SUMMARY
Parkinson’s disease (PD) is a progressive neurological disorder predominantly characterized by motor symptoms including bradykinesia and resting tremor. The gold standard of treatment for PD remains dopamine replacement therapy, which eventually fails due to continued progression of the disease and the development of debilitating side effects. Recent breakthroughs are providing the first major advances in the development of fundamentally new pharmacological strategies for the treatment of PD that do not rely on dopamine replacement strategies, but rather aim to reduce the overactive indirect pathway within the basal ganglia. In this article, we will review the role of metabotropic glutamate receptors within the basal ganglia and discuss the potential for modulation of metabotropic glutamate receptors as a treatment for PD.
Parkinson’s disease (PD) is the second most common neurological disorder, affecting approximately 1% of the population over 60 years of age, and is characterized by debilitating motor symptoms including resting tremor, rigidity, postural instability and bradykinesia [1–3]. These primary motor symptoms of PD arise as a result of dopaminergic neuron degeneration in the substantia nigra pars compacta (SNc), resulting in the loss of dopamine release to the target striatum (the brain region that receives the bulk of the dopaminergic inputs) within the basal ganglia. In the normally functioning basal ganglia, there is a balance between the signals that promote movements (thought to be transmitted to the basal ganglia output nuclei via the ‘direct’ pathway of the basal ganglia), and those that suppress movement (thought to be transmitted via the ‘indirect’ pathway of the basal ganglia) [4,5]. The activation of D1 receptors of the direct pathway in the striatum stimulates inhibitory neurons and leads to a direct inhibitory effect on the GABAergic neurons of the globus pallidus pars interna (GPi; entopeduncular nucleus in nonprimates) and substantia nigra pars reticulata (SNr) [5,6]. The binding of dopamine to D2 receptors in the indirect pathway of the striatum inhibits the projections to the GP pars externa (GPe) and results in a disinhibition of the sub-thalamic nucleus (STN), and a net excitatory effect of the projections from the STN to the GPi/SNr [5,6]. This balance between the excitatory projections from the STN and inhibitory signals from the direct pathway act to modulate the degree of inhibition from the GPi/SNr GABAergic projections that are exerted on the thalamus, which projects to motor areas of the cerebral cortex [5,6]. In PD, the loss of dopaminergic input into the circuit disrupts this delicate balance. In the direct pathway, there is a loss of inhibition of the GPi/SNr, whereas the loss of dopamine in the indirect pathway leads to excessive inhibition of the GPe, leading to disinhibition of the STN and an increase in excitatory output by the STN onto the GPi/SNr [5,6–8]. The current gold standard of treatment for PD patients aims to replace the loss of dopaminergic tone in the basal ganglia with the dopamine precursor, levodopa (L-DOPA). Unfortunately, these current therapies are designed to address the primary symptoms of PD but do not slow down the continued progression of this neuro-degenerative disorder. Thus, the development of therapies designed to slow or stop the progression of PD, while also reversing the symptoms of this disease, would greatly improve the quality of life for PD patients. One promising alternative strategy for the treatment of PD symptoms and progression includes manipulating targets that reduce transmission from the indirect pathway. In support of this hypothesis, lesions or high-frequency stimulation of the STN normalize excitatory output and produce antiparkinsonian effects [9–13]. While effective, surgical therapies may not be available to all patients owing to cost and the invasive nature of the procedure. Thus, pharmacological intervention that reduces the aberrant signaling from the indirect pathway remains an active target for the development of novel nondopaminergic therapies for the treatment of PD. Furthermore, such therapies could reduce the economic burden of care for PD patients, which is currently estimated at US$10.8 billion [3].
In the CNS, glutamate is the major excitatory neurotransmitter and can signal through activation of ionotropic glutamate receptors or G protein-coupled receptors. While ionotropic glutamate receptors mediate fast excitatory synaptic transmission, metabotropic glutamate receptors (mGluRs) play a neuromodulatory role in synaptic transmission [14,15]. There are eight mGluR subtypes, which have been classified into three major groups based on sequence homologies, coupling to second-messenger systems and ligand selectivity. Group I mGluRs (mGlu1 and 5) couple primarily to Gq and increases in phosphoinositide hydrolysis, whereas group II (mGlu2 and 3) and III (mGlu4, 6, 7 and 8) mGluRs couple to Gi/o and associated signaling pathways, such as the inhibition of adenylyl cyclase [16,17]. mGluRs are differentially expressed within the basal ganglia and throughout the rest of the CNS (Table 1) [18]. Group I mGluRs are highly expressed throughout the basal ganglia and have pre- and postsynaptic cellular localization. Specifically, mGlu1 is expressed on dopaminergic neurons within the SNc and dopaminergic fibers within the striatum [19]. In the striatum, mGlu1 is also expressed on medium spiny neurons (MSNs) and GABAergic interneurons [20]. The expression of mGlu5 has been localized postsynaptically to striatal MSNs and GABAergic interneurons [19,20]. Postsynaptic mGlu5 is also found in the GPe, STN and GPi/SNr [20]. Group II mGluRs are primarily localized presynaptically to corticostriatal fibers, SNc dopaminergic neurons and excitatory terminals originating in the STN [21–24]. For group III mGluRs, while mGlu6 is limited to the retina, mGluRs 4, 7 and 8 all have mostly presynaptic distributions within the basal ganglia. Expression of mGlu4 and 7 are found on terminals of the corticostriatal, striatopallidal and striatonigral pathways, and on terminals from the STN to the SNc and SNr [25–30]. The expression of mGlu8 is the least well characterized but may be located in the terminals of striatonigral and STN–SNr synapses [29]. The cellular localization and modulatory role for mGluRs make them prime targets for pharmacological intervention within the indirect pathway of the basal ganglia (Figure 1).
