Abstract
Benzo[a]pyrene (BaP) is activated by xenobiotic-metabolizing enzymes to highly mutagenic and carcinogenic metabolites. Previous studies in this laboratory have shown that benzo(a)pyrene quinones (BPQs), 1,6-BPQ and 3,6-BPQ, are able to induce epidermal growth factor receptor (EGFR) cell signaling through the production of reactive oxygen species. Recently, we have reported that BPQs have the potential to induce the expression of genes involved in numerous pathways associated with cell proliferation and survival in human mammary epithelial cells. In the present study we demonstrated that BPQs not only induced EGFR tyrosine autophosphorylation, but also induced EGFR-dependent tyrosine phosphorylation of phospholipase C-γ1 and several signal transducers and activators of transcription (STATs). The effects of BPQs were evaluated in a model of EGF withdrawal in MCF10-A cells. We found that BPQs (1 μM), induced EGFR tyrosine phosphorylation at positions Y845, Y992, Y1068, and Y1086. PLC-γ1 phosphorylation correlated with the phosphorylation of tyrosine-Y992, a proposed docking site for PLC-γ1 on the EGFR. Additionally, we found that BPQs induced the activation of STAT-1, STAT-3, STAT-5a and STAT-5b. STAT5 was shown to translocate to the nucleus following 3,6-BPQ and 1,6-BPQ exposures. Although the pattern of phosphorylation at EGFR, PLC-γ1 and STATs were quite similar to those induced by EGF, an important difference between BPQ-mediated signaling of the EGFR was observed. Signaling produced by EGF ligand produced a rapid disappearance of EGFR from the cell surface, whereas BPQ signaling maintained EGFR receptors on the cell membrane. Thus, the results of these studies show that 1,6-BPQ and 3,6-BPQ can produce early events as evidenced by EGFR expression, and a prolonged transactivation of EGFR leading to downstream cell signaling pathways.
Keywords: Benzo[a]pyrene, EGFR, phosphorylation, STAT, PLCγ
INTRODUCTION
Breast cancer is one of the most common cancers and the second leading cause of cancer-related deaths for women in the United States. Environmental factors, including diet, are believed to play important roles in breast cancer development (Shi et al., 2004; Brody et al., 2007). Polycyclic aromatic hydrocarbons (PAHs), ubiquitous environmental carcinogens, have been associated with several types of cancer (Bostrom et al., 2002), including breast cancer (Brody et al., 2007). Occupational exposure to benzo[a]pyrene (BaP), a prototype PAH, has been the subject of concern for many years because it has been implicated in many cancers (Bosetti et al., 2007). An additional important source of exposure to PAHs for many people is through the diet (Lee et al., 2007). BaP is metabolized to four ultimate carcinogenic forms of reactive (+)- or 9-benzo[a]pyrene diol epoxides (BPDEs) that covalently bind to DNA, proteins, or lipids, which might be mechanistically related to BaP mutagenesis and carcinogenesis (Pelkonen and Nebert, 1982; Cavalieri and Rogan,1985; Conney et al., 1994). BPQs (1,6-BPQ and 3,6-BPQ) are also metabolites of BaP that have been demonstrated to activate signaling pathways associated with increased intracellular Ca2+, protein tyrosine kinase signaling, cell proliferation, and increased cell survival through the generation of reactive oxygen species (ROS) (Tannheimer et al., 1998, 1999; Burdick et al., 2003, 2006). Additionally, recent studies from this laboratory have demonstrated that 1,6-BPQ and 3,6-BPQ activate dioxin response elements (DRE) and anti-oxidant response elements (ARE) in human mammary epithelial cells (Burchiel et al., 2007). Based on the patterns of genes induced, we concluded that three main pathways are activated by BPQs: (1) AhR-dependent pathways associated with activation of DRE, signaling, and metabolism of PAHs and steroids; (2) oxidative stress pathways likely associated with Nrf2 and ARE activation; and (3) epidermal growth factor receptor (EGFR) activated pathways.
Redox-dependent post-translational modification of proteins is emerging as a key signaling system, conserved through evolution, and influencing many aspects of cellular homeostasis (Cecarini et al., 2007). Recent data provide new insights into the interplay between phosphorylation-and redox dependent signaling. Reactive oxygen species (ROS) have been included among intracellular signal transducers for the epidermal growth factor receptor (Chiarugi et al., 2007). It is well known that ROS act as chemical messengers in cellular processes such as mitogenic signal transduction, gene expression, cell proliferation, senescence and apoptosis (Martinez-Sanchez and Giuliani, 2007; Carreras et al., 2007).
EGFR stimulates tumor growth and progression by activating several signaling pathways associated with cell proliferation, angiogenesis, invasion, metastasis and inhibition of apoptosis (Sebastian et al., 2006). EGFR exerts its function in the cellular environment mainly, if not exclusively, via its tyrosine kinase activities. In general, tyrosine autophosphorylation serves to stimulate the catalytic activity of EGFR and to generate docking sites for recruitment of substrate proteins. Tyrosine phosphorylation of cellular substrates is thus the first and crucial step in transducing EGFR-mediated signals (Herbst, 2004). It has been reported that several non-physiologic agents such as radiation, oxidants and alkylating agents induce ligand-independent activation of numerous receptor tyrosine kinases at the inner side of the plasma membrane, including EGFR (Knebel et al., 1996). Because in our previous studies, BPQs (1,6-BPQ and 3,6-BPQ) enhanced EGFR pathway activation in mammary epithelial cells by producing ROS (Burdick et al., 2003), the purpose of the present study was to analyze the EGFR tyrosine phosphorylation patterns induced by BPQs. We addressed the role of phosphorylation on specific EGFR tyrosine residues and the phosphorylation-dependent activation of major intracellular signaling pathways associated with this receptor.
