Dear Editor
CD44 is a transmembrane glycoprotein expressed on the surface of many cell types including the majority of myeloid cells and early thymic T-cell progenitors (1). As a cell adhesion molecule, CD44 is differentially required for the adhesion and mobility of different leukemia initiating cells. In animal models of BCR-ABL induced human hematopoietic malignancies, CD44 is required for the homing and engraftment of chronic myeloid leukemia initiating cells but is dispensable for the engraftment of B-cell lymphoblastic leukemia initiating cells (2). In contrast, in an animal model of human acute myeloid leukemia, a CD44 activating antibody eradicates leukemia initiating cells partially by interfering with their interaction with the microenvironment (3). CD44 is also an important regulator of cell signaling and regulates signaling cascades in various ways (1). For example, CD44 can provide specialized platforms for growth factors and matrix metalloproteinases, act as a co-receptor in many receptor complexes, and/or organize signaling cascades through association with cytoskeleton.
During tumorigenesis, various signals have been implicated in regulating CD44 expression and/or its alternative splicing. A positive feedback loop has been identified to couple the activation of Ras/ERK signaling and induction of CD44 expression, in particular expression of its splicing variant v6(4). Ras signaling promotes CD44v6 expression and in turn CD44v6 sustains late Ras signaling. Consistent with this finding, we and others previously reported that CD44 is invariably overexpressed in acute T-cell lymphoblastic leukemia/lymphoma (T-ALL) induced by endogenous oncogenic Kras (Kras G12D) or oncogenic Nras (5–7).
Under physiologic conditions, CD44 is only transiently expressed in early thymic T-cell progenitors at the CD4, CD8-double negative (DN) 1 and DN2 stages. However, in TALL patients CD44 is often expressed in the tumor T-cells. Expression of CD44 correlates with increased numbers of circulating blasts as well as tissue infiltration (8) and is a negative prognostic factor (9). Activation of CD44 enhances DNA repair and thus protects T-ALL cells from chemo/radiation therapy-induced apoptosis. Consistent with this finding, blocking CD44 function by IM7 antibody sensitizes T-ALL cells to dexamethasone-induced apoptosis. Despite the apparently necessary role of CD44 in some types of cancers, CD44 is dispensable in normal cells as mice develop and survive well in the absence of CD44 (10). This makes CD44 an attractive target for treating CD44+ cancers.
Given the important role of CD44 in homing and engraftment of tumor cells as well as in modulating cytokine signaling, we asked whether and how CD44 deficiency affects Kras G12D-induced hematopoietic malignancies. To address these questions, we generated LSL Kras G12D/+; Mx1-Cre mice and LSL Kras G12D/+; Mx1-Cre; CD44−/− mice (Fig. S1A). Administration of polyinosinic-polycytidylic acid (pI-pC) in these compound mice induces expression of Kras G12D. We refer to these pI-pC-treated compound mice as Kras G12D and Kras G12D; CD44−/− mice respectively, and pI-pC-treated Mx1-Cre or wild-type mice as control mice throughout this manuscript.
After acute induction of Kras G12D expression in whole bone marrow cells, both Kras G12D and Kras G12D; CD44−/− mice showed marked splenomegaly, which is characteristic of myeloproliferative neoplasm (MPN) (Fig. 1A and Fig. S1B). However, the average spleen weight of Kras G12D; CD44−/− mice was significantly lower than that of Kras G12D mice, suggesting that CD44 deficiency attenuates but does not completely prevent acute MPN development in Kras G12D mice. Consistent with this finding, Kras G12D; CD44−/− mice indeed survived significantly longer than Kras G12D mice (Fig. 1B). At the moribund stage, these two groups of mice showed comparable MPN phenotypes (Fig. S2).
Figure 1. Loss of CD44 alleviates the acute MPN phenotypes in Kras G12D mice and attenuates aberrant GM-CSF signaling in Kras G12D cells.
