Abstract
The hematopoietic stem cell (HSC) niche in the bone marrow has been studied extensively over the past few decades, yet the bone marrow microenvironment that supports the growth of metastatic prostate cancer (PCa) has only been recently considered to be a specialized ‘niche' as well. New evidence supports the fact that disseminated tumor cells (DTCs) of PCa actually target the HSC niche, displace the occupant HSCs and take up residence in the pre-existing niche space. This review describes some of the evidence and mechanisms by which DTCs act as molecular parasites of the HSC niche. Furthermore, the interactions between DTCs, HSCs and the niche may provide new targets for niche-directed therapy, as well as insight into the perplexing clinical manifestations of metastatic PCa disease.
Keywords: dormancy, hematopoietic stem cell, metastasis, niche, osteoblasts, prostate cancer
Introduction to cancer metastasis and the niche
In the field of bone metastasis, Stephen Paget's famous observation that tumor ‘seeds' seek out ‘fertile soil'1 represents a well-known paradigm for why certain cancers including breast and prostate cancer (PCa) localize to the bone marrow with a strikingly high propensity. The bone marrow microenvironment has been well established as a regulatory site for hematopoietic function.2, 3, 4, 5, 6 In the marrow, hematopoietic stem cells (HSCs) are believed to localize to the specific microenvironment, or the niche, to engage in a quiescent state to preserve their self-renewal capacity, or divide and differentiate to populate their corresponding lineages.3 Two such niches have been described: a vascular niche and an endosteal niche. The majority of HSCs as detected by immunohistochemical techniques appear to be in the vascular niche, which is compromised of endothelial cells in the sinusoids of the marrow. The sinusoids are venous areas of marrow that facilitate extravasation of HSCs into circulation.5, 7 In the vascular niche, it is thought that HSCs are engaged in active surveillance of hematopoietic output, such that these HSCs are able to rapidly respond to hematopoietic demands. HSCs in the endosteal niche are thought to represent a more quiescent or reserve population,3, 4, 8 although these data have been hotly debated.2, 5, 9 The principal regulatory cells of the HSC niche are thought to be osteoblasts, endothelial cells, adipocyctes, mesenchymal stem cells and CXCL12-abundant reticular cells. Osteoblasts appear to be major regulators of endosteal niche function, as they have been shown to express a milieu of cytokines, growth factors and adhesion molecules critical to HSC regulation.3, 4, 8
Parallel to the concept of the HSC niche, growing evidence have suggested that disseminated tumor cells (DTCs) also localize and take up residence in the bone marrow niche.2, 10 The niche provides signals for localization, adhesion molecules and mechanisms to regulate dormancy and survival. In the bone marrow, the proximity of the niche to the endosteal surface also provides access to active bone, which is a locale rich in growth factors and other soluble factors conducive to tumor survival and growth. In addition to these abundant growth factors, cytokines and nutrients, the tumor niche is composed of many different cell types. Mesenchymally derived cells including osteoblasts, fibroblasts and reticular cells are believed to provide the physical framework of the bone marrow tumor niche.2 Hematopoetically derived cells including HSCs, macrophages, lymphocytes and osteoclasts are also present in the marrow and interact with tumor cells, creating a dynamic ecosystem.
Once a DTC has engaged the niche, it may initiate a state of dormancy or proliferate to populate a metastatic mass.11 However, not all areas of the marrow possess the ability to support tumor growth, as metastases are rarely seen in the diaphyses, or areas of long bone, while they are commonly detected in the trabecular bone of epiphyses.12, 13 Contrary to the idea of the bone marrow as a homogenous field of soil where DTC seeds would be able to survive and grow equally in all parts, metastases grow in highly complex and specialized microenvironments within the marrow that are well equipped for tumor survival. Clinically, metastatic tumor cells may be found as a detectable and overt mass of cells dominating a portion of the bone marrow space, rapidly dividing and consuming nutrients, while initiating angiogenesis to fuel their growth. In addition, clinically silent micro-metastases and DTCs have also been found in the marrow of PCa patients.14, 15 These DTCs are genetically heterogeneous, while overt metastases show a high degree of genetic homogeneity among the cells in the tumor mass, indicating growth has proceeded through clonal expansion of a single DTC.16 The genetic heterogeneity of DTCs suggests that the tumor cells circulating through the bloodstream are selected for survival in a Darwinian manner.17 As such, a major determinant of this selection is dictated by the environmental pressures specified by the bone marrow. The bone and bone marrow itself is heterogeneous, as different areas of the bone marrow have different rates of turnover, cytokine levels and cellular populations. Metastatic cells have a high propensity for areas of the marrow with high bone turnover, such as trabecular bone, indicating that there is a preferred microenvironment for these cells, commonly referred to as the ‘niche'.