In the present study we used a previously characterized model of EGF withdrawal in MCF-10A cells (Burdick et al., 2003) to investigate how phosphorylation of specific tyrosine residues on the EGFR induce downstream signaling pathways following BPQ exposure. Here, we show that BPQs are able to increase EGFR tyrosine phosphorylation and autophosphorylation, induce the phospholipase C-γ1 and activation of STATs. Importantly, specific phosphorylation of EGFR tyrosine residues was correlated with activation of these intracellular signaling pathways. These findings suggest potential mechanisms whereby BaP metabolites can activate EGFR in human mammary epithelial cells to promote carcinogenesis.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma (St. Louis, MO), unless otherwise indicated. 1,6-BPQ and 3,6-BPQ were purchased from Midwest Research Institute (Kansas City, MO) at >99% purity and maintained as stock solutions in anhydrous tissue culture grade DMSO. Except where noted, the final concentration of DMSO in all experiments was 0.1%.
Antibodies
An antibody against EGFR was purchased from Cell Signaling Technology (Boston, MA) as were antibodies against phospho-EGFR (Tyr1045 and Tyr845). Antibodies against phospho-EGFR (Tyr992, Tyr1068 and Tyr1086) were purchased from Biosource (Camarillo, CA). Antibodies specific against STAT1, phospho-STAT1 (Tyr701), STAT 3, phospho-STAT 3 (Tyr705), STAT 5a (Tyr694, and STAT 5b (Tyr699) were purchased from Biosciences (San Jose, CA). Anti-phospholipase C γ1 and anti- phospho- phospholipase C γ1(Tyr783) antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). The anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
MCF-10A Cell Culture and treatments
MCF-10A cells are a spontaneously immortalized and growth factor-dependent mammary epithelial cell line that is grown on a Type I collagen-coated (PureCol Inamed Biomaterials, Fremont, CA) 100×20 mm dishes (Corning Glass, Corning, NY, USA) in serum-free, growth factor-defined media at 10% CO2 and 37°C, as described elsewhere (Burdick et al., 2003; Davis et al., 2003). For treatments, MCF-10A cells, passage 56 (and higher), were plated into fifteen 100mm dishes at 3.5×105 cells per dish in 5ml SFIHE (serum free media containing growth factors insulin, hydrocortisone, epidermal growth factor) with 2% FBS, after 24 hr the media was removed and fresh SFIHE was added. On day 5, media was removed and cells were placed in serum free media containing hydrocortisone and insulin (SFIH), and were without EGF for approximately 24 hr. Cells in a EGF-deficient medium (SFIH medium) were then incubated with different treatment regimens (as listed below). Treatments were run in triplicate at 1 μM 1,6-BPQ and 3,6-BPQ, and 10 ng/ ml EGF. All treatments, except EGF, contained 0.1% DMSO. DMSO was not found to alter cell growth or phosphorylation of any of the proteins examined. Cells remained in treatment media for 18 hr or 15 min and were then lysed for further studies.
EGFR Immunoprecipitation and Western blot analysis
Lysates were prepared by scraping cells off of the plastic plates in lysis buffer (20 mM HEPES, 2mM EGTA, 50 mM β-glycerophosphate, 5mM sodium fluoride, 50 μM DTT, 100 mM PMSF, 1% Triton X-100, 10% glycerol and 0.1% protease and phosphatase inhibitor cocktails (Sigma, St. Louis, MO) and cleared by centrifugation. Protein concentrations were determined with the BCA protein assay (Biorad Laboratories, Hercules, CA), and the remaining lysate was boiled in Laemmli sample buffer (Jorissen et al., 2003). EGFR immunoprecipitations were carried out essentially as described in Burdick et al., 2003. Three hundred μg of protein were incubated with 2 μg of anti-EGFR antibody with Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 4 hr at 4°C. Beads were collected by centrifugation, washed four times with lysis buffer, and resuspended in 2× sample buffer. For Western analysis, samples were electrophoresed through 7% gels and transferred to PVDF membranes (Millipore, Bedford, MA). Immunoblot analyses were carried out using the appropriate antibodies. Specific phosphotyrosines were detected with Western Lightning Enhanced Chemiluminescence reagents (Perkin Elmer Life Science, Boston, MA). The intensity of each band was imaged and quantified by using a Kodak Imager system (Kodak Co. Rochester, NY).
Anti-phosphotyrosine-Western blots
Eighty μg aliquots of lysates were separated by SDS-polyacrylamide gel electrophoresis (10% gel for anti-phospholipase C-γ1 and 7% gel for STAT1, STAT 3, STAT 5a and STAT 5b) and transferred to PVDF membranes (Millipore, Bedford, MA). Membranes were blocked for 1 hr in Tris-buffered saline (50 mM Tris, 150 mM NaCl, pH 7.4) with 0.05% Tween 20 (TBST) and 5% nonfat dry milk and exposed to the primary antibodies listed above overnight at 4°C in 5% BSA in TBST using the manufacturers' suggested dilutions. Blots were then washed (3 × 5 min) in TBST, exposed to secondary antibody where appropriated for 1 hr at a 1:2000 dilution in 5% nonfat dry milk in TBST. Bound horseradish peroxidase was detected with the Western Lightning Enhanced Chemiluminescence reagents (Perkin Elmer Life Science, Boston, MA). The intensity of each band was imaged and quantified by using a Kodak Imager scanning system (Kodak Co. Rochester, NY).
Immunofluorescence
MCF-10A cells were grown on Vitrogen coated microscope slides for 24h in complete media (SFIHE). Cells were placed in SFHI media overnight and then treated. Cells were fixed with 2% paraformaldehyde for 15 min on ice. Slides were washed with PBS and then incubated for 10 min with 0.5% Triton X-100 to permeabilize cells. All slides were then incubated with a10% BSA block to decrease non-specific binding. Slides were incubated with primary antibodies overnight at 4°C. To detect EGFR, an anti-EGFR antibody was used (#2232, Cell Signal Technologies) and for STAT 5 an anti-STAT 5 antibody (Biosciences, San Jose, CA) was used. Slides were washed thrice in PBS, stained with secondary antibody for 1 h, washed thrice in PBS, and then mounted with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Fluorescence microscopy analysis was performed on a Zeiss Axioplan 2 microscope located in the UNM Cancer Center Core facility lab.
Statistical Analyses
Data were analyzed for statistical differences (p < 0.05) between control and treated groups using SigmaStat software (Jandel Scientific, San Rafael, CA). ANOVA followed by Dunnett's t tests was performed on sample means.