Table 1.
Expression of metabotropic glutamate receptors in the basal ganglia.
| Group | Signaling pathway | Receptor | Basal ganglia localization | Synaptic localization | Antiparkinsonian effects in animal models | Neuroprotective effects |
|---|---|---|---|---|---|---|
| I | Coupled to Gq and PI hydrolysis | mGlu1 | SNc, striatum, GPe, GPi/SNr, STN | Pre- and postsynaptic | Limited beneficial effects of antagonists | No data |
| mGlu5 | Striatum, GPe, GPi/SNr, STN | Predominately postsynaptic | Antagonists have moderate symptomatic effects and may be beneficial for LIDs | Yes | ||
|
| ||||||
| II | Coupled to Gi/o and inhibition of adenylyl cyclase | mGlu2/3 | SNc, striatum, GPi/SNr | Predominately presynaptic | Agonists demonstrate antiparkinsonian effects in acute but not chronic PD models | Yes |
|
| ||||||
| III | Coupled to Gi/o and inhibition of adenylyl cyclase | mGlu4 | SNc, striatum, GPe, GPi/SNr | Predominately presynaptic | Group III agonists, mGlu4- preferring agonists and mGlu4 PAMs have antiparkinsonian effects in several animal models of PD | Yes for group III agonists and mGlu4 PAMs |
| mGlu6 | Not expressed in basal ganglia | – | – | – | ||
| mGlu7 | Striatum, GPe, GPi/SNr | Predominately presynaptic | Agonists demonstrate antiparkinsonian effects in animal models of PD | No data | ||
| mGlu8 | Striatum, GPi/SNr | Predominately presynaptic | No antiparkinsonian effects reported | No data | ||
GPe: Globus pallidus pars externa; GPi: Globus pallidus pars interna; LID: Levodopa-induced dyskinesias; mGlu: Metabotropic glutamate; PAM: Positive allosteric modulator; PD: Parkinson’s disease; PI: Phosphoinositide; SNc: Substantia nigra pars compacta; SNr: Substantia nigra pars reticulata; STN: Subthalamic nucleus.
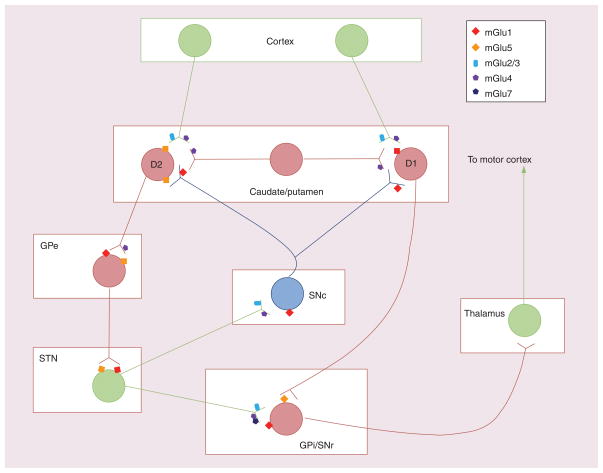
Figure 1. Potential targets for metabotropic glutamate receptor modulation within the basal ganglia.
