38 weeks of pregnancy | 7% (7%) | 23% (20%) | 0.008 | 0.03 |
| 3-month postpartum | 14% (14%) | 20% (17%) | 0.31 | 0.55 |
| Sustained abstinence | 7% (5%) | 5% (5%) | 0.70 | 0.94 |
CBT+NRT = Cognitive-Behavioral Therapy + Nicotine Replacement Therapy
Analysis controlled for number of completed counseling sessions.
Sustained abstinence was defined as reported 7-day point prevalence abstinence at all three timepoints
Table 3.
Unadjusted and adjusted percentages of biochemically validated women’s 7-day point prevalent abstinence
| Arm | p value | |||
|---|---|---|---|---|
| CBT-only | CBT+NRTa | Unadj. | Adjb | |
| Time-point | N=59 | N=122 | ||
| Unadj (Adj.) | Unadj (Adj.) | |||
| 7-weeks post-randomization | 3% (8%) | 18% (24%) | 0.006 | 0.02 |
| 38 weeks of pregnancy | 2% (7%) | 14% (18%) | 0.01 | 0.04 |
CBT+NRT = Cognitive-Behavioral Therapy + Nicotine Replacement Therapy
Analysis controlled for number of completed counseling sessions
The relationship between length of NRT use and cessation was assessed. Logistic regression was conducted with counseling dose and length of NRT use regressed on cessation at 7-weeks post-randomization. Among the 122 women, for every 7 days of NRT use, they were 1.25 times (95% CI=1.08–1.47, p=0.003) more likely to self-report 7-day point prevalent abstinence. Dose of intervention was no longer predictive of cessation (OR=1.29, 95% CI=0.44–3.73, p=0.64).
Birth outcomes
There was no difference between the CBT+NRT and CBT-only arms in mean birthweight: 3061 (±661) vs 3132 (±688), respectively, p=0.51. Likewise there was no arm difference in mean gestational age: 37.9 (±3.1) vs 38.6 (±2.7), respectively, p=0.14.
Serious adverse events
Ten women had missing birth outcome data. Forty-four of the 171 women with data had at least one serious adverse event. Adverse events were, ordered from most to least frequent: preterm birth (<37 weeks), Neonatal Intensive Care Unit (NICU) admissions, small-for-gestational-age, placenta abruption, and fetal demise (Table 4). Serious adverse events occurred in 34 of 113 (30%) women assigned to CBT+NRT versus 10 of 58 (17%) women assigned to CBT-only (RD=0.13, 95% CI=0.00–0.26, p=0.07). After adjusting for prior history of preterm birth, these adverse event rates changed to 27% versus 18% for the CBT+NRT and CBT-only arms, respectively (RD=0.09, 95% CI=0.05–0.21, p=0.26). Women with adverse events had a mean cotinine value of 189.3 (SD=223.9) versus those who did not have an adverse event of 140.8 (SD=149.4), p=0.85. Women who reported smoking had higher cotinine values (M=201.0, SD=158.7) than the women reporting using NRT (M=94.5, SD=129.0).
Table 4.
Adverse events
| Treatment details | Pregnancy outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gestational age (GA) at Rand | GA at last sessiona | Smoking status at last session | Pre-eclampsia | Placental abnormality | Preterm birth | Final GA | SG A | Birthweight (g) | NICU admission | Fetal loss GA |
| CBT only | |||||||||||
| A | 21.7 | 27.4 | Smoking | x | 36.9 | 3289 | |||||
| B | 22.4 | 35.4 | Smoking | x | 36.3 | 2410 | x | ||||
| C | 23.9 | 29.9 | Relapsed | 36.3 | Unknown | 36.3f | |||||
| D | 17.1 | 30.0 | Relapsed | x | 36.1 | 3402 | |||||
| E | 14.4 | 27.6 | Smoking | x | 36.1 | 2863 | |||||
| F | 23.6 | 34.9 | Smoking | x | 36.0 | 2807 | |||||
| G | 16.6 | 32.1 | Relapsed | x | 34.6 | 2240 | x | ||||
| H | 13.1 | 20.1 | Smoking | x | 34.3 | 2410 | x | ||||
| I | 23.0 | 27.1 | Smoking | x | x | 31.9 | 1503 | ||||
| J | 20.9 | 20.9b | Unknown | x | x | 25.4 | 312 | x | 27.0 | ||