Targeting the osteoblastic niche
Osteoblasts have been studied in detail to understand their role in the HSC niche, but the role of osteoblasts as major regulators of the bone marrow tumor niche has only recently been appreciated. Osteoblasts in particular play a critical regulatory role and may specifically identify the spatial and functional location of both the quiescent HSC and PCa.3, 18, 19, 20, 21 The parallels between the HSC niche and the PCa niche have become increasingly apparent, and it has recently been demonstrated that metastatic PCa cells actually use the same niche as HSCs. Recent study has provided evidence that DTCs target and displace HSCs out of their niche, and establish the niche space as metastatic microfoci.22 Using an in vivo micrometasasis model,23 DTCs were introduced into immunodeficient mice and allowed to take up residence in the bone marrow. After this establishment of micro-metastases, the mice were given bone marrow transplantations to determine the ability of HSCs to engraft into their marrow niches. Significantly less HSC engraftment was seen in mice harboring tumor cells in their marrow, suggesting that the tumor cells were occupying the niches and preventing HSC localization and engraftment.22 More importantly, a competitive engraftment was performed to determine if DTCs directly out-compete HSCs for niche space. In this experiment, existing niches were cleared by lethally irradiating mice. Equal amounts of HSCs were given to all mice via bone marrow transplantation to rescue the mice from the lethal irradiation, and in some groups, PCa cells were also implanted. The PCa cells were irradiated to rule out proliferation in the marrow as a variable for differences in engraftment. Mice receiving tumors cells showed markedly less engraftment and thus less survival, indicating that PCa cells did in fact out-compete HSCs for their niches.22 These findings have lead to the concept of DTCs as ‘parasites' of the HSC niche.22
Osteoblasts express a milieu of secretory factors and adhesion molecules that have shown to be important for PCa metastasis to the bone marrow.21 In vitro and in vivo studies confirm that they furthermore regulate essential niche activity. In vivo expansion of the population of osteoblasts using parathyroid hormone resulted in a higher capacity for metastatic implantation.22 Conditional ablation osteoblasts in subcutaneous bone implants resulted in significantly less tumor metastasis to the bone implants in comparsion to bone implants where osteoblasts remained viable.22 Thus, osteoblasts were demonstrated to be imperative for supporting metastatic tumors.22
Homing and localization to the niche through the stromal cell derived factor-1 (SDF-1)/CXCR4 axis
Relative to other cells in the microenvironment, osteoblasts secrete particularly high levels of the cytokine SDF-1, also known as CXCL12. SDF-1 is a chemokine ligand that binds to receptors CXCR4 and CXCR7. It serves as a strong chemoattractant for cells expressing either of its receptors. The SDF-1/CXCR4 axis strongly regulates PCa invasion and localization to the bone marrow.24, 25, 26 PCa cells have been shown to express both CXCR4 and CXCR7, and upregulation of these receptors correlates with a poor prognosis.26, 27 This localization is furthermore enhanced by signaling through CXCR4. Engagement of CXCR4 receptors on PCa cells induces expression of the adhesion molecules αVβ3-integrin28 and CD164.29 Both of these molecules support tight binding of the tumor cell to the osteoblast. Importantly, blockade of CXCR4 receptors on PCa renders less metastatic load in the bones of mice,25 while endogenous enhancement of SDF-1 secretion results in greater tumor burden in the bone.30 In parallel, HSCs must ‘home' to the osteoblast in order to engage the endosteal niche. Homing activity is an essential stem cell behavior, and is especially important for engraftment of stem cell transplants.31, 32, 33 The SDF-1/CXCR4 axis is the key regulator of homing activity. HSCs express CXCR4 on their surface, and use the gradient of SDF-1 generated by osteoblasts to localize to their surface. Depletion of SDF-1 and blockade of CXCR4 have both been shown to severely reduce HSC homing and thus engraftment.34, 35 It was noted that PCa DTCs use the SDF-1/CXCR4 pathway as mechanism to parasitize the HSC niche.22 This was demonstrated by disrupting SDF-1/CXCR4 activity in two ways: by degrading SDF-1 in vivo using recombinant granulocyte colony-stimulating factor (G-CSF), and also by use of AMD3100, a small molecule antagonist of the CXCR4 receptor. Both methods revealed that PCa cells could be mobilized out of the HSC niche and into the blood using these HSC-mobilizing agents.22 Thus, DTCs hijack this same molecular pathways used by HSCs to gain access to the niche. The SDF-1/CXCR4 pathway is just one example of a well-studied axis that is shared by HSCs. It is likely that DTCs also utilize other shared localization pathways that are less well understood.