Group I mGluRs are widely distributed throughout the basal ganglia. When activated, group I mGluRs counteract the response of the basal ganglia to dopamine (both directly in medium spiny neurons and indirectly via other nuclei within the basal ganglia). Antagonists of group I mGluRs (specifically mGlu5) have antiparkinsonian effects in animal models of Parkinson’s disease, which is thought to be due to reduced output from the STN and/or activity of the striatopallidal synapse. Group II mGluRs are localized presynaptically to corticostriatal terminals, STN–SNr synapses and STN–SNc synapses. Agonists of group II mGluRs may have antiparkinsonian effects due to reduced corticostriatal glutamate release and reduced activation of the STN. Group III mGluRs are also expressed in multiple basal ganglia nuclei. Electrophysiological and behavioral studies have revealed that activation of mGlu4 at the striatopallidal synapse has profound antiparkinsonian effects. Activation of mGlu4 also modulates the corticostriatal, STN–SNr and intrastriatal GABAergic synapses. While mGlu7 is expressed in many of the same nuclei as mGlu4, current data only support effects of mGlu7 at SNr synapses. Red neuron: GABAergic; green neuron: glutamatergic; blue neuron: dopaminergic.
GPe: Globus pallidus pars externa; GPi: Globus pallidus pars interna; mGluR: Metabotropic glutamate receptor; SNc: Substantia nigra pars compacta; SNr: Substantia nigra pars reticulata; STN: Subthalamic nucleus.
Group I mGluRs
The expression of group I mGluRs at several of the key synapses within the basal ganglia indicates that modulation of group I mGluRs may be beneficial for parkinsonian symptoms. Group I mGluR activation counteracts the effects of dopamine in the basal ganglia via a direct interaction in D1- and D2-expressing MSNs, and indirectly by increasing the excitability of the GPe, STN and SNr; thus, it is predicted that antagonism of these receptors could have antiparkinsonian activity by reducing the increased excitatory drive of the indirect pathway [18,31–33]. Much of the focus for potential antiparkinsonian activity of group I mGluRs remains on the antagonism of mGlu5. The few studies evaluating the use of mGlu1 antagonists have yielded limited beneficial effects and thus it is not thought to be a viable target for the treatment of motor symptoms in PD [34]. There are several studies highlighting the antiparkinsonian activity of the mGlu5-negative allosteric modulators (NAMs) 2-methyl-6-(phenylethynyl) pyridine (MPEP) and 3-[(2-methyl-1,3-thia-zol-4-yl)ethynyl]pyridine (MTEP) in animal models of PD. For example, MPEP and MTEP reverse haloperidol-induced catalepsy and attenuate deficits in the reaction time task (a measure of akinesia) in 6-hydroxydopamine (6-OHDA)-lesioned animals [35–38]. Although the exact mechanism for the antiparkinsonian activity of mGlu5 antagonism is not clearly understood, it is hypothesized that a reduction in striatopallidal and/or STN activity may play a role. Consistent with this hypothesis, activation of mGlu5 has excitatory effects and potentiates NMDA receptor currents on STN neurons [31,39]. A role for actions in the STN is further supported by data indicating that direct STN infusions of MPEP reduce asymmetric motor behavior in 6-OHDA-lesioned rats [40]. The suggestion of a possible role for mGlu5 in the GP comes from findings that systemic administration of mGlu5 NAMs attenuate proenkephalin mRNA (a marker of striatopallidal activity) in dopamine-depleted rats [41]. Interestingly, electrophysiological studies reveal that antagonism of mGlu5 can increase GP activity by reducing desensitization of excitatory responses mediated by mGlu1 activation in GP projection neurons [42]. Reduced desensitization of mGlu1 could increase GP neuron activity and could contribute to an anti-parkinsonian effect of mGlu5 NAMs. Thus, antagonism of mGlu5 remains a pharmacological target for the motor symptoms associated with PD. However, many effects of mGluR5 activation are altered in dopamine-depleted animals, indicating that mGlu1 and mGlu5 have redundant roles when dopamine function is lost [32,43]. For example, under basal conditions, mGlu5 activation depolarizes neurons within the STN, whereas mGlu1 activation depolarizes SNr neurons [32]. After dopamine depletion due to haloperidol treatment, both mGlu1 and mGlu5 activation induce depolarization for these basal ganglia nuclei [32]. The combination of dopamine depletion-related alterations in mGlu5 and the effects that mGlu5 activation can have in the direct pathway, could counteract the beneficial effects of mGlu5 NAMs on primary motor symptoms in PD patients [18,32,44]. Consistent with this, the effects of the mGlu5 NAM AFQ056 (Novartis, Basel, Switzerland) on primary motor symptoms in PD patients are relatively modest [45].