| CBT+NRT | |||||||||||
| K | 13.9 | 13.9b,c | Unknown | e | Unknown | e | |||||
| L | 13.0 | 30.3 | Quit | 40.9 | 3033 | x | |||||
| M | 16.9 | 35.1d | Relapsed | 39.3 | x | 2466 | |||||
| N | 14.9 | 32.6 | Relapsed | 39.1 | 4508 | x | |||||
| O | 18.9 | 37.6c | Relapsed | 39.0 | x | 2466 | |||||
| P | 21.3 | 21.3c | Unknown | 39.3 | 2977 | x | |||||
| Q | 14.3 | 27.3 | Relapsed | x | 39.0 | 3995 | |||||
| R | 24.0 | 30.4 | Quit | x | 37.7 | 2523 | |||||
| S | 14.1 | 14.1b | Unknown | 37.4 | x | 2466 | |||||
| T | 22.3 | 31.9 | Smoking | 37.0 | x | 2296 | |||||
| U | 19.0 | 26.3 | Relapsed | x | 36.9 | 2665 | |||||
| V | 15.3 | 15.3c | Unknown | x | 36.7 | 3260 | |||||
| W | 16.1 | 18.0 | Quit | x | 36.7 | 2977 | x | ||||
| X | 17.9 | 32.6 | Relapsed | x | 36.6 | 3289 | |||||
| Y | 21.9 | 28.9 | Relapsed | x | 36.6 | 2126 | |||||
| Z | 22.1 | 33.0 | Relapsed | x | 36.1 | 2722 | |||||
| AA | 15.9 | 23.7 | Relapsed | x | 36.0 | 2580 | |||||
| BB | 21.7 | 35.1d | Smoking | x | x | 36.0 | 2410 | ||||
| CC | 16.6 | 34.0 | Relapsed | x | 35.9 | 2637 | x | 35.9 | |||
| DD | 18.9 | 25.7 | Relapsed | x | 35.0 | 2381 | |||||
| EE | 17.6 | 27.1 | Relapsed | x | 35.0 | 2325 | |||||
| FF | 15.4 | 27.7 | Quit | x | 35.0 | 1361 | x | ||||
| GG | 19.4 | 34.9c | Smoking | x | 34.9 | 2722 | |||||
| HH | 14.4 | 32.1d | Relapsed | x | 34.0 | 2608 | |||||
| II | 14.4 | 27.4 | Relapsed | x | 34.9 | 3090 | x | ||||
| JJ | 14.6 | 29.0 | Relapsed | x | x | 33.9 | 2296 | x | |||
| KK | 20.9 | 34.0 | Quit | x | x | 33.4 | 1701 | x | |||
| LL | 19.1 | 19.1b | Unknown | x | x | 33.3 | 1928 | ||||
| M | 18.4 | 25.3c | Smoking | x | 2098 | x | |||||
| M | 33.1 | ||||||||||
| NN | 18.9 | 32.0c | Quit | x | x | 32.4 | 1106 | x | |||
| OO | 16.0 | 23.3 | Quit | x | 30.7 | 1673 | x | ||||
| PP | 24.0 | 26.9 | Relapsed | x | 29.6 | twins | |||||
| 14.0 | 23.9 | Relapsed | x | 24.0 | 700 | x | |||||
| RR | 19.1 | 22.1 | Relapsed | x | 23.0 | 567 | 23.3 | ||||
Nicotine replacement therapy (NRT) use during the study. None of the participants in the cognitive-behavioral therapy (CBT)-only arm who had adverse events used NRT.
NRT use is unknown due to no contact after randomization. Baby died at 9 days old.
Participant in CBT+NRT arm did not use NRT.
Gestational age at last contact. Participant still reported using NRT. Participant may have used NRT after session but use is unknown.
Participant randomized at 13 weeks; prior to receiving any intervention, underwent ultrasound in which embryonic demise at approximately 7 weeks diagnosed.
Gestational age was approximated from obituary.
DISCUSSION
This is the first large trial to show effectiveness of NRT in helping pregnant women quit smoking. The findings from this trial differ from another large efficacy trial36; compliance with NRT in the previous trial was very low, and no differences in cessation rates resulted. Women in the Baby Steps trial used NRT more than 1 week longer than the women in the Wisborg trial. Further, length of NRT use was positively related to cessation, indicating that higher compliance might have contributed to the higher cessation rates. In addition, efficacy of CBT+NRT was nearly threefold over CBT-only; this effect is greater than what has been found in the general population.30 These results confirm findings from a small pilot study in which three of 20 women versus zero of 20 women quit smoking in the NRT patch and control arms, respectively.38 The results from Baby Steps are encouraging given the dearth of effective interventions for smoking cessation during pregnancy.
