ADENOSINE MONOPHOSPHATE ACTIVATED PROTEIN KINASE
Adenosine monophosphate (AMP) -activated protein kinase (AMPK), a heterodimer consisting of a catalytic α subunit and regulatory β and γ subunits, is a key molecular player in energy homeostasis at both the cellular and whole-body levels.[1–3] Generally, activated peripheral AMPK enhances catabolic pathways and suppresses anabolic pathways, and central AMPK has a direct appetite-regulating effect. AMPK is activated by food deprivation and is inhibited by re-feeding.[4] Hypothalamic AMPK is regulated by orexigenic and anorexigenic signals. Thus, hypothalamic AMPK activity is suppressed by anorexigenic peptides, such as leptin, which affect the AMPK α2 activity. The inactivation of AMPK in the hypothalamus reduces the phosphorylation of acetyl-coenzyme A (CoA) carboxylase (ACC), which results in the increased production of malonyl CoA to inhibit food intake.[5] However, AMPK is also important in the peripheral metabolism of skeletal muscle, liver, fat, myocardium and other tissues.[3] For example, AMPK plays a key role in regulating lipid metabolism in peripheral tissues. Activated AMPK phosphorylates and inhibits ACC1 and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), suppresses fatty acid synthase (FAS) expression and activates malonyl-CoA carboxylase, resulting in a decrease in fatty acid and cholesterol synthesis. AMPK also controls hepatic gluconeogenesis by inhibiting the transcription of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). AMPK α 2-knockout (KO) mice were shown to have glucose intolerance and fasting-induced hyperglycemia, possibly caused by enhanced gluconeogenesis associated with activated PEPCK and G6Pase. Additionally, activated AMPK in skeletal muscle phosphorylates and inhibits glycogen synthase, thereby decreasing the synthesis of glycogen. The regulation of peripheral metabolism has been linked to hypothalamic AMPK, suggesting that AMPK is a key enzyme in coordinating the interaction between peripheral and central energy regulation. Leptin, an anorexigenic peptide, directly increases AMPK activity in the skeletal muscle, but decreases AMPK activity in the arcuate nucleus (ARC) and paraventricular nucleus (PVN). By increasing peripheral fatty acid consumption and reducing the appetite, leptin leads to an overall negative energy balance and a reduction in body weight [Figure 1].
Figure 1.
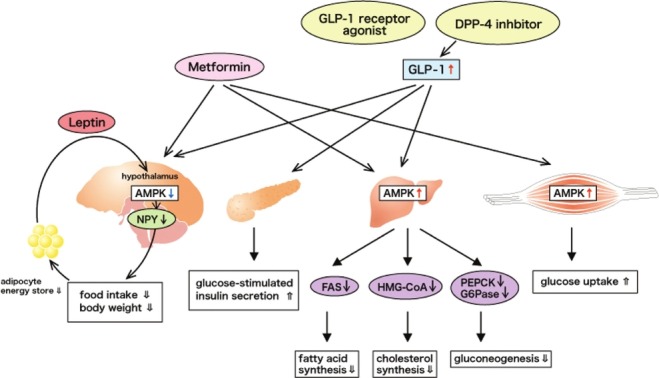
Metformin and Glucagon-like peptide-1 (GLP-1) inhibit Adenosine monophosphate activated protein (AMPK) in the hypothalamus and activate AMPK in peripheral tissues. Hypothalamic AMPK activity is uppressed by leptin. Through metformin, low glucose-induced AMPK phosphorylation is inhibited, and the Messenger Ribonucleic acid expression of neuropeptide Y is suppressed in the hypothalamus. In peripheral tissues, activated AMPK inhibits 3-hydroxy-3- methylglutaryl-coenzyme A and suppresses fatty acid synthase expression, resulting in a decrease in fatty acid and cholesterol synthesis. AMPK also controls hepatic gluconeogenesis by inhibiting phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. Additionally, metformin stimulates AMPK in muscle and induces glucose uptake
METFORMIN
Metformin is widely used as an oral antidiabetic agent for type 2 diabetes.[6] Metformin inhibits gluconeogenesis, delays the gastrointestinal absorption of glucose, and reduces food intake preventing body weight gain in obese patients with type 2 diabetes. Recently, it has been revealed that these actions of metformin are related to the activation of AMPK.[7] [Figure 1] The main effect of this drug is a decrease in hepatic glucose production through mild suppression of the mitochondrial respiratory chain complex I.[8] Metformin activates AMPK indirectly by increasing the AMP: ATP ratio as a result of inhibiting the respiratory chain complex I.[3] Activated AMPK also decreases fatty acid and cholesterol synthesis, as mentioned previously, and induces hepatic fatty acid oxidation. In muscle, metformin stimulates AMPK and induces glucose uptake. In contrast, metformin inhibits AMPK in the hypothalamus.[3] Through metformin, low glucose-induced AMPK phosphorylation is inhibited, and the Messenger Ribonucleic acid (mRNA) expression of neuropeptide Y (NPY), an onexigenic peptide, is suppressed in the hypothalamus. Anorectic effects of metformin may be explained by inhibited AMPK and NPY expression.