While mGlu5 antagonism may have limited potential as a symptomatic therapy for the treatment of PD, recent evidence suggests that coadministration of mGlu5 antagonists with adenosine A2A antagonists could have more robust antiparkinsonian effects than either treatment alone. Antagonists of adenosine A2A receptors reverse parkinsonian motor symptoms in animal models and have now progressed to Phase III clinical trials for symptomatic treatment in PD patients [46–51]. Within the striatum, mGlu5 and A2A adenosine receptors can heterodimerize and are colocalized with dopamine D2 receptors on MSNs where they function to oppose the cellular response to dopamine [52]. Coadministration with MPEP and A2A antagonists have resulted in a synergistic relationship as evidenced by submaximal doses of combined MPEP and A2A antagonists significantly attenuating parkinsonian motor symptoms [36,53]. Thus, the use of combination therapy of multiple targets within the overactive indirect pathway may be a viable nondopaminergic therapy for the treatment of PD. Beside symptomatic relief, there is also evidence demonstrating that mGlu5 antagonism is neuroprotective and, thus, potentially a disease-modifying therapy. Mice lacking mGlu5 are less sensitive to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration, indicating that reduced signaling through mGlu5 may be neuroprotective against neurotoxin insults to the nigrostratal pathway [54]. In addition, chronic administration of MPEP significantly reduces the nigrostriatal damage due to 6-OHDA injection, indicating that pharmacological intervention with mGlu5 antagonists is neuroprotective for neurotoxin models of PD [55]. Furthermore, in MPTP-treated monkeys, chronic MTEP administration significantly attenuated the toxin-induced dopaminergic and noradrenergic cell death [56]. Interestingly, there is evidence of additional symptomatic benefits for mGlu5 antagonism other than the motor symptoms associated with PD. In one study, the systemic administration of MPEP reversed cognitive deficits in bilaterally 6-OHDA-lesioned mice [57]. Moreover, antagonism of mGlu5 has demonstrated efficacy in preclinical models of anxiety and depression [58].
Although the efficacy of mGlu5 NAMs in the treatment of primary motor symptoms in PD patients is relatively modest, exciting new results suggest that mGlu5 NAMs may have robust efficacy in reducing L-DOPA-induced dyskinesias (LIDs). Expression of mGlu5 is upregulated in the putamen and GP of MPTP-treated monkeys and this increase in mGlu5 expression is associated with the development of LIDs [59]. Furthermore, in 6-OHDA-lesioned rats chronically treated with L-DOPA, the systemic administration of MTEP prevents the formation of, and attenuates, LIDs; thus supporting the hypothesis that mGlu5 antagonism reduces LIDs [60–62]. Similar effects of mGlu5 NAMs on LIDs have now been observed in parkinsonian nonhuman primates [59]. Finally, exciting clinical studies have now been reported by investigators at Novartis showing that AFQ056 has robust efficacy in reducing LIDs in PD patients [45]. Also encouraging is a recent report that Addex Pharmaceuticals (Geneva, Switzerland) has completed a Phase I clinical trial and has announced the start of a Phase II proof-of-concept trial for the use of the mGlu5 antagonist ADX48261 for the treatment for LIDs in PD patients [63]. The data from these studies could further validate the use of mGluR modulation as an adjunct therapy to L-DOPA for symptomatic treatment, and could indicate a potential benefit of mGlu5 antagonism treatment prior to the formation of LIDs in PD patients. Taken together, these data signify that mGlu5 antagonism may have multiple beneficial effects for PD patients.
Group II mGluRs
The localization of group II mGluRs presynaptically at STN–SNr and corticostriatal synapses leads to the hypothesis that activation of these receptors may attenuate activity through the indirect pathway at the level of the striatum, and could be beneficial for reducing parkinsonian motor symptoms. Consistent with this, electrophysiological recordings in the striatum reveal that group II agonists reduce the activity of corticostriatal synapses and inhibit glutamate release as demonstrated by in vivo microdialysis studies [22,64,65]. Group II agonists acutely reduce excitatory postsynaptic currents and induce long-term depression at the STN–SNr synapse, thus attenuating the activity of the STN towards the output structures of the basal ganglia [66,67]. In animal models of PD, systemic administration of the group II agonist LY354740 (Eli Lilly, IN, USA) significantly decreased haloperidol-induced catalepsy and muscle rigidity, indicating a potential benefit as a symptomatic treatment for PD [68,69]. Moreover, intracerebroventricular administration of the group II agonist LY379268 (Eli Lilly, IN, USA) reversed reserpine-induced akinesia [69]. In 6-OHDA lesioned animals, the expression of mGlu2/3 receptor proteins is increased and the potency of mGlu2/3 agonist-induced depression of coticostriatal transmission is increased [70]. This indicates that dopamine depletion induces compensatory changes in mGlu2/3 expression and activation that may be relevant to the treatment of PD with mGlu2/3 agonists. However, electrophysiology studies reveal that the effects of group II mGluR agonists on STN–SNr synapses are reduced in dopamine-depleted animals [71]. This is further supported by behavioral studies in which systemic administration of group II mGluR agonists were unable to reverse motor deficits in chronically dopamine-depleted animals [72]. This inability of mGlu2/3 agonism to reverse motor deficits in chronically dopamine-depleted animals could indicate a limited therapeutic potential for the treatment of PD patients. Group II agonists have also demonstrated anxiolytic and antidepressant activity, thus, they may be a potential adjunct therapy addressing the psychiatric nonmotor symptoms associated with PD [58].