Several factors other than NRT (open-label and increased utilization with different forms) may have contributed to the observed improved effectiveness of the CBT+NRT. Also, although the women assigned to the CBT+NRT arm had more total counseling sessions than the women in the CBT-only arm, adjustment for number of sessions did not substantially change the increased cessation observed with NRT. Thus, the observed arm effect was not due to the fact that the arms differed in the mean number of counseling sessions. A comprehensive examination of the effect of counselor adherence to counseling protocols, both in terms of content and Motivational Interviewing techniques, will be published in a forthcoming manuscript.
Cessation effects during pregnancy were not statistically significant at 3-months postpartum. This was due, in part, to more women in the CBT-only arm quitting smoking postpartum. Cessation rates in the CBT-only arm went from 10% and 7% during pregnancy to 14% at 3-months postpartum, a doubling from the late pregnancy assessment. Cessation rates for the women in the CBT+NRT arm had a more linear pattern with rates at 29%, 23%, and 20%, at the 7-week, 38-week, and 3-months postpartum assessments, respectively. These results suggest that use of NRT during pregnancy does not improve the likelihood of permanent smoking cessation.
The cessation rates during pregnancy found in this trial are clouded by the DSMB suspension of enrollment. Although this study was not powered to detect differences in adverse pregnancy outcomes, it is hypothesized that improved smoking cessation might lead to a detectable improvement in pregnancy outcomes. Thus, a stopping rule was included, in the event of a twofold excess in adverse events. At an interim analysis, the a priori stopping rule was met with more adverse events occurring in the women in the CBT+NRT arm. However, the DSMB reported that adverse events likely were not associated with NRT use. This is partially confirmed by the lower cotinine values found among the women who reported using NRT compared to those who reported smoking. The observed arm difference in the adverse event rate was partially confounded by the fact that a greater proportion of women in the CBT+NRT arm had a history of preterm birth than women in the CBT-only arm. In the final analysis, after controlling for this known risk factor for subsequent preterm birth,45 the adverse event rates of the two arms was not statistically different. Further, many of the observed “serious adverse events” were preterm births between 35 and 37 weeks, with no clinical sequelae to the baby. Physiologic studies of NRT in pregnancy31–33 and previous clinical trials36–38 have not suggested an adverse effect of NRT itself in pregnancy. The data from the trial cannot support or negate the previous literature about the harm of NRT use during pregnancy. Future trials are needed to determine the safety and benefit of NRT to the fetus.
This study has several limitations. Even at the originally planned sample size, power to detect differences in specific pregnancy outcomes was lacking. In hindsight, randomization should have been stratified on history of negative pregnancy outcomes. The fact that the women lost to follow-up had more previous negative birth outcomes, smoked more, and had tried to quit during a prior pregnancy may affect generalizability. Generalizability also may be limited by medically excluding those for whom NRT was not advisable. The open-label design also may have influenced cessation rates. In addition, the unique family stresses introduced by the wars in Iraq and Afghanistan, which affected a large proportion of the participants from the Fayetteville NC clinical site, were unanticipated at the beginning of the study and may also have affected generalizability. Another potential limitation is that although not statistically significant, more women in the CBT+NRT arm completed surveys; in intent-to-treat analyses, this may have inflated the difference between arms. However, the women in the CBT arm were less engaged than those in the CBT+NRT arm in the study, in general, as indicated by their lower number of counseling sessions completed. This low engagement may be a sign of inability to quit smoking but also may be due to their disappointment in not being offered free NRT. Loss to follow-up was relatively high, but consistent with other studies of smoking cessation in this population.25
Many women who smoke recognize the risks of continuing to smoke during pregnancy, as evidenced by relatively high quit rates during the first trimester.46 For those unable to quit on their own, adding NRT to CBT may improve smoking cessation rates during pregnancy. These results are promising for future trials; however, more data are needed to determine the safety and benefit of NRT use during pregnancy.