Induction of quiescence and dormancy by the niche
Both HSCs and DTCs can engage in a state of reversible cell cycle arrest once they have localized to the niche. This concept has been referred to as ‘quiescence' in the field of stem cells,8 and ‘dormancy' in the context of metastatic tumor cells.36 Although quiescence and dormancy should not be considered equivalent processes, they do share some similarities, especially in regard to mechanisms of induction.37 The ability to engage in quiescence is a hallmark of stem cell function. Quiescence allows HSCs to evade the damage of cellular toxins and stresses, and thus maintain a reserve population of viable stem cells.38 Importantly, these cells can be induced out of cell cycle arrest, so they can go on to differentiate into more mature progenitors that will populate the entire hematopoietic system.3 Dormancy is the process that is believed to allow tumor cells to become resistant to traditional chemotherapies, as most of these agents work by targeting rapidly dividing cells. By engaging in reversible cell cycle arrest, dormant tumor cells are not susceptible to chemotherapeutic drugs, and may go on to survive and begin dividing and proliferating. The ability to switch between cell cycle arrest and activity is believed to allow tumor cells to generate and/or repopulate a tumor mass or metastasis.11, 36 One molecule that is believed to be implicated in the induction of reversible cell cycle arrest of HSCs and DTCs in the marrow is annexin II (Anxa2). Anxa2 is expressed on the cell surface of osteoblasts. In conjunction with its corresponding receptor, Anxa2R, Anxa2 is used in a similar manner to the SDF-1/CXCR4 to regulate homing. Anxa2R is expressed on both HSCs and PCa cells alike.39, 40 In HSCs, short-term lodging, engraftment and survival of declines when the Anxa2/Anxa2R axis is disrupted.39 In PCa, Anxa2R has been shown to be upregulated in on metastatic cells, and the degree of expression correlates with a poor outcome.40, 41 Furthermore, blockade of the Anxa2/Anxa2R axis results in significantly reduced PCa tumor burden in mice.40 In addition to localization, both HSCs and PCa adhere to the niche through the Anxa2/Anxa2R axis. It was demonstrated that both HSCs and PCa cells bind significantly better to osteoblasts expressing Anxa2 than those isolated from Anxa2−/− animals.22 Once a cell is anchored into its niche through adhesion, both HSCs and metastatic PCa cells may use the Anxa2/Anxa2R axis to initiate a state of cell cycle arrest. It has been proposed that this occurs in part through signaling by the growth arrest specific-6 (GAS6)/Axl axis. Recent work by our group has also shown that Anxa2 binding induces the expression of Axl, the receptor to GAS6.42 GAS6 is expressed by osteoblasts, and its receptors include the tyrosine kinases Axl, Sky and Mer.42, 43 Axl is expressed on both HSCs43, 44 and PCa42 alike. Engagement of GAS6 to Axl on PCa induces growth arrest and confers drug resistance,42 and may thus result in chemotherapy-resistant dormant tumor cells. In addition to Anxa2 and GAS6, other molecules have also been implicated in HSC quiescence and tumor dormancy, yet the evidence for shared pathways between HSCs and PCa specifically remains largely unexplored. Further research will undoubtedly provide more knowledge on this topic and exciting new insight.
Implications
The understanding that DTCs target the HSC niche and acts as parasites provides important new directions for further research and targets for drug therapy. In addition, the new paradigm of PCa invading the HSC niche may provide more insight to lesser understood phenomena that are clinically seen in metastatic PCa.