Electrophysiology studies demonstrating that activation of presynaptic group II mGluRs at the STN–SNc synapse reduces excitatory transmission in rat SNc neurons indicates that group II mGluR activation may be neuroprotective due to reduced glutamate excitotoxicity [21,72]. Chronic systemic dosing of LY379268 reduced nigral dopaminergic cell death following a 6-OHDA lesion and reduced dopamine cell degeneration in MPTP-treated mice, supporting the neuroprotective activity of group II agonists for the nigrostriatal system [69,73]. Although the mechanism of neuroprotection is not fully understood, agonists of group II mGluRs may reduce glutamate excitotoxicity and can increase the production of neuroprotective growth factors [72,74–77]. Thus, group II mGluR activation may not be an optimal symptomatic therapy for PD but may be used in combination with other symptomatic treatments as a potentially disease-modifying therapy.
Group III mGluRs
Recently, there has been an increased interest in generating pharmacological agents that target group III mGluRs as a potential treatment for PD. Group III mGluRs are expressed at multiple synapses within the basal ganglia (except mGlu6, which is restricted to the retina), where their activation decrease transmission [25]. Using the pan group III mGluR agonist (2S)-2-amino-4-phosphonobutanoic acid (L-AP4), it has been demonstrated that group III mGluR agonism significantly reduces synaptic transmission at both the striatopallidal and STN–SNr synapses in rat brain slices [78–83]. The mGlu4 subtype of group III mGluRs is highly localized at the striatopallidal synapse [25], suggesting that mGlu4 could be responsible for this effect at this key synapse. Consistent with this, the effect of L-AP4 at the striatopallidal synapse is absent in mGlu4-knockout mice. Thus, mGlu4 activation is solely responsible for inhibiting transmission at this first synapse in the indirect pathway. Furthermore, pallidal infusions of L-AP4 or another group III agonist, L-serine-O-phosphate, significantly reduce evoked GABA release in the GP in vivo [82]. These studies suggest that activation of mGlu4 in the GP could have antiparkinsonian effects through reduced activity of the indirect pathway. In support of this hypothesis, intracerebroventricular administration of L-AP4 reverses parkinsonian motor dysfunction in several animal models of PD, including reserpine-induced akinesia and haloperidol-induced catalepsy [81]. In the same study, L-AP4 administration was as equally efficacious as L-DOPA at reversing the behavioral motor deficits caused by a unilateral 6-OHDA lesion [81]. Intrapallidal infusions of group III agonists reverse haloperidol-induced catalepsy, reserpine-induced akinesia and akinetic effects of 6-OHDA in the reaction time task, indicating that the antiparkinsonian effects of group III agonists may be the result of reduced transmission at the striatopallidal synapse in vivo [81,84–87]. More recently, the orthosteric mGlu4-preferring group III agonists (LSP1-2111 and 3081, which have higher potency for mGlu4 than any of the other group III mGluRs) have demonstrated reduced motor deficits in 6-OHDA-lesioned animals after striatal or pallidal infusions [88,89]. Taken together with studies suggesting that mGlu4 is the group III mGluR responsible for modulating transmission at the striatopallidal synapse, these studies suggest that mGlu4 may be primarily responsible for the antiparkinsonian activity of group III agonists and, thus, may be a potential target for pharmacological intervention.