Five-seven week old mice were injected with pI-pC as described in Supplementary Materials and Methods. Two days after the 2nd pI-pC injection, various hematopoietic tissues were collected for analysis. Kras G12D and Kras G12D; CD44−/− refer to pI-pC treated compound mice. (A) Splenomegaly in Kras G12D and Kras G12D; CD44−/− mice. Results are presented as scatter plots of the spleen weight of individual animals with mean ± s.d.. Student’s t-test was performed: * p < 0.05. (B) Kaplan-Meier survival curves were plotted against days after the 1st pI-pC injection. P value was determined by the Log-rank test. (C) Total bone marrow cells were serum- and cytokine- starved for 1 hour and stimulated with various concentrations of GM-CSF (0, 0.16, and 2 ng/ml) or IL-3 (0, 1, and 10 ng/ml) at 37°C for 10 minutes. Levels of phosphorylated ERK1/2 and STAT5 were measured using phospho-specific flow cytometry. Non-neutrophil bone marrow cells were gated for data analysis. Myeloid progenitors are enriched in c-Kit+ Lin−/low cells (R1), whereas myeloid precursors are enriched in c-Kit− Lin−/low cells (R2). To quantify the activation of ERK1/2 and STAT5, median intensities of p-ERK or p-STAT5 at different GM-CSF or IL-3 concentrations in different groups of animals are compared to those of their respective control cells at 0 ng/ml, which are arbitrarily set at 1. Data from more than three independent experiments are shown as mean ± s.d.. Red asterisks indicate significant differences of Kras G12D cells compared to the control cells (unspecified or specified with brackets) or Kras G12D; CD44−/− cells (specified with brackets), while blue asterisks indicate significant differences of Kras G12D; CD44−/− cells compared to the control cells (p<0.05).
To investigate whether the diminished MPN phenotypes observed in Kras G12D; CD44−/− mice are due to reduced cytokine signaling, we analyzed GM-CSF- and IL-3-evoked ERK1/2 and STAT5 activation in both c-Kit+ Lin−/low cells (R1, enriched for myeloid progenitors) and c-Kit− Lin−/low cells (R2, enriched for myeloid precursors) (11) as well as SCF-evoked AKT activation in R1 cells. Our results show that CD44 deficiency greatly attenuates aberrant GM-CSF signaling in Kras G12D myeloid progenitor/precursor cells, while it has no significant effect on IL-3- and SCF-evoked signaling in Kras G12D cells (Fig. 1C and Fig. S3). Together, these results demonstrate that CD44 deficiency compromises some but not all of cytokine signaling in Kras G12D cells, which might contribute to the moderate attenuation of MPN phenotypes in Kras G12D; CD44−/− mice.
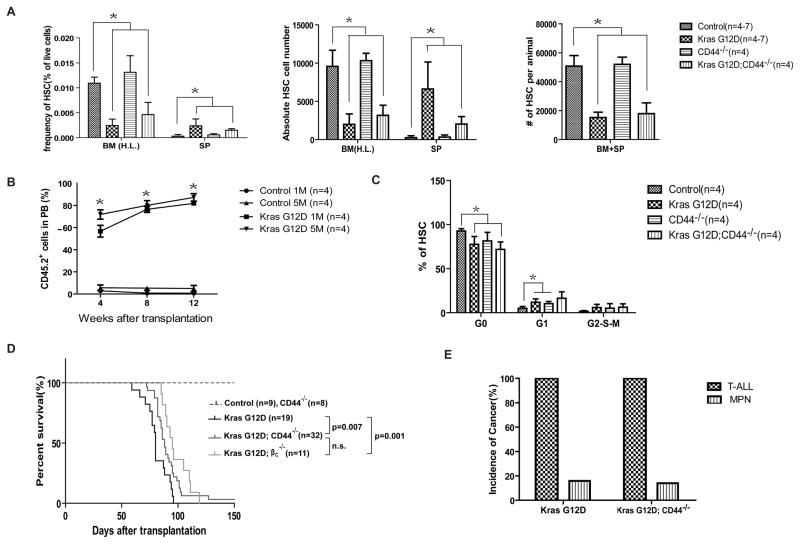
In a bone marrow transplantation model, mice receiving Kras G12D cells develop a highly penetrant T-ALL with a low incidence of MPN. The possible reasons leading to this drastic phenotypic switch are extensively discussed in previous publications (6, 12). We found that only Kras G12D hematopoietic stem cells (HSCs) but not other lineage-committed progenitors initiate both MPN (6) and T-ALL in recipient mice (Table S1), indicating that in Kras G12D model, genetically altered HSCs are the cell origin for both MPN and T-ALL. Therefore, we next determined whether loss of CD44 affects the properties of Kras G12D HSCs. Surprisingly, CD44 is dispensable in Kras G12D HSCs. CD44 deficiency does not affect the depletion (Fig. 2A), mobilization (Fig. 2A and 2B), hyperproliferation (Fig. 2C), and survival (Fig. S4) of Kras G12D HSCs. In addition, although recipient mice with Kras G12D; CD44−/− cells survived significantly longer (Fig. 2D), all these mice developed T-ALL and 19% of them developed MPN simultaneously, similar to the mice injected with Kras G12D cells (Fig. 2E and Fig. S5). These results suggest that CD44 is also dispensable for the homing and engraftment of Kras G12D HSCs.