GLUCAGON-LIKE PEPTIDE-1
Glucagon-like peptide-1 (GLP-1), an incretin, is a neuropeptide that is endogenously produced from ‘L’ cells in the gastrointestinal tract and in the brain stem and hypothalamus.[9] GLP-1 exerts an inhibitory role on gastric emptying and small intestinal transit.[10] Such inhibitory effects of GLP-1 are directly mediated via the GLP-1 receptor on smooth muscles and indirectly via neuronal pathways. Glucose metabolism and energy balance are also partly regulated by GLP-1 [Figure 1]. GLP-1, which is degraded by dipeptidyl peptidase-4 (DPP-4), stimulates insulin secretion in response to nutrients.[11] Through GLP-1 treatment of type 2 diabetes, the fasting blood glucose levels are normalized, and the postprandial glucose levels are suppressed.[4] In addition to glycemic regulation, either peripheral or central GLP-1 activation suppresses lipogenesis in hepatocytes and inhibits food intake.[11] In hepatocytes, cyclic AMP (cAMP) levels are increased by GLP-1, resulting in the phosphorylation of AMPK, and lipogenesis is suppressed. Indeed, in hepatocytes expressing a dominant negative Ad-DN-AMPK, attenuated GLP-1 effects on both AMPK phosphorylation and its downstream lipogenic targets are observed.[9] However, GLP-1 prevents fasting - induced increases in AMPK expression in the hypothalamus.[5] GLP-1 also affects the expression of appetite-regulating peptides in the hypothalamus such as NPY, agouti-related peptide (AgRP), pro-opiomelanocotin (POMC), and amphetamine-regulated transcript (CART). GLP-1 reduced orexigenic peptides (NPY and AgRP) and increased anorexigenic peptides (POMC and CART) at the mRNA level in the hypothalamus of fasted rats that displayed elevated NPY/AgRP and decreased POMC/CART. Thus, GLP-1 and its agonists may be effective for the treatment of obesity. It has been reported that metformin significantly increases active plasma GLP-1 levels in normal rats in the presence of a DPP-4 inhibitor.[6] In addition, combined treatment with metformin and a DPP-4 inhibitor decreased food intake and body weight gain. This result may be explained by the significant increase in plasma GLP-1 levels. The combination therapy seems to be effective for the treatment of type 2 diabetes with obesity.
CONCLUSIONS
In conclusion, AMPK is one of the key regulators of energy homeostasis and affects several metabolic hormones. AMPK is now recognized as a potential target for the treatment of diabetes, obesity, and associated co-morbidities. The mechanisms underlying how diabetic agents mediate AMPK should be studied further, and new therapies targeting AMPK are expected.
REFERENCES
- 1.Grzybowska M, Bober J, Olszewska M. Metformin-mechanisms of action and use for the treatment of type 2 diabetes mellitus. Postepy Hig Med Dosw. 2011;65:277–85. doi: 10.5604/17322693.941655. [DOI] [PubMed] [Google Scholar]
- 2.Derosa G, Maffioli P. Glp-1 agonists exenatide and liraglutide: A review about their safety and efficacy. Curr Clin Pharmacol. 2012 doi: 10.2174/157488412800958686. [DOI] [PubMed] [Google Scholar]
- 3.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signaling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 4.Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol Neurobiol. 2012;45:348–61. doi: 10.1007/s12035-012-8239-z. [DOI] [PubMed] [Google Scholar]
- 5.Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like- peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J. 2008;55:867–74. doi: 10.1507/endocrj.k08e-091. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda N, Inoue T, Nagakura T, Yamazaki K, Kira K, Saeki T, et al. Metformin causes reduction of food intake and body weight gain and improvement of glucose intolerance in combination with dipeptidyl peptidase IV inhibitor in Zucker fa/fa rats. J Pharmacol Exp Ther. 2004;310:614–9. doi: 10.1124/jpet.103.064964. [DOI] [PubMed] [Google Scholar]
- 7.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes Obes Metab. 2011;13:1097–104. doi: 10.1111/j.1463-1326.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- 8.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molocular mechanisms of metformin: An overview. Clin Scin (Lond) 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake suppressive effects of hindbrain glucagon-like-peptode-1 receptor activation. Cell Metab. 2011;13:320–30. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2000;21:1565–82. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Shiomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–23. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]