ACKNOWLEDGEMENTS
We would like to thank our collaborators at Womack Army Medical Center, Lieutenant Colonel Jeff Kingsbury, M.D. and Lieutenant Colonel Carol Goins, R.N. We also would like to thank Dr. Neal Benowitz and Dr. Susan Curry for their expertise in designing the study. In addition, we are indebted to the many clinical sites from which we recruited pregnant smokers. We want to thank GlaxoSmithKline for donating the nicotine replacement therapy. We want to acknowledge our collaboration with Duke’s General Clinical Research Center, Protocol 906, M01-RR-30. Finally, we want to acknowledge our Data and Safety Monitoring Board: Chair: Katherine E. Hartmann, MD, PhD, Statistician: Carl F. Pieper, DrPH, Members: Paul Whitecar, MD, Kimberly S. Yarnall, MD, and Vickie White, RN.
This work was supported by National Cancer Institute grant R01CA089053 and operated under IND # 67,259. No financial disclosures were reported by the authors of this paper. Trial Registration: ClinicalTrials.gov, Registration Number NCT00224419 http://www.clinicaltrials.gov/ct/show/NCT00224419
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstet Gynecol. 1995;85:625–630. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- 2.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and Sudden Infant Death Syndrome. J Fam Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Davis RO, Cliver SP, et al. Maternal risk factors and their influence on fetal anthropometric measurements. Am J Obstetr Gynecol. 1993;168:1197–1203. doi: 10.1016/0002-9378(93)90369-t. [DOI] [PubMed] [Google Scholar]
- 4.Haglund B, Cnattingius S. Cigarette smoking as a risk factor for Sudden Infant Death Syndrome: A population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews T, Rivera C. Smoking during pregnancy—United States, 1990–2002. MMWR Morb Mortal Wkly Rep. 2004;53:923–924. [PubMed] [Google Scholar]
- 6.Butler CC, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: A randomized trial. Br J Gen Pract. 1999;49:611–616. [Google Scholar]
- 7.Colby SM, Monti PM, Barnett NP, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. J Consult Clin Psychol. 1998;66:574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- 8.Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy: Effects on pregnancy outcomes and cessation efforts. Ann Rev Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. Am J Public Health. 2000;90:786–789. doi: 10.2105/ajph.90.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann KE, Thorp JM, Jr, Pahel-Short L, Koch MA. A randomized controlled trial of smoking cessation intervention in pregnancy in an academic clinic. Obstet Gynecol. 1996;84:621–626. doi: 10.1016/0029-7844(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 11.Kendrick JS, Zahniser SC, Miller N, et al. Integrating smoking cessation into routine public prenatal care: The smoking cessation in pregnancy project. Am J Pub Health. 1995;85:217–222. doi: 10.2105/ajph.85.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride CM, Curry SJ, Grothaus L, Nelson JC, Lando H, Pirie PL. Partner smoking status and pregnant smoker's perceptions of support for and likelihood of smoking cessation. Health Psychol. 1998;17:63–69. doi: 10.1037//0278-6133.17.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Pletsch PK. Reduction of primary and secondary smoke exposure for low-income black pregnant women. Nurs Clin North Am. 2002;37:315–329. doi: 10.1016/s0029-6465(01)00011-1. [DOI] [PubMed] [Google Scholar]
- 14.Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA. 1984;251:911–915. [PubMed] [Google Scholar]
- 15.Stotts AL, DeLaune KA, Schmitz JM, Grabowski J. Impact of a motivational intervention on mechanisms of change in low-income pregnant smokers. Addict Behav. 2004;29:1649–1657. doi: 10.1016/j.addbeh.2004.02.063. [DOI] [PubMed] [Google Scholar]
- 16.Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one: a motivational intervention for resistant pregnant smokers. Addict Behav. 2002;27:275–292. doi: 10.1016/s0306-4603(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 17.Valanis B, Lichtenstein E, Mullooly JP, et al. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med. 2001;20:1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- 18.Tappin DM, Lumsden MA, Gilmour WH, et al. Randomised controlled trial of home based motivational interviewing by midwives to help pregnant smokers quit or cut down. BMJ. 2005;331:373–377. doi: 10.1136/bmj.331.7513.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira-Borges C. Effectiveness of a brief counseling and behavioral intervention for smoking cessation in pregnant women. Prev Med. 2005;41:295–302. doi: 10.1016/j.ypmed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking cessation advice in pregnancy result in long-term quitters? 18-month postpartum follow-up of a randomized controlled trial. Addiction. 2005;100:107–116. doi: 10.1111/j.1360-0443.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 21.Solomon LJ, Flynn BS. Telephone support for pregnant smokers who want to stop smoking. Health Promot Pract. 2005;6:105–108. doi: 10.1177/1524839903260642. [DOI] [PubMed] [Google Scholar]