Due to the highly conserved glutamate binding site, attempts to develop highly specific orthosteric agonists of group III mGluR subtypes (more specifically for mGlu4) have been difficult. An alternative strategy is to develop compounds that modulate the receptors at an allosteric site, thus increasing the specificity of the compound. One example of this strategy is the development of N-phenyl-7-(hydroxyimino)cyclopropa[b] chromen-1a-carboxamide (PHCCC), a positive allosteric modulator that selectively potentiates mGlu4 signaling when compared with other mGluR subtypes [90]. Positive allosteric modulators (PAMs) like PHCCC enhance mGlu4 activation in the presence of glutamate by binding to mGlu4 at a site distinct from that of the glutamate binding site, but have no intrinsic ability to activate the receptor [91]. Brain slice electrophysiology reveals that PHCCC potentiates the L-AP4-mediated inhibition of inhibitory postsynaptic currents at the striatopallidal synapse while having no effect on inhibitory postsynaptic currents alone [86]. PHCCC also reverses reserpine-induced akinesia after intra-cerebroventricular or systemic administration, indicating the potential antiparkinsonian effects of mGlu4 PAMs [86,87]. More recently, other mGlu4 PAMs have been generated and have confirmed the earlier antiparkinsonian data, indicating that enhanced mGlu4 activation may be a viable target for the treatment of PD [92–94]. One of these mGlu4 PAMs is VU0364770 (Vanderbilt University, TN, USA) and is highly potent and systemically active. Systemic administration of VU0364770 has antiparkinsonian activity in haloperidol-induced catalepsy and in the 6-OHDA model of PD [93]. While the stand-alone efficacy of VU0364770 against 6-OHDA motor dysfunction was modest, VU0364770 significantly potentiated a subthreshold dose of L-DOPA, indicating that this mGlu4 PAM could be an adjunct therapy, thereby reducing the need for L-DOPA [93]. The cellular localization within the striatopallidal synapse of mGlu4 and adenosine A2A receptors, and the individual anti-parkinsonian activity of adenosine A2A receptor antagonists and mGlu4 PAMs make them exciting targets for a combination therapy. An early study raised the possibility that coadministration of A2A antagonists and group III mGluR agonists further reduce catalepsy after haloperidol treatment, suggesting that combination therapy with these agents may provide more robust efficacy than either approach alone [95]. Similarly, administration of VU0364770 significantly potentiates the anticataleptic effects of an A2A antagonist (preladenant) in the haloperidol-induced catalepsy model of preclinical PD [93]. Thus, mGlu4 PAMs have standalone symptomatic effects for PD and may potentiate the effects of L-DOPA or A2A antagonists as an adjunct therapy for PD. Recent data indicate activation of mGlu7 may also have antiparkinsonian activity in vivo. Systemic administration of the mGlu7 agonist, AMN082 (Novartis, Basel, Switzerland), attenuates haloperidol-induced catalepsy and akinetic deficits in 6-OHDA-lesioned rats [96]. These effects are likely mediated by the SNr as demonstrated by reduced reserpine-induced akinesia following intranigral AMN082 injections [97]. Interestingly, intranigral infusion of the selective mGlu8 agonist (S)-3,4-dicarboxyphenylglycine induces catalepsy, suggesting that mGlu8 activation could counteract antiparkinsonian effects of mGlu4 activation [98]. Thus, highly selective activators of mGlu4 could be preferable to less selective group III mGluR agonists for treating PD symptoms. Similar to mGlu5 antagonists and group II agonists, group III agonists may also have anxiolytic/antidepressant properties [58].
There are several lines of evidence based on cellular and physiological data that predict that group III mGluR agonists or PAMs may also have neuroprotective effects for PD patients. Activation of mGlu4 at the striatopallidal synapse reduces the activity of the indirect pathway, which should reduce subsequent excitotoxicity of the SNc due to STN overactivation [81]. In addition, activation of mGlu4 decreases transmission at synapses from the STN on to dopaminergic neurons in the SNc [99]. This could further reduce excitatory drive and any component of dopamine cell death that involves excitotoxicity. In addition to the potential protection against excitotoxicity, mGlu4 activation may reduce neuroinflammation and thus participate in multiple potential mechanisms of neuroprotection [100]. For instance, activation of mGlu4 reduces cytokine release from reactive astrocytes [101]. Also, activation of mGlu4 and mGlu4 PAMs reduces neuroinflammation by actions on peripheral immune cells [102]. In animal models of PD, both acute and subchronic intranigral infusions of L-AP4 reduce the extent of 6-OHDA toxicity in the rat SNc [55,103]. Moreover, systemic or intrapallidal PHCCC administration protects the dopaminergic nigrostriatal system against MPTP toxicity in mice [87]. The neuroprotective effects of PHCCC are lost in mice lacking mGluR4, thus supporting the selective activation of mGlu4 as a strategy for neuroprotection in PD [87]. Recently, a study demonstrated that chronic coadministration of MPEP with L-AP4 was significantly more neuroprotective than either compound alone, indicating that combination therapy targeting multiple mGluRs as a potential disease-modifying therapy for the treatment of PD [104]. Overall, these data show that mGlu4 activation may be utilized as a symptomatic and disease-modifying treatment for PD.
Conclusion & future perspective
Preclinical evidence has validated mGluRs as pharmacological targets for the treatment of parkinsonian motor symptoms. Most notably, mGlu5 antagonism and mGlu4 activation appear to have promise as potential treatments for PD patients. Antagonists of mGlu5 reverse some motor dysfunction and have neuroprotective effects in animal models of PD. The use of mGlu5 antagonists may be most beneficial at attenuating LIDs in PD patients. Activation of group III mGluRs (with particular interest in mGlu4) reduces activity of the indirect pathway and reverses motor symptoms in several animal models of PD. Activation of mGlu4 has also been shown to be neuroprotective in animal models of PD. Thus, mGlu4 PAMs are being developed to target the motor symptoms associated with PD. Data are now emerging that indicate mGlu5 antagonists or mGlu4 PAMs interact with adenosine A2A antagonists, potentiating their anti-parkinsonian effects. Thus, combined pharmacological therapy with modulators of mGluRs and adenosine receptors may be necessary to normalize the aberrant indirect pathway in PD patients. This type of treatment could reduce the need for dopamine replacement therapy and significantly modify the current therapeutic strategy for the treatment of PD.