RESULTS
BPQs stimulate EGF-Receptor tyrosine phosphorylation at specific phosphostyrosine residues
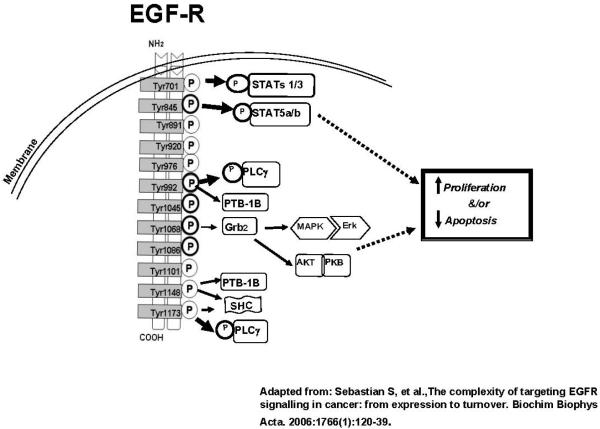
Based on our previous observations that BPQs can mimic EGFR signaling to induce cell proliferation (Burdick et al., 2003), we examined the effects of 1,6-BPQ and 3,6-BPQ on EGFR activation, as measured using anti-phosphotyrosine blots. The rapid phosphorylation and autophosphorylation of various tyrosine residues present in the EGFR have been identified as important triggers of the activation of signaling pathways that occur in cells following treatment with EGF (Jorissen et al., 2003). Since many diverse agents, including X-rays, UVA, UVB, mitomycin-C, alkylating agents, oxidants and antioxidants have also been reported to induce gene transcription and to activate EGFR signal transduction pathways (Reynolds et al., 2003), we examined whether BPQs could also trigger EGFR activation. As shown in Figure 1, there are numerous tyrosine residues on the EGFR that can be phosphorylated leading to downstream signaling (Sebastian et al., 2006).
Figure 1.
Sites of tyrosine phosphorylation on the human EGF receptor leading to downstream signaling. Figure based on Sebastian et al., 2006.
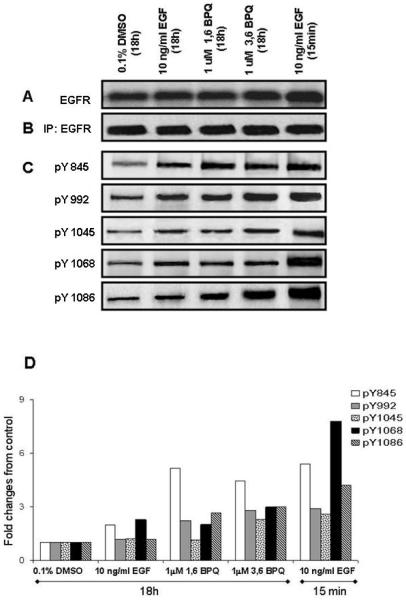
A series of experiments were performed to determine whether exposure of cells to BPQs result in changes in the phosphorylation status of specific EGFR tyrosines. First, the effects of BPQs were examined following exposure of intact cells and examination of either whole cell lysates (Fig. 2A) or after lysate immunoprecipitation of the EGFR with anti-EGFR antibody (Fig. 2B). For these experiments, MCF-10A cells were exposed to 1 μM 1,6-BPQ, 1 μM 3,6-BPQ, or 10 ng/ml EGF for 18 hr. We used the 1 μM concentrations of BPQs and the 18 hour time point to examine phosphorylation changes because these conditions have been shown in previous studies to be optimal (Burdick et al., 2003). Previous kinetics studies have been performed indicating that tyrosine phosphorylation by EGF was optimal at 15 min. Thus, both the 15 min and 18 hr time points were used in these studies for comparison of EGF effects. Whole cells were lysed and equal amounts of total protein lysates were subjected to SDS-PAGE analysis. An increase in the signal was observed only in those whole cell lysates treated with10 ng/ml EGF for 15 min (Fig. 2A). No changes in the intensity of the EGFR protein in immunoprecipitates were observed (Fig. 2B). However, significant changes were detected in EGFR tyrosine phosphorylation in the anti-phosphotyrosine blots (Fig. 2C). Densitrometic analysis revealed that BPQs and EGF induced EGFR tyrosine phosphorylation, but at different specific tyrosine residues (Fig. 2D). 1 μM 1,6-BPQ induced a significant increase in phosphorylation at Y845 and only modestly increased Y992, Y1068 and Y1086 phosphorylation; no effect was observed on phosphotyrosine Y1045. In contrast, we found that 1 μM 3,6-BPQ induced the phosphorylation at all EGFR tyrosines examined. The most important effects were observed on phosphotyrosine Y845 and to a lesser degree at tyrosine Y992, Y1045, Y1068 and Y1086. Interestingly, 10 ng/ml EGF produced a different effect on EGFR tyrosine phosphorylation in MCF-10A cells depending on the time of exposure. EGF produced a rapid (15 min) and transient increase in tyrosine phosphorylation of EGFR, most notable at Y1068 and Y845. EGF also increased phosphorylation at Y992, Y1045 and Y1086. Only a slight increase in phosphorylation on Y845 and Y1068 in EGF-treated cells for 18 hr was observed. These results indicate that BPQs induce EGFR tyrosine phosphorylation at many of the same tyrosine residues as occur with EGF; however, the kinetics of tyrosine phosphorylation are quite different with maximal effect of EGF occurring at ~15 min and maximal effects for BPQs occurring at 18 hr.
Figure 2.
Effect of BPQs on phosphorylation of the EGFR phosphosites under growth factor-dependent conditions. (A) Cell lysates were prepared in lysis buffer, subjected to Western Blot analysis. (B, C) EGFR was immunoprecipitated from MCF-10A cells treated as was specified in “Material and Methods”. Immunocomplexes were separated by SDS-PAGE and subjected to Western blot analysis for EFGR phosphorylation using an anti-EGFR or site-specific antibodies as indicated. (D) Densitometric analysis of the bands was used in order to make a semi-quantitative analysis of phosphorylation of EGFR phosphosites. The relative phosphorylation levels were estimated as compared to control. These results are representative of several independent replicates performed for each of the different phosphorylation sites.