- 22.Pbert L, Ockene JK, Zapka J, et al. A community health center smoking-cessation intervention for pregnant and postpartum women. Am J Prev Med. 2004;26:377–385. doi: 10.1016/j.amepre.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Polanska K, Hanke W, Sobala W, Lowe JB. Efficacy and effectiveness of the smoking cessation program for pregnant women. Int J Occup Med Environ Health. 2004;17:369–377. [PubMed] [Google Scholar]
- 24.McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89:706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumley J, Oliver S, Chamberlin C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2006:1. doi: 10.1002/14651858.CD001055.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Adams MA, Brogan DJ, Kendrick JS, Shulman HB, Zahniser SC, Bruce FC. Pregnancy risk assessment monitoring system working group. Smoking, pregnancy, and source of prenatal care: Results from the pregnancy risk assessment monitoring system. Obstet Gynecol. 1992;80:738–744. [PubMed] [Google Scholar]
- 27.Ershoff DH, Mullen PD, Quinn VP. A randomized trial of a serialized self-help smoking cessation program for pregnant women in an HMO. Am J Public Health. 1989;79:182–187. doi: 10.2105/ajph.79.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost FJ, Cawthon ML, Tollestrup K, Kenny FW, Schrager LS, Nordlund DJ. Smoking prevalence during pregnancy for women who are and women who are not Medicaid-funded. Am J Prev Med. 1994;10:91–96. [PubMed] [Google Scholar]
- 29.O'Campo P, Faden RR, Brown H, Gielen AC. The impact of pregnancy on women's prenatal and postpartum smoking behavior. Am J Prev Med. 1992;8:8–13. [PubMed] [Google Scholar]
- 30.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2006:1. [Google Scholar]
- 31.Oncken CA, Hardardottir H, Hatsukami DK, Lupo VR, Rodis JF, Smeltzer JS. Effects of transdermal nicotine or smoking on nicotine concentrations and maternal–fetal hemodynamics. Obstet Gynecol. 1997;90:569–574. doi: 10.1016/s0029-7844(97)00309-8. [DOI] [PubMed] [Google Scholar]
- 32.Oncken CA, Hatsukami DK, Lupo VR, Lando HA, Gibeau LM, Hansen RJ. Effects of shortterm use of nicotine gum in pregnant smokers. Clin Pharmacol Therap. 1996;59:654–661. doi: 10.1016/S0009-9236(96)90005-3. [DOI] [PubMed] [Google Scholar]
- 33.Wright LN, Thorp JMJ, Kuller JA, Shrewsbury RP, Ananth C, Hartmann K. Transdermal nicotine replacement in pregnancy: Maternal pharmacokinetics and fetal effects. Am J Obstet Gynecol. 1997;176:1090–1094. doi: 10.1016/s0002-9378(97)70407-1. [DOI] [PubMed] [Google Scholar]
- 34.Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24:277–322. doi: 10.2165/00002018-200124040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Coleman T, Antoniak M, Britton J, Thornton J, Lewis S, Watt K. Recruiting pregnant smokers for a placebo-randomised controlled trial of nicotine replacement therapy. BMC Health Serv Res. 2004;4:29. doi: 10.1186/1472-6963-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: A randomized controlled study. Obstet Gynecol. 2000;96:967–971. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- 37.Hegaard H, Hjaergaard H, Moller L, Wachmann H, Ottesen B. Multimodel intervention raises smoking cessation rate during pregnancy. Acta Obstet Gynecol Scand. 2003;82:813–819. doi: 10.1034/j.1600-0412.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 38.Hotham E, Gilbert A, Atkinson E. A randomized-controlled pilot study using nicotine patches with pregnant smokers. Addict Behav. 2006;31:641–648. doi: 10.1016/j.addbeh.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 39.Pollak K, Oncken C, Lipkus I, et al. Challenges and solutions for recruiting pregnant smokers into a nicotine replacement therapy trial. Nic Tob Res. 2006;8(4):547–554. doi: 10.1080/14622200600789882. [DOI] [PubMed] [Google Scholar]
- 40.Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- 41.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: Effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64:202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 42.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- 43.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 44.Bandura A. Self-efficacy mechanism in physiological activation and health-promoting behavior. In: Madden JM, editor. Adaptation, learning and affect. New York: Raven Press; 1986. [Google Scholar]
- 45.Moutqin J-M. Classification and heterogeneity of preterm birth. Br J Obstet Gynaecol. 2003;110:30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 46.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during and after pregnancy. Am J Public Health. 1990;80:541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]