Minimal residual disease
One of the most dreaded complications of PCa is metastatic relapse. Clinical observations show that many patients have viable DTCs present in their bone marrow, even after removal of the primary prostate tumor and chemotherapeutic treatment.45, 46 This phenomenon is known as minimal residual disease (MRD). These viable DTCs are thought to persist in a state of tumor dormancy.11 They evade attack from the immune system and the toxicity of chemotherapy through their engagement in reversible growth arrest. It remains unclear what factors specifically induce these dormant cells out of growth arrest and into a proliferative state, and thus allow the cancer to recur. In order to fully eradicate the presence of cancer in a patient's body, it is critical that we learn how to fully eradicate MRD in addition to overt tumors and metastatic lesions. MRD and tumor dormancy is an ongoing topic of research, as the mechanisms by which tumors evade chemotherapy and silently persist in the marrow appear complex and multifaceted. Considering that tumor cells hijack the HSC niche and utilize similar molecular pathways, it is possible that the same mechanisms that regulate HSC quiescence may have a role in regulating tumor dormancy and allowing MRD to persist. As more understanding is developed regarding HSC quiescence, this new knowledge will also be valuable in providing potentials strategies toward combating MRD.
Osteoblastic activity
An interesting aspect of PCa is that it is the only metastatic cancer known to form predominantly osteoblastic lesions in bone.47, 48 Other cancers that metastasize to the bone marrow often show an osteolytic phenotype. This is due to activation of the osteoclasts in the marrow, which degrade the bone and cause painful fractures. When osteoclasts degrade bone, they release the sequestered nutrients and growth factors that are otherwise tied up in the bone and unavailable to tumor cells. While osteolytic PCa is not uncommon, PCa is unique in its high prevalence of creating osteoblastic lesions. Through numerous mechanisms, including inhibition of osteoblast apoptosis and expression of the molecules bone morphogenic proteins, parathyroid hormone, parathyroid hormone related protein, which increase osteoblast populations and osteoblastic activity, PCa has a characteristic ability induce rapid bone formation.21 This PCa-induced bone is often disorganized, and variable in density,49 inevitably leading to fractures and bone pain.49, 50 From a biological standpoint, the rationale for why bone formation may be advantageous for metastatic progression has been unclear. In light of the fact that DTCs use osteoblasts as niche cells, this induction of bone by PCa may be a way to further support niche availability and activity. It has been well established that cancers can induce neoangiogenesis to further support their growth,51, 52 so it may accordingly follow that PCa can induce new bone growth to support an expanding tumor mass in a similar fashion. One direct mechanism through which PCa induces bone growth is through phosphoglycerate kinase 1. Phosphoglycerate kinase 1 is expressed by metasatic PCa, and induces bone marrow stromal cells to differentiate into osteoblasts upon contact.53 In addition to direct stimulation of osteoblastic differentiation, PCa has also been shown to alter the phenotype of other cells present in the niche's vicinity. It has been demonstrated that HSCs actively participate in osteoblastic differentiation of marrow mesenchymal stem cells by expressing bone morphogenic proteins when they encountered hematologic stress.54 Similarly, PCa cells were recently shown to induce HSCs to express bone morphogenic proteins, which then could stimulate bone marrow stromal cells to differentiate into osteoblasts.56 This study shows that in the presence of PCa, HSCs are induced to become unwitting mediators in the stimulation of osteoblastic activity, and perhaps further contributing to the malicious augmentation of niche availability. Better understanding of niche expansion and its nuances may provide therapeutic targets to combat metastasis growth and skeletal compromise.