BPQs-induced activation of PLC-γ1
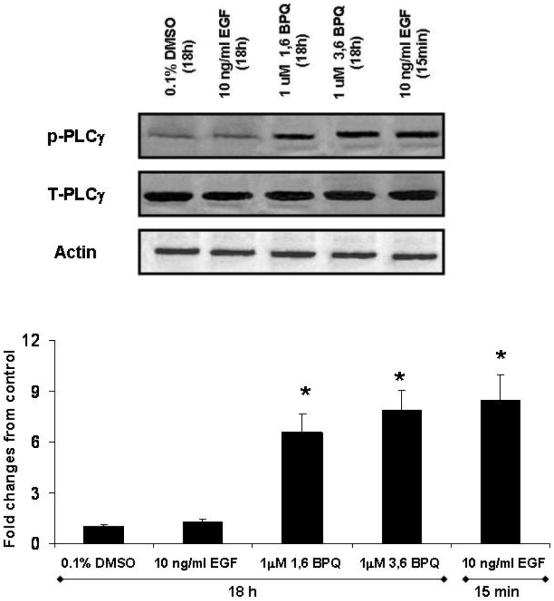
Phospholipase C-γ1 (PLC-γ1) is known to be activated in response to growth-factor stimulation by a mechanism that relies on tyrosine phosphorylation, and it plays an important role in regulating cell proliferation (Wang et al., 2006). PLC-γ1 activation has also been reported to enhance cell survival during the cellular response to oxidative stress (Bai et al., 2002). PLCγ1 activation is associated with Y992 phosphorylation on the EGFR (Sabastian et al., 2006). To determine whether the increase in phosphorylation on specific EGFR tyrosines led to downstream signaling from receptor, we analyzed PLC-γ1 expression and activation in MCF-10A cells. Cells were treated basically as described in “Material and Methods”. Whole cell lysates were subjected to an immunoblot analysis by using anti-phospholipase C-γ1 antibody. Our results showed that cell lysates did not demonstrate changes in total PLC-γ1 protein levels with any treatment (Fig. 3, top). The effect of BPQs on PLC-γ1 activation was also investigated by subjecting whole cell lysates to immunoblot analysis with antibodies anti-phosphotyrosine-PLC-γ1 (Tyr783). Fig. 3, (bottom) shows the quantitative analysis of bands with all treatments. We found that both BPQs induced PLC-γ1 phosphosphorylation. The effect on PLC-γ1 phosphorylation was similar for both 1,6-BPQ and 3,6-BPQ. In order to compare the magnitude of PLC-γ1 activation induced by BPQs with EGF, we stimulated MCF-10A cells with 10 ng/ml EGF for 15 min and 18 hr. A significant increase in PLC-γ1 tyrosine phosphorylation was only observed in cells exposed at 15 min. It was interesting to note that the level of PLC-γ1 phosphorylation produced by BPQs at 18 hr was similar in magnitude to that produced by EGF at early time points (15 min).
Figure 3.
BPQs induce Phospholipase C (PLC-γ1) activation in MCF-10A cells. MCF-10A cells were cultured overnight in media without EGF and treated with 1 μM BPQs or 10 ng/ml EGF for 18h or for 15 min with 10 ng/ml EGF. Cell lysates were collected, separated with 7% SDS-PAGE, and immunoblotted either with antiphospho- PLC-γ1 (Y783) or antiphospholipase C-γ1 antibodies. Representative immunoblot and quantitative data of PLC-γ1 activation in MCF-10A cells. Statistical analyses are for this individual experiment. Values represent means ± S.E. * p < 0.05 as compared with control group.
STAT-1 activation is induced by BPQs in MCF-10A cells
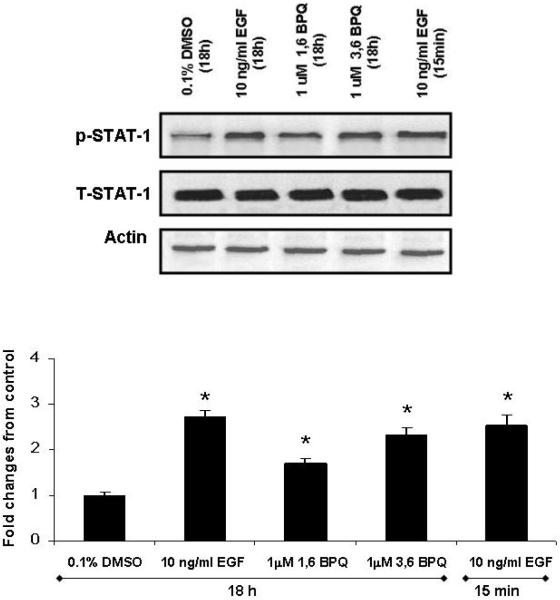
It has been shown that EGF induces STAT-1 phosphorylation, a known downstream target of this receptor (Leaman et al., 1996). STAT-1 signaling pathway has been found to be vital for proliferation following EGFR activation. To determine whether BPQ activation of EGFR signaling led to downstream STAT-1 signaling, we compared the effect of BPQs and EGF in MCF-10A cells obtained from EGF-deficient media. Treatment of MCF-10A cells with 1 μM BPQ did not modify the total amount of STAT-1 protein; however, 1,6-BPQ and 3,6-BPQ were able to induce STAT-1 phosphorylation (See Fig. 4). The effect of 3,6-BPQ on STAT-1 tyrosine phosphorylation was of a similar magnitude to that seen in cells treated at 10 ng/ml EGF for 15 min. In contrast to our findings of transient PLC-γ1 phosphorylation produced by EGF, we observed STAT activation in cells treated at both the 15 min and 18 hr time points.
Figure 4.
STAT 1 activation is induced by BPQs in MCF-10A cells. Representative blot and quantified data show that STAT-1 phosphorylation in MCF-10A cells in media without EGF treated with 1 μM BPQs or 10 ng/ml EGF for 18 hr. Cell lysates were blotted with phosphotyrosine antibody to determine the phosphorylation status of STAT-1, the membrane was stripped of signal, and total STAT-1 levels were determined. 10 ng/ml EGF (15 min exposure) is included as positive control. Statistical analyses are for this individual experiment. Values are means ± S.E. * p< 0.05 as compared with control group.