Figure 2. CD44 deficiency does not alter the properties of Kras G12D HSCs.
Different tissues were isolated and analyzed two days after the 2nd pI-pC injection. HSCs are defined as [CD41 CD48 B220 Gr1 TER119]− CD150+ cKit+ Sca1+ cells. (A) Quantitative analysis of HSCs in hind limb bone marrow [BM (H.L.)] and spleen (SP) of control, Kras G12D, CD44−/−, and Kras G12D; CD44−/− mice. The absolute HSC numbers in BM (H.L.) and spleen were calculated based on bone marrow or spleen cell numbers and HSC frequencies. Because BM (H.L.) represents 25% of whole body bone marrow, the total number of HSCs per animal was calculated as the sum of HSC number in spleen and four-fold of HSC number in BM (H.L.). Data are presented as average from at least 4 mice each group + s.d.. (B) One million (1M) or five million (5M) splenocytes of control or Kras G12D mice were transplanted into individual recipient mice as described in the Supplementary Materials and Methods. The percentages of donor derived cells (CD45.2+) in the peripheral blood of recipient mice were examined at multiple time points after transplantation. Data are presented as mean ± s.d.. Student’s t-test was performed against controls: *p<0.01. (C) Cell cycle analysis of HSCs in bone marrow of control, Kras G12D, CD44−/−, and Kras G12D; CD44−/− mice. Results are presented as mean + s.d.. Student’s t-test was performed: *p < 0.05. (D) Lethally irradiated mice (CD45.1+) were transplanted with 2.5 × 105 total bone marrow cells of control, Kras G12D, CD44−/−, Kras G12D; CD44−/−, or Kras G12D; βc−/− mice along with same number of competitor cells through retro-orbital injections. Kaplan-Meier survival curves of reconstituted mice were plotted against days after transplantation. Log-rank test was performed: n.s., not significant. (E) Disease distribution patterns in recipient mice transplanted with Kras G12D/+ or Kras G12D; CD44−/− cells.
To determine whether the prolonged survival observed in recipient mice with Kras G12D; CD44−/− cells is due to reduced cytokine signaling, for example, GM-CSF signaling, we repeated the same transplantation experiment using Kras G12D cells deficient for the common β subunit of GM-CSF receptor (Kras G12D; βc−/− cells) (Fig. 2D). Consistent with our signaling and survival studies in primary non-transplanted mice (Fig. 1), these recipient mice indeed survived longer than those with Kras G12D cells but comparable to those with Kras G12D; CD44−/− cells. Therefore, our data suggest that loss of CD44 prolongs the survival of recipient mice in a cell-autonomous manner and the extended survival is likely through downregulating GM-CSF signaling rather than impairing the homing and engraftment of Kras G12D HSCs.
Although overexpression of CD44 is observed in all Kras G12D-induced T-ALL tumors (5, 6), apparently T-ALL formation can occur in the absence of CD44 (Fig. 5). Because multiple types of Notch1 mutations have been reported in mouse T-ALL tumors (13), including Kras G12D induced T-ALL (5, 7, 12), we examined Notch1 mutations in Kras G12D; CD44−/− induced T-ALL tumors (Fig. S6). We found that 6/6 tumors bear heterozygous Type 1 deletion, 1/6 tumors carries Type 2 deletion, and 4/6 tumors have insertions at the PEST domain of exon 34. These Notch1 mutation frequencies and types are similar as those identified in Kras G12D T-ALL (our unpublished data), suggesting that hyperactivation of Notch1 co-operates with Kras G12D to drive T-ALL formation regardless of the expression status of CD44. Moreover, the majority of tumor samples have two different types of Notch1 mutations. It is not clear whether this is due to incremental Notch1 activation in the same cells and/or presence of multiple clones in these tumor samples. However, based on the relative intensities of these mutations, we favor the possibility that Type 1 deletion and exon34 mutations target almost all T-ALL cells, whereas Type 2 deletion is only acquired in a fraction of tumor cells.