Hematologic complications
Another complication of PCa is anemia, leukopenia and bone marrow failure associated with metastatic disease.56 As an example, PCa bone metastases can cause myelophthisis, or filling of the marrow space. Myelophthisis is associated with leukoerythroblastic anemia, which is characterized by partially differentiated hematopoietic cells in the peripheral blood.57, 58 In addition, dysregulation of hematopoietic differentiation is also observed. In leukoerythroblastic anemia, large numbers of immature leukocytes are present, and erythrocytes retain their nuclei, indicating a defect in differentiation. This results in nonfunctional erythrocytes and causes anemia. Despite the fact that phenomenon is a recognized clinical complication of PCa, it has not been studied in detail and its pathogenesis concerning PCa is not completely understood. In light of the fact that PCa cells occupy HSC niches, leukoerythroblastic anemia may be a manifestation of HSCs that are displaced out of their niches and forced into differentiation. This idea is supported by a recent study in which, more hematopoietic progenitor cells were found in peripheral blood of PCa patients with bone metastasis than either PCa patients with no evidence of bone metastasis or healthy age-matched controls.22 This is further supported by the observation that more hematopoietic progenitor cells and less HSCs were recovered from the blood and the marrow of tumor-bearing animals, respectively.22 An increased ratio of blood hematopoietic progenitor cells to marrow HSCs indicates that HSCs have lost their self-renewal capacity and are being forced to undergo differentiation and become lineage-specific progenitors. These observations suggest that the HSCs had been displaced from their niches, and lost their ability to maintain quiescence and self-renewal. Clinically, this may translate to a lack of signaling from the niche, which may result in the deregulated differentiation and nonfunctional hematopoietic cells that are observed.
The niche as a novel target for therapy
Finally, inhibition of the SDF-1/CXCR4 axis may provide a new approach to therapy. Filgrastim, commonly known as the drug Neupogen, is a G-CSF analog used to mobilize HSCs out of niches to increase the number of HSCs in peripheral blood in order to be collected for stem cell transplants. The small molecule AMD3100, known as plerixafor, is also used clinically and often in conjunction with G-CSF to mobilize stem cells.59, 60, 61, 62 G-CSF mobilizes HSCs from their niches by decreasing levels of SDF-1 in the marrow.63 AMD3100 is a selective CXCR4 antagonist, and prevents cells from interacting with SDF-1.64 It has recently been shown that AMD3100 and G-CSF also severe the connections between PCa and the niche, and similarly mobilize the tumor cells out in to the peripheral blood.22 In the peripheral blood, the tumor cells are no longer protected by the niche, and may be forced to exit a dormant state that renders them impervious to chemotherapy. Similar to how HSCs are induced to differentiate once displaced from the niche, PCa cells may re-enter the cell cycle and cease from growth arrest. Cycling cells are the main target of chemotherapy, so mobilization from the niche may increase the sensitivity of metastatic PCa cells to these drugs. Currently, this idea for therapy has yet to be considered clinically and requires more research, especially considering mobilizing dormant PCa cells may risk inducing metastatic proliferation. Nonetheless, this concept has already been reported to be effective in a mouse model of hematologic cancer.65 This recent study used AMD3100 to mobilize acute myeloid leukemia out of the marrow has shown to increase the chemosensitivity of the cells. Another study shows a similar effect in multiple myeloma.66 These reports show that other malignant cells may also hijack the HSC niche using the SDF-1/CXCR4 axis, and indicate that tumor–niche interactions may be important targets for improving current chemotherapy regimens. As an alternative to enhancing the action of current chemotherapy, another potential area of exploration may be to target the SDF-1/CXCR4 pathway through enhancing the activity CD26, which degrades SDF-1 in vivo.30 Other potential niche targets may include Anxa2, as this may interfere with PCa niche targeting and binding. Targeting Anxa2 may also enhance the effect of targeting the SDF-1/CXCR4 axis, as Anxa2 has recently been found to have a new role in which it anchors SDF-1 to facilitate the presentation of SDF-1 to HSCs during localization.67 In addition, there are numerous other adhesion molecules, growth factors, and chemoattractants that have similarly been indicated in the niche function, many of which may be considered as potential targets for therapy.17, 68 Targeting niche molecules is currently an active area of research.