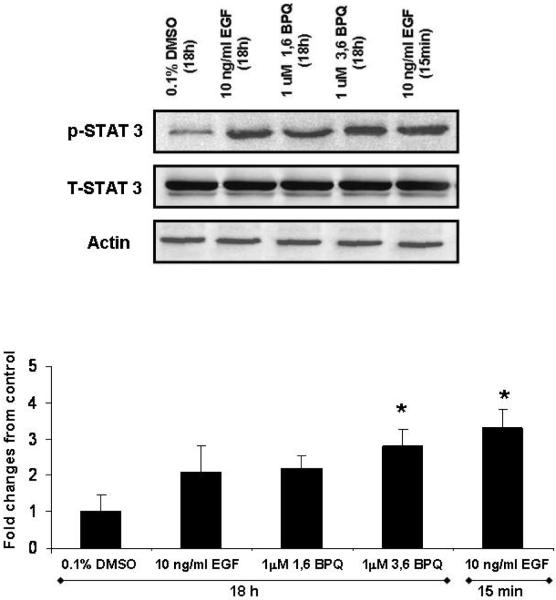
STAT-3 activation is induced by 3,6-BPQ but not by 1,6-BPQ in MCF-10A cells
It has been reported that constitutive activation of STAT-3 contributes to oncogenesis by promoting proliferation and inhibiting apoptosis of tumor cells (Grandis et al., 2000; Kijima et al., 2002). Tyrosine 705 on STAT-3 is phosphorylated by upstream tyrosine kinases, such as EGFR. Because of the important role of STAT-3 in tumor growth and survival in breast cancer, we evaluated the status of STAT-3 expression and activation. To explore the effect of BPQs on STAT-3 activation, MCF-10A cells were treated in EGF deficient media at 1 μM 1,6-BPQ, 3,6-BPQ, or 10 ng/ml EGF for 15 min or 18 hr. Fig. 5 shows the Western blot results for total STAT-3 expression and activation in MCF-10A cells. Constitutive STAT-3 phosphorylation and expression was detectible in MCF-10A cells. STAT-3 activation was only observed in cells treated with 1 μM 3,6-BPQ, and no significant effects were observed at 1 μM 1,6-BPQ or 10 ng/ml EGF for 18 hr. Total STAT-3 levels were similar in all treatments, indicating that the differences in constitutive activation of STAT-3 between treatments could not be attributed to simple variation in protein expression. As previously reported EGF (10 ng/ml) induced STAT-3 phosphorylation, but this was only observed at short times (15 min). Our findings suggest that 3,6-BPQ is able to induce STAT-3 phosphorylation after long periods of time (18 hr) and its effect is quantitatively similar to the rapid (15 min) activation produced by EGF.
Figure 5.
STAT-3 activation is induced by 3,6-BPQ but not by 1,6-BPQ and EGF in MCF-10A cells. Cells were treated with 1 μM BPQs or 10 ng/ml EGF for 18 hr and 10 ng/ml EGF for 15 min. Whole cell lysates (80 μg) were used for Western blot analysis and detected with anti-phosphorylated STAT-3, stripped and reprobed with anti STAT-3 and actin antibodies. 3,6-BPQ induced STAT-3 activation to the same extent as EGF (15 min). Statistical analyses are for this individual experiment. Bars show the means ± S.E. * p<0.05 as compared with control.
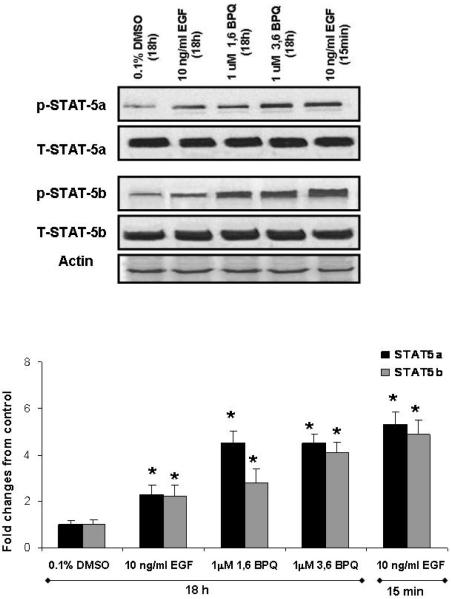
BPQs can differentially induce STAT-5a and STAT-5b activation in MCF-10A cells
The role of the STAT-5a and STAT-5b transcription factors in breast cancer has been studied in different models (Biscardi et al., 2000). STAT-5a and STAT-5b proteins are normally involved in a variety of cellular processes including cell proliferation, differentiation and apoptosis. Previous studies have also demonstrated that STAT-5a and STAT-5b activation is dependent on the kinase activities of the EGFR and c-Src, and the c-Src mediated EGFR tyrosine phosphorylation (Y845) (Kloth et al., 2003). We found that Y845 EGFR phosphorylation, which has been correlated with STAT-5 activation (Sebastian et al., 2006), was increased significantly after treatment with BPQs for 18 hr (Figure 2). Therefore, we examined the phosphorylation status of STAT-5a and STAT-5b in MCF-10A cells treated with 1 μM 1,6-BPQ, 1 μM 3, 6-BPQ, or 10 ng/ml EGF for 18 hr. As shown in Fig. 6 (top), BPQs did not alter the expression of STAT-5 at the total protein level; however, BPQs were able to induce STAT-5a and STAT-5b phosphorylation. 1,6-BPQ induced STAT-5a and STAT-5b in tyrosine phosphorylation, as did 3,6-BPQ. The induction of the STAT-5a activation correlated with EGFR tyrosine phosphorylation at Y845.
Figure 6.
BPQs can induce differentially to STAT-5a and STAT-5b activation in MCF-10A cells. (A) Subconfluent MCF-10A cells were subjected to 18 hr treatments with 1 μM BPQs or 10 ng/ml EGF, in EGF-deficient media. Lysates were analyzed by Western blotting with anti-phosphotyrosine anti-STAT-5a or anti-STAT-5b to determine the relative contribution of BPQs in STAT-5 activation. Membranes were stripped and reprobed with STAT-5a, STAT-5b, or actin antibodies. 10 ng/ml EGF (15 min exposure) is included as positive control. BPQs induced STAT-5a activation in the same degree that EGF (15 min) but not STAT-5b activation. (B) Densitometric analysis are the results of triplicate analyses for this individual experiment, which is representative of others that were conducted. Shown is the mean –fold induction ± S.E. * p < 0.05 as compared with control.