Practice Points.
Reducing the activation of the indirect pathway within the basal ganglia represents a potential therapeutic strategy for Parkinson’s disease (PD) that is not dependent on dopamine replacement.
Antagonism of metabotropic glutamate receptor subtype 5 reduces motor dysfunction and attenuates levodopa-induced dyskinesias in animal models of PD.
Group III metabotropic glutamate receptor activation reduces striatopallidal activation and has antiparkinsonian activity in vivo.
Chronic administration of group I, II or III metabotropic glutamate receptor modulators demonstrates neuroprotection for the dopaminergic nigrostriatal system.
Agonists of group III metabotropic glutamate receptors or metabotropic glutamate receptor subtype 5 antagonists are even more efficacious when coadministered with adenosine A2A antagonists in animal models of PD.
Modulation of metabotropic glutamate receptors may also be beneficial for psychiatric nonmotor symptoms associated with PD.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
PJ Conn is employed by Vanderbilt University (TN, USA). Over the past year, he has served as a consultant or received honoraria from Millipore Corp. (MA, USA), Karuna Pharmaceuticals (MA, USA), Seaside Therapeutics (MA, USA), NIH (MD, USA), Michael J Fox Foundation (NY, USA), Stanley Center of the Broad Institute (MIT/Harvard, MA, USA), Leiber Institute (Johns Hopkins, MD, USA), Washington University (MO, USA) and the University of Montreal (QC, Canada). PJ Conn holds equity in Seaside Therapeutics and Karuna Pharmaceuticals. He has received research support from NIH (grant no. R01 NS031373-15), Seaside Therapeutics, Janssen Pharmaceutica (Beerse, Belgium), Michael J Fox Foundation and Vanderbilt University. PJ Conn is an inventor on multiple issued and pending patents for novel ligands that interact with neurotransmitter receptors and transporters. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72(21):S1–S13. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 3.Chen JJ. Parkinson’s disease: health-related quality of life, economic cost, and implications of early treatment. Am J Manage Care. 2010;16:S87–S93. [PubMed] [Google Scholar]
- 4.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 5.Bartels AL, Leenders KL. Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex. 2009;45(8):915–921. doi: 10.1016/j.cortex.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15(12):8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 8.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 9.Limousin P, Pollak P, Benazzouz A, et al. Bilateral subthalamic nucleus stimulation for severe Parkinson’s disease. Mov Disord. 1995;10(5):672–674. doi: 10.1002/mds.870100523. [DOI] [PubMed] [Google Scholar]
- 10.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol. 1997;42(3):283–291. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 11.Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345(8942):91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 12.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249(4975):1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 13.Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci. 2010;32(7):1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 14.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 15.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi S. The molecular diversity of glutamate receptors. Prog Clin Biol Res. 1994;390:85–98. [PubMed] [Google Scholar]
- 17.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6(10):787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 19.Smith Y, Charara A, Hanson JE, Paquet M, Levey AI. GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat. 2000;196 (4):555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74(5):271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Wigmore MA, Lacey MG. Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br J Pharmacol. 1998;123(4):667–674. doi: 10.1038/sj.bjp.0701662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGluR2 or 3. J Neurophysiol. 1995;73(3):1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229(3):161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 24.Cozzi A, Attucci S, Peruginelli F, et al. Type 2 metabotropic glutamate (mGlu) receptors tonically inhibit transmitter release in rat caudate nucleus: in vivo studies with (2S,1′S,2′S,3′R)-2-(2′-carboxy-3′-phenylcyclopropyl)glycine, a new potent and selective antagonist. Eur J Neurosci. 1997;9(7):1350–1355. doi: 10.1111/j.1460-9568.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 25.Bradley SR, Standaert DG, Rhodes KJ, et al. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407(1):33–46. [PubMed] [Google Scholar]
- 26.Kosinski CM, Risso Bradley S, Conn PJ, et al. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol. 1999;415(2):266–284. [PubMed] [Google Scholar]