Although CD44 is not required for the homing and engraftment of Kras G12D HSCs, blocking CD44 activity using CD44 blocking antibodies (IM7 and KM114) in Kras G12D T-ALL cells greatly impaired their homing to bone marrow (Fig. S7), suggesting a gained-dependence on CD44 function in tumor cells. IM7 is a pan-CD44 blocking antibody that sheds membrane expression of CD44 (14), while KM114 only blocks the interaction of CD44 to hyaluronic acid (15). After normalized to the ratio of input cells, the homing index was less than 0.2 in T-ALL cells incubated with IM7 and ~0.5 in cells incubated with KM114. These results indicate that CD44-mediated homing is partially through the interaction of CD44 to hyaluronan and interaction of CD44 to other cellular matrix proteins is also important in this process. However, due to the limitation of the experimental systems, it is difficult to further evaluate the significance of this short-term homing defect on long-term tumor development in vivo.
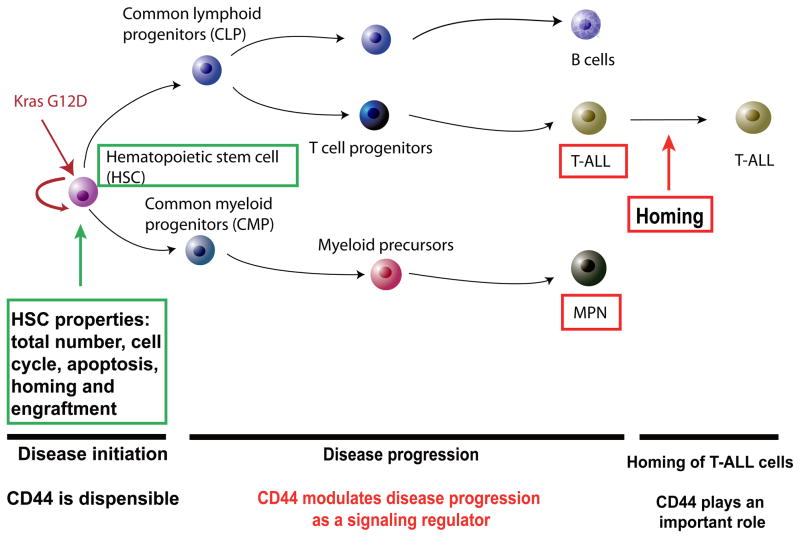
In summary, our results elucidate the function of CD44 at different stages and in different populations of cells during Kras G12D-mediated leukemogenesis (Fig. 3). In contrast to previous reports that CD44 plays an important role in human AML (3) and CML (2) as a cell adhesion molecule, we found that CD44 modulates Kras G12D-mediated MPN and T-ALL as a signaling regulator. Our results emphasize the complex functions of CD44 during leukemogenesis induced by different genetic lesions and provide an additional avenue for exploration of CD44 as a target for leukemia therapy.
Figure 3.
Schematic picture illustrating the role of CD44 in Kras G12D-induced hematopoietic malignancies.
Supplementary Material
Acknowledgments
We thank Drs David A. Tuveson and Tyler E. Jacks for generously providing us with conditional oncogenic Kras mice and Bruce Bagley for helping us detect Type 2 deletion in Notch1. We are grateful to Drs. Qiang Chang, Norman Drinkwater, and Shannon Kenney for helpful discussion and critical comments on the manuscript. We would like to thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services to complete this research. This work was supported by a Howard Temin Award and a R01 grant 1R01CA152108 from the National Cancer Institute, a Shaw Scientist Award from the Greater Milwaukee Foundation, an ASH Scholar Award from the American Society of Hematology, a V Scholar Award from the V Foundation for Cancer Research, and an Investigator Initiated Grant from UWCCC to J.Z.. This work was also supported in part by NIH/NCI P30 CA014520--UW Comprehensive Cancer Center Support.