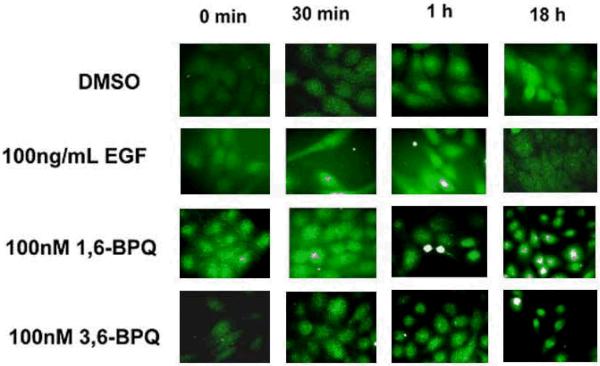
BPQs induce STAT-5 translocation to the cell nucleus
Because we found that STAT-5a and STAT-5b were phosphorylated following BPQ exposure, we next examined the cellular localization of STAT-5a/b following treatments with EGF and BPQs to determine if the increased phosphorylation of STAT-5 correlated with translocation of the protein to the nucleus. As shown in Figure 7, STAT-5 was seen in the nucleus of permeabilized MCF-10A cells after treatment with BPQs, as detected by the nuclear localization of STAT-5 protein using anti-STAT-5 antibodies. Following either 1,6-BPQ or 3,6-BPQ treatments, STAT-5 protein appeared in the nucleus beginning 1 hr after treatment and persisted for 18 hrs. EGF also translocated the STAT-5 protein to the nucleus within 30 min and persisted for 1 hr, while STAT-5 protein was present in the cytoplasm by 18 hr. These results are consistent with the prolonged EGFR signaling produced by BPQs on STAT-5 signaling pathways discussed above.
Figure 7.
Immunofluorescence staining of STAT 5 after treatment in SFIH shows localization of the STAT 5 in the MCF-10A cell nucleus over time.
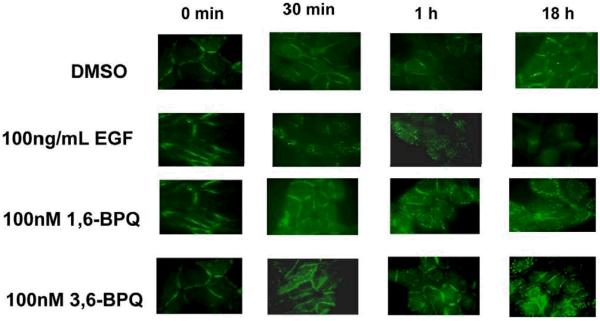
BPQs maintain EGFR expression on the surface of MCF-10A cells
EGF is known to cause EGFR receptor internalization and recycling in epithelial cells (Levkowitz et al., 1999). Because of the difference in kinetics of EGFR activation and several signaling pathways discussed above it was of interest to determine whether BPQs influence EGFR internalization in MCF-10A cells. EGFR localization was determined by immunofluorescence in SFIH media following treatment with EGF or BPQs (Figure 8). After treatment with EGF, EGFR internalization was detected by punctuate staining and decreased EGFR expression on the cell surface by 18 hr. However, following treatment with 1,6-BPQ or 3,6-BPQ, surface staining was maintained at 18 hr. This result was confirmed by flow cytometry (data not shown). Thus, the delayed phosphorylation of EGFR produced by BPQs may be responsible for maintenance of the protein on the cell surface of MCF-10A cells.
Figure 8.
Immunfluorescence analysis of EGFR in MCF-10A cells grown in SFIH media (without EGF). Cells were treated with EGF, 1,6-BPQ, or 3,6-BPQ for up to 18 hrs. EGFR was rapidly lost from the cell surface in EGF treated cells, whereas BPQs maintained EGFR expression for up to 18 hrs.
DISCUSSION
We previously reported that two BPQs (1,6-BPQ and 3,6-BPQ) increase cell proliferation and transactivate the EGFR in human mammary epithelial cells (Burdick et al., 2003). In an attempt to explain how BPQs can induce EGFR activation and lead to downstream signaling, we demonstrate in the present studies that 1,6-BPQ and 3,6-BPQ increase tyrosine phosphorylation at numerous sites on the EGFR leading to activation of phospholipase C-γ1 and several signal transducers and activators of transcription (STATs). Both 1,6-BPQ and 3,6-BPQ induced the EGFR tyrosine phosphorylation at Y845, Y992, Y1068 and Y1086 residues. 3,6-BPQ also induced phosphorylation of Y1045. However, the magnitude of tyrosine phosphorylation and profile of tyrosine residues activated were different for these agents compared to EGF. Because we have previously shown that BPQs cause tyrosine phosphorylation by a ROS-dependent mechanism that is sensitive to catalase and anti-oxidants (Burdick et al., 2003), the present results strongly suggest that downstream signaling by EGFR is also associated with oxidative stress.
It has been shown that various ligands and transactivator agents can differentially induce specific tyrosine phosphorylations on EGFR to activate downstream signal transduction pathways (Knebel et al., 1996; Herbst, 2004; Sebastian et al., 2006). The phosphorylation of tyrosine residues plays a key in the regulation of signal transduction during a plethora of eukaryotic cell functions (Chiarugi, 2005; Lee et al., 2004). The phosphorylation states in the cell environment are governed by the opposing activities of protein tyrosine phosphatases (PTPs) and protein tyrosine kinase (PTKs) in vivo; tyrosine phosphorylation is a reversible and dynamic process. Previous studies by this group have shown that halogenated aromatic hydrocarbons, such as 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons, such as benzo[a]pyrene (BaP) and their metabolites, are able to increase tyrosine phosphorylation of PTKs, such as Lyn, Syk, IGF-IR, Shc, IRS-1 and EGFR (Tannheimer et al., 1998; Burdick et al., 2003, 2006). In the last two decades, evidence in the literature has shown the participation of redox sensitive proteins in the regulation of EGFR tyrosine phosphorylation in the activation of cell signaling (Burdick et al., 2003, Knebel et al., 1996; Wang et al., 2000; Khan et al., 2006; Rhee, 2006). Interestingly, it has been reported that H2O2 induces phosphorylation preferentially at tyrosine residues on EGFR in intact cells (Goldkorn et al., 1998). Previous studies by our group have shown that BPQs (1,6-BPQ and 3,6-BPQ) produce reactive oxygen species (such as superoxide anion and H2O2), and transactivate the EGFR, leading to strong proliferative activity in human mammary epithelial cells (Burdick et al., 2003, 2006). In addition, we have recently shown evidence that 1,6-BPQ and 3,6-BPQ activate response elements in promoter sequences of oxidative stress-associated genes (HMOX1, GCLC, GCLM and SCL7A11) (Burchiel et al., 2007). The HMOX-1 gene is activated within 2 hrs of exposure to BPQs. Therefore, oxidative stress appears to be the major mechanism by which 1,6-BPQ and 3,6-BPQ might induce the protein phosphorylation.