Conclusions
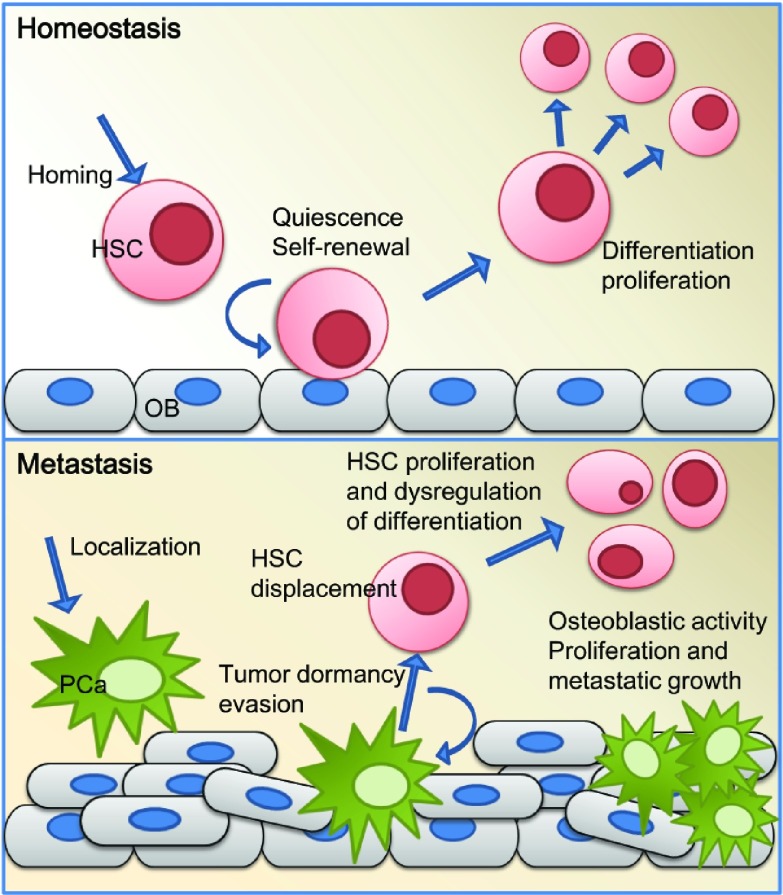
The striking similarities between the PCa niche and the HSC niche have been well established in the literature, but only recently have the niches been shown to be one in the same (Figure 1). It is now understood that all areas of the bone are not alike, and thus marrow is not a homogeneous soil able to support random DTC growth. Instead, the DTCs seek out established osteoblastic microenvironments that have already been selected by HSCs as conducive to survival, and take over these niches for their own activities. More research and characterization of the defining and most important niche components will without a doubt lead to a better understanding of the complex cellular interactions that occur in bone metastases. In order to develop therapies targeted at tumor–niche interactions, it is imperative that effective in vitro and in vivo models are developed and refined. As niche biology unfolds, it will undoubtedly provide us with new ideas and innovative strategies for better understanding and treating metastasis, tumor dormancy, and PCa complications.
Figure 1.
In the top panel, HSC function under homeostatic conditions is depicted. HSCs home to the endosteal niche using the SDF-1/CXCR4 axis. HSCs utilize the adhesion molecules and soluble factors produced by the OB in the endosteal niche to induce quiescence and regulate self-renewal. They can be easily mobilized into the vascular niches of the bone marrow sinusoids to proliferate and differentiate when needed. The bottom panel depicts the endosteal niche when disseminated PCa cells metastasize to bone. PCa localizes to the endosteum and takes up residence in the osteoblastic HSC niche, hijacking the pathways and mechanisms used by HSCs. The HSCs are displaced from their niches and lose their regulatory niche interactions. Once the PCa has parasitized the HSC niche, it is postulated that these tumor cells can then engage in a state of dormancy, allowing PCa to evade chemotherapy and the immune system. In addition, PCa can induce osteoblastic activity, leading to dsyregulated bone formation and the formation of new niches. HSC, hematopoietic stem cell; OB, osteoblasts; PCa, prostate cancer.
Acknowledgments
This work is directly supported by the National Cancer Institute (CA093900, KJP and RST, CA163124, YS, KJP and RST), NIDDK (DK08248, RT), The Fund for Cancer Research (RST), United States Department of Defense (KJP, YS and RST), and the Prostate Cancer Foundation (YS, KJP and RST). KJP receives support as an American Cancer Society Clinical Research Professor, NIH SPORE in prostate cancer grant P50 CA69568, and the Cancer Center support grant P30 CA46592.
The authors declare no competing financial interests.