Mouse genotype abbreviations
- Control
pI-pC treated Mx1-Cre or wild-type
- Kras G12D
recombined Kras G12D heterozygous (pI-pC treated LSL Kras G12D/+; Mx1-Cre)
- CD44−/−
CD44 knockout
- Kras G12D; CD44−/−
recombined Kras G12D heterozygous deficient for CD44 (pI-pC treated LSL Kras G12D/+; Mx1-Cre; CD44−/−)
- Kras G12D; βc−/−
recombined Kras G12D heterozygous deficient for common β subunit of GM-CSF receptor (pI-pC treated LSL Kras G12D/+; Mx1-Cre; βc −/−).
Footnotes
Author Contributions
The contributions of individual authors are listed below: Juan Du for experimental design & execution as well as writing manuscript; Yangang Liu, Benjamin Meline, Guangyao Kong, Li Xuan Tan, Juinn Cherng Lo, Jinyong Wang, Li Zhang, Yuan-I Chang, Myung-Jeom Ryu, and Jingfang Zhang for experimental execution; Erik A. Ranheim for histopathology analysis; Jing Zhang for experimental design and writing manuscript.
Conflict of Interest Disclosures
We declare no competing financial interests.
References
- 1.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003 Jan;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 2.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006 Oct;12(10):1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 3.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006 Oct;12(10):1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 4.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006 Jul 1;20(13):1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindler T, Cornejo MG, Scholl C, Liu J, Leeman DS, Haydu JE, et al. K-RasG12D-induced T-cell lymphoblastic lymphoma/leukemias harbor Notch1 mutations and are sensitive to gamma-secretase inhibitors. Blood. 2008 Oct 15;112(8):3373–3382. doi: 10.1182/blood-2008-03-147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Wang J, Liu Y, Sidik H, Young KH, Lodish HF, et al. Oncogenic Kras-induced leukemogeneis: hematopoietic stem cells as the initial target and lineage-specific progenitors as the potential targets for final leukemic transformation. Blood. 2009 Feb 5;113(6):1304–1314. doi: 10.1182/blood-2008-01-134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JY, Liu YG, Li ZY, Wang ZD, Tan LX, Ryu MJ, et al. Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a dose- and cell type-dependent manner. Blood. 2011;118(2):368–379. doi: 10.1182/blood-2010-12-326058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalcanti GB, Savino W, Pombo-de-Oliveira MS. CD44 expression in T-cell lymphoblastic leukemia. Braz J Med Biol Res. 1994 Sep;27(9):2259–2266. [PubMed] [Google Scholar]
- 9.Chen C, Chang MC, Hsieh RK, Chang YF, Lin J, Tsan KW. Activation of CD44 facilitates DNA repair in T-cell lymphoma but has differential effects on apoptosis induced by chemotherapeutic agents and ionizing radiation. Leuk Lymphoma. 2005 Dec;46(12):1785–1795. doi: 10.1080/10428190500232501. [DOI] [PubMed] [Google Scholar]
- 10.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999 Nov 1;163(9):4917–4923. [PubMed] [Google Scholar]
- 11.Wang JY, Liu YG, Li ZY, Du J, Ryu MJ, Taylor PR, et al. Endogenous oncogenic Nras mutation leads to aberrant GM-CSF signaling in granulocytic/monocytic precursors in a murine model of chronic myelomonocytic leukemia. Blood. 2010;116(26):5991–6002. doi: 10.1182/blood-2010-04-281527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabnis AJ, Cheung LS, Dail M, Kang HC, Santaguida M, Hermiston ML, et al. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol. 2009 Mar 17;7(3):e59. doi: 10.1371/journal.pbio.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashworth TD, Pear WS, Chiang MY, Blacklow SC, Mastio J, Xu L, et al. Deletion-based mechanisms of Notch1 activation in T-ALL: key roles for RAG recombinase and a conserved internal translational start site in Notch1. Blood. 2010 Dec 16;116(25):5455–5464. doi: 10.1182/blood-2010-05-286328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, Dennis K, Peschon JJ, Chandrasekaran R, Mikecz K. Antibody-induced shedding of CD44 from adherent cells is linked to the assembly of the cytoskeleton. J Immunol. 2001 Jul 1;167(1):123–131. doi: 10.4049/jimmunol.167.1.123. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Katoh S, He Q, Oritani K, Miyake K, Lesley J, et al. Monoclonal antibodies to CD44 and their influence on hyaluronan recognition. J Cell Biol. 1995 Jul;130(2):485–495. doi: 10.1083/jcb.130.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.