In the present studies, we found that BPQs induce EGFR tyrosine phosphorylation, as well as several other proteins associated with EGFR downstream signaling. We found that 1,6-BPQ and 3,6-BPQ, were able to induce the phosphorylation and activation of PLC-γ1 in MCF-10A cells. While the kinetics of BPQ activation (18 hrs) were different than EGF(15 min), similar magnitudes of activation were observed. PLC-γ1 is known to be activated in response to growth-factor stimulation by a mechanism that relies on tyrosine phosphorylation (Carpenter, 1999). PLC-γ1 binds directly to the autophosphorylated EGFR via Y1173 and Y992 and is phosphorylated by EGFR kinase. We found that there was a correlation between the pattern of EGFR tyrosine phosphorylation at Y992 and the pattern of PLC-γ1 tyrosine phosphorylation in cells treated with 1,6-BPQ and 3,6-BPQ. These results suggest that PLC-γ1 activation could be part of downstream signaling cascades activated after EGFR phosphorylation.
We also found that 1,6-BPQ and 3,6-BPQ induced the activation of signal transducers and activators of transcription, specifically STAT-1, STAT-3, STAT-5a and STAT-5b. STAT proteins can bind to and become activated by EGFR through the SH2 domain (Leaman et al., 1996). In addition, STATs can be activated by several EGFR-independent mechanisms including other growth factor receptors and non-receptor tyrosine kinases. The SH2 domain of STAT is, in fact, required for activation by tyrosine phosphorylation most commonly at Y701, Y705, or Y694, respectively for STAT-1, STAT-3, and STAT-5 (Sebastian et al., 2006; Herbst, 2004). Our results showed that there was a differential activation of STATs induced by BPQs. 3,6-BPQ not only induced the activation of all STATs studied, but the magnitude of phosphorylation was greater than in 1,6-BPQ-treated cells. Observations by other groups (Xia et al., 2002) indicate that the EGFR cytoplasmic tail contains docking sites for STAT-1 and STAT-3. However, EGFR phosphorylation appears to not be directly involved in STAT-1 and STAT-3 activation. JAK activation is required for complex formation STAT1/STAT3 with JAK1/JAK2, which is essential for STAT activation (Andl et al., 2004). In contrast, STAT-5a and STAT-5b are tyrosine phosphorylated in response to EGFR phosphorylation at Y845 (Sebastian et al., 2006). Tyrosine 845 is not an EGFR autophosphorylation site; instead its phosphorylation requires the association of EGFR with c-Src and the kinase activity (Boerner et al., 2005). c-Src kinase modulates STAT5 activation in at least two ways: (1) by direct phosphorylation of STAT; and (2) by phosphorylating the EGFR at Y845; and on the other hand, STAT 1 and STAT 3 are dependent on JAK activation. We demonstrated that 1,6-BPQ and 3,6-BPQ induced EGFR tyrosine phosphorylation, and the most important effect was observed at tyrosine 845; however, we do not know whether JAK and c-Src are directly activated by BPQs. Given that we found a significant increase in STAT-1, STAT-3 and STAT-5 phosphorylation, our findings suggest that BPQs may play an important role to activate other tyrosine kinases both upstream and downstream in the EGFR-STAT pathway. ROS-induced activation of c-Src and JAK2 has been previously described (Garcia et al., 2001).
A key finding in the present study was that EGFR recycling following activation was different in EGF versus BPQ-activated cells. EGFR internalization is seen after EGF activation by tyrosine phosphorylation and downstream signaling (Levkowitz et al., 1999). This process results in rapid, but transient signaling by EGF. Our results confirmed that EGF rapidly induces the disappearance of EGFR from MCF-10A cell membranes. BPQs also produced a rapid modulation of EGFR, beginning as early as 30 min after treatment and resulting in prolonged (18 hr) expression of EGFR on the cell surface. Because we know that BPQs produce rapid oxidative stress in MCF-10A cells, as evidenced by increased HMOX-1 expression within 2 hrs of exposure to BPQs (Burdick et al., 2003), we hypothesize that oxidative stress produced by BPQs is responsible for the changes in EGFR expression. Whether the changes in EGFR expression is due to altered tyrosine kinase activity is still under investigation. Finally, we have recently observed that like TCDD (Davis et al., 2003), BPQs induce AhR activation in MCF-10A cells leading to the expression of TGF-alpha which is a known agonist for the EGFR. Thus, we believe that some of the 18 hr signaling events associated with BPQs are the result of the autocrine production of TGF-alpha.
In summary, the experimental evidence presented here clearly support the hypothesis that 1,6-BPQ and 3,6-BPQ can transactivate EGFR and activate downstream cell signaling in MCF-10A cells, probably mediated by oxidative stress. The patterns of EGFR, PLC-γ1 and STATs phosphorylation found after treatment of cells with BPQs were quite similar to those induced by the natural EGF ligand, and in fact some of the effects of BPQs may be due to the autocrine upregulation of TGF-alpha. Further characterization and understanding of the cellular mechanisms involved in ROS signal transduction via tyrosine kinase activation and protein tyrosine phosphatase inhibition, as well as Ah receptor-dependent signaling may provide further insights into the mechanisms by which BPQs contribute to tumor progression.
Acknowledgments
We thank Dr. Jun Gao for his expert advice and assistance. This work was supported by RO1 ES-07945, NIEHS P30 ES-012072, and NCI P30 CA-118100.