References
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–50. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–9. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–32. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- Doan PL, Chute JP.The vascular niche: home for normal and malignant hematopoietic stem cells Leukemiae-pub ahead of print 2 September2011. doi: 10.1038/leu.2011.236. [DOI] [PubMed]
- Arai F, Yoshihara H, Hosokawa K, Nakamura Y, Gomei Y, et al. Niche regulation of hematopoietic stem cells in the endosteum. Ann NY Acad Sci. 2009;1176:36–46. doi: 10.1111/j.1749-6632.2009.04561.x. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–9. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbriaco M, Larson SM, Yeung HW, Mawlawi OR, Erdi Y, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the Bone Scan Index. Clin Cancer Res. 1998;4:1765–72. [PubMed] [Google Scholar]
- Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, et al. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–81. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- Klein CA, Blankenstein TJ, Schmidt-Kittler O, Petronio M, Polzer B, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–9. doi: 10.1016/S0140-6736(02)09838-0. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–64. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiva K, Sun YX, Taichman RS. The role of osteoblasts in regulating hematopoietic stem cell activity and tumor metastasis. Braz J Med Biol Res. 2005;38:1449–54. doi: 10.1590/s0100-879x2005001000001. [DOI] [PubMed] [Google Scholar]
- Jung Y, Wang J, Havens A, Sun Y, Jin T, et al. Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine. 2005;32:155–62. doi: 10.1016/j.cyto.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Keller ET, Zhang J, Cooper CR, Smith PC, McCauley LK, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333–49. doi: 10.1023/a:1015599831232. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–80. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- Sun YX, Schneider A, Jung Y, Wang J, Dai J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. . J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. . J Cell Biochem. 2003;89:462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, et al. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- Havens AM, Jung Y, Sun YX, Wang J, Shah RB, et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer. 2006;6:195. doi: 10.1186/1471-2407-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Pedersen EA, Shiozawa Y, Havens AM, Jung Y, et al. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin Exp Metastasis. 2008;25:765–76. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–9. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–31. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5:1744–50. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- Pedersen EA, Shiozawa Y, Taichman RS.Structure and function of the solid tumor niche Front Biosci.In Press [DOI] [PMC free article] [PubMed]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Jung Y, Wang J, Song J, Shiozawa Y, Havens A, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Waisman DM, Sloane BF. Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta. 2000;1477:215–30. doi: 10.1016/s0167-4838(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–27. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolayers that overexpress growth arrest-specific gene-6 (GAS6) Proc Natl Acad Sci USA. 2000;97:12260–5. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer A, Fiebeler A, Graham DK, O'Bryan JP, Schmidt CA, et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84:1931–41. [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2012;15:677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzenmaier J, Ellis WJ, Hawley S, Arfman EW, Klein JR, et al. The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol Oncol. 2007;25:214–20. doi: 10.1016/j.urolonc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Zetter BR. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990;322:605–12. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]
- Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–44. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–60. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastham JA. Bone health in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2007;177:17–24. doi: 10.1016/j.juro.2006.08.089. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, Wang Z, Pedersen EA, et al. Expression of PGK1 by prostate cancer cells induces bone formation. Mol Cancer Res. 2009;7:1595–604. doi: 10.1158/1541-7786.MCR-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–51. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Shiozawa Y, Jung Y, Kim JK, Pedersen E, Mishra A, Zalucia JL, Wang J, Keller ET, Pienta KJ, Taichman RS.Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Mol Cancer Res 2012 Jan12[Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Spivak JL. Cancer-related anemia: its causes and characteristics. Semin Oncol. 1994;21 (2 Suppl 3:3–8. [PubMed] [Google Scholar]
- Eriksson S, Killander J, Wadman B. Leuco-erythroblastic anaemia in prostatic cancer. Report of two cases with complete haematological remission. Scand J Haematol. 1972;9:648–53. doi: 10.1111/j.1600-0609.1972.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Shamdas GJ, Ahmann FR, Matzner MB, Ritchie JM. Leukoerythroblastic anemia in metastatic prostate cancer. Clinical and prognostic significance in patients with hormone-refractory disease. Cancer. 1993;71:3594–600. doi: 10.1002/1097-0142(19930601)71:11<3594::aid-cncr2820711121>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–53. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, de Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–14. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, Patel LR, Havens AM, et al. Annexin-2 is a regulator of stromal cell-derived factor-1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol. 2011;39:151–66.e1. doi: 10.1016/j.exphem.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res. 2011;17:5553–8. doi: 10.1158/1078-0432.CCR-10-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]