REFERENCES
- Andl CD, Mizushima TM, Oyama KM, Bowser M, Nakagawa H, Rustgi AK. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol Liver Physiol. 2004;287:G1227–1237. doi: 10.1152/ajpgi.00253.2004. [DOI] [PubMed] [Google Scholar]
- Bai XC, Deng F, Liu AL, Zou ZP, Wang Y, Ke ZY, Ji QS, Luo SQ. Phospholipase C-gamma1 is required for cell survival in oxidative stress by protein kinase C. Biochem J. 2002;363:395–401. doi: 10.1042/0264-6021:3630395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Boffetta P, La Vecchia C. Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: a quantitative review to 2005. Ann Oncol. 2007;18:431–446. doi: 10.1093/annonc/mdl172. [DOI] [PubMed] [Google Scholar]
- Bostrom CE, Gerde P, Hanberg A, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air, Environ. Health Perspect. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JG, Moysich KB, Humblest O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer. 2007;109:2667–26711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associated genes by benzo(a)pyrene 3,6 quinone and benzo(a)pyrene 1,6-quinone in MCF-10A cells human mammary epithelial cells. Toxicol App Pharmacol. 2007;221:203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Davis JW, Liu KJ, et al. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–7833. [PubMed] [Google Scholar]
- Burdick AD, Ivnitski-Steele ID, Lauer FT, Burchiel SW. PYK2 mediates anti-apoptotic AKT signaling in response to benzo[a]pyrene diol epoxide in mammary epithelial cells. Carcinogenesis. 2006;27:2331–2340. doi: 10.1093/carcin/bgl083. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Ji Q. Phospholipase C γ1 as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- Carreras MC, Poderoso JJ. Mitochondrial nitric oxide in the signaling of cell integrated responses. Am J Physiol Cell Physiol. 2007;292:1569–1580. doi: 10.1152/ajpcell.00248.2006. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Rogan E. Role of radical cations in aromatic hydrocarbon carcinogenesis. Environ Health Perspect. 1985;64:69–84. doi: 10.1289/ehp.856469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim Biophys Acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, PTPs versus PTKs The redox side of the coin. Free Radical Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslational modifications. Antioxidants & redox signaling. 2007;9:1–23. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- Conney AH, Chang RL, Jerina DM, Wei SJ. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab Rev. 1994;26:125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- Davis JW, Burdick AD, Lauer FT, Burchiel SW. The aryl hydrocarbon receptor antagonist, 3'methoxy-4'nitroflavone, attenuates 2,3,7,8-tetrachlorodibenzo-p-dioxin-dependent regulation of growth factor signaling and apoptosis in the MCF-10A cell line. Toxicol Appl Pharmacol. 2003;188:42–49. doi: 10.1016/s0041-008x(03)00012-7. [DOI] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Matsukuma K, et al. EGF-receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998;19:786–798. doi: 10.1165/ajrcmb.19.5.3249. [DOI] [PubMed] [Google Scholar]
- Grandis JR, Drenning SD, Zeng Q, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal Growth Factor Receptor Exposed to Oxidative Stress Undergoes Src- and Caveolin-1-dependent Perinuclear Trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- Kijima T, Niwa H, Steinman RA, et al. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13:355–362. [PubMed] [Google Scholar]
- Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM. STAT5b, a Mediator of Synergism between c-Src and the Epidermal Growth Factor Receptor. J Biol Chem. 2003;278:1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinase as target of regulation by radiation, oxidants or alkylanting agents. The EMBO Journal. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- Leaman DW, Pisharody S, Flickinger TW, et al. Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol Cell Biol. 1996;16:369–375. doi: 10.1128/mcb.16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BM, Shim GA. Dietary exposure estimation of benzo(a)pyrene and cancer risk assessment. J Toxicol Environ Health A. 2007;70:1391–1394. doi: 10.1080/15287390701434182. [DOI] [PubMed] [Google Scholar]
- Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC, Huang CJ, Kandaswami C, Lee PP, Lee MT. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavinoids: effect on invasive potential of human carcinoma cells. Biochem Pharmacol. 2004;67:2103–2114. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Martínez-Sánchez G, Giuliani A. Cellular redox status regulates hypoxia inducible factor-1 activity. Role in tumor development. J Exp Clin Cancer Res. 2007;26:39–50. [PubMed] [Google Scholar]
- Pelkonen O, Nebert DW. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- Reynolds AR, Tischer C, Verveer PJ, Rocks O, Bastiaens PIH. EGFR activation coupled to inhibition of tyrosine phosphatase causes later signal propagation. Nat Cell Biol. 2003;5:447–453. doi: 10.1038/ncb981. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wang LE, Bondy ML, Brewster A, Singletary SE, Wei Q. Reduced DNA repair of benzo[a]pyrene diol epoxide-induced adducts and common XPD polymorphisms in breast cancer patients. Carcinogenesis. 2004;25:1695–1700. doi: 10.1093/carcin/bgh167. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Lauer FT, Lane J, Burchiel SW. Factors influencing elevation of intracellular Ca2+ in the MCF-10A human mammary epithelial cell line by carcinogenic polycyclic aromatic hydrocarbons. Mol Carcinog. 1999;25:48–54. doi: 10.1002/(sici)1098-2744(199905)25:1<48::aid-mc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Ethier SP, Caldwell KK, Burchiel SW. Benzo[a]pyrene- and TCDD-induced alterations in tyrosine phosphorylation and insulin-like growth factor signaling pathways in the MCF-10A human mammary epithelial cell line. Carcinogenesis. 1998;19:1291–1297. doi: 10.1093/carcin/19.7.1291. [DOI] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, Holbrook NK. Epidermal growth factor receptor-dependent Akt activation by oxidative stress cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu J, Wang Z. Akt Binds to and Phosphorylates Phospholipase C-γ1 in Response to Epidermal Growth Factor. Mol Biol Cell. 2006;17:2267–2277. doi: 10.1091/mbc.E05-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Wang L, Chung AS, et al. Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation. J Biol Chem. 2002;277:30716–30723. doi: 10.1074/jbc.M202823200. [DOI] [PubMed] [Google Scholar]