- 27.Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110(3):403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 28.Duty S. Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson’s disease. Br J Pharmacol. 2010;161(2):271–287. doi: 10.1111/j.1476-5381.2010.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messenger MJ, Dawson LG, Duty S. Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43(2):261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 30.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14(5):3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20(21):7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino MJ, Awad-Granko H, Ciombor KJ, Conn PJ. Haloperidol-induced alteration in the physiological actions of group I mGlus in the subthalamic nucleus and the substantia nigra pars reticulata. Neuropharmacology. 2002;43(2):147–159. doi: 10.1016/s0028-3908(02)00097-7. [DOI] [PubMed] [Google Scholar]
- 33.Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21(18):7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull. 2006;69(3):318–326. doi: 10.1016/j.brainresbull.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22(13):5669–5678. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29(8):1451–1461. doi: 10.1038/sj.npp.1300444. Demonstrates that combined antagonism of adenosine A2A and metabotropic glutamate (mGlu)5 receptors increases symptomatic efficacy in preclinical models of Parkinson’s disease (PD) [DOI] [PubMed] [Google Scholar]
- 37.Ossowska K, Konieczny J, Wolfarth S, Pilc A. MTEP, a new selective antagonist of the metabotropic glutamate receptor subtype 5 (mGluR5), produces antiparkinsonian-like effects in rats. Neuropharmacology. 2005;49(4):447–455. doi: 10.1016/j.neuropharm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Ossowska K, Konieczny J, Wolfarth S, Wieronska J, Pilc A. Blockade of the metabotropic glutamate receptor subtype 5 (mGluR5) produces antiparkinsonian-like effects in rats. Neuropharmacology. 2001;41(4):413–420. doi: 10.1016/s0028-3908(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 39.Pisani A, Gubellini P, Bonsi P, et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JM, Lam HA, Ackerson LC, Maidment NT. Blockade of mGluR glutamate receptors in the subthalamic nucleus ameliorates motor asymmetry in an animal model of Parkinson’s disease. Eur J Neurosci. 2006;23(1):151–160. doi: 10.1111/j.1460-9568.2005.04550.x. [DOI] [PubMed] [Google Scholar]
- 41.Wardas J, Pietraszek M, Wolfarth S, Ossowska K. The role of metabotropic glutamate receptors in regulation of striatal proenkephalin expression: implications for the therapy of Parkinson’s disease. Neuroscience. 2003;122(3):747–756. doi: 10.1016/j.neuroscience.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23(1):122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poisik OV, Smith Y, Conn PJ. D1- and D2-like dopamine receptors regulate signaling properties of group I metabotropic glutamate receptors in the rat globus pallidus. Eur J Neurosci. 2007;26(4):852–862. doi: 10.1111/j.1460-9568.2007.05710.x. [DOI] [PubMed] [Google Scholar]
- 44.Spooren WP, Gasparini F, Bergmann R, Kuhn R. Effects of the prototypical mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine on rotarod, locomotor activity and rotational responses in unilateral 6-OHDA-lesioned rats. Eur J Pharmacol. 2000;406(3):403–410. doi: 10.1016/s0014-2999(00)00697-x. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Berg D, Godau J, Trenkwalder C, et al. AFQ056 treatment of levodopa-induced dyskinesias: result of 2 randomized controlled trials. Mov Disord. 2011;26(7):1243–1250. doi: 10.1002/mds.23616. Results from a Phase II clinical trial evaluating the use of a mGlu5 receptor negative allosteric modulator for the treatment of levodopa-induced dyskinesias in PD patients. [DOI] [PubMed] [Google Scholar]
- 46.Fenu S, Pinna A, Ongini E, Morelli M. Adenosine A2A receptor antagonism potentiates L-DOPA-induced turning behaviour and c-fos expression in 6-hydroxydopamine-lesioned rats. Eur J Pharmacol. 1997;321(2):143–147. doi: 10.1016/s0014-2999(96)00944-2. [DOI] [PubMed] [Google Scholar]
- 47.Kanda T, Tashiro T, Kuwana Y, Jenner P. Adenosine A2A receptors modify motor function in MPTP-treated common marmosets. Neuroreport. 1998;9(12):2857–2860. doi: 10.1097/00001756-199808240-00032. [DOI] [PubMed] [Google Scholar]
- 48.Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology (Berl) 1999;147(1):90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- 49.Koga K, Kurokawa M, Ochi M, Nakamura J, Kuwana Y. Adenosine A2A receptor antagonists KF17837 and KW-6002 potentiate rotation induced by dopaminergic drugs in hemi-parkinsonian rats. Eur J Pharmacol. 2000;408(3):249–255. doi: 10.1016/s0014-2999(00)00745-7. [DOI] [PubMed] [Google Scholar]
- 50.Hauber W, Neuscheler P, Nagel J, Muller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2A receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14(8):1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- 51.Hauser RA, Cantillon M, Pourcher E, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a Phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10(3):221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 52.Fuxe K, Agnati LF, Jacobsen K, et al. Receptor heteromerization in adenosine A2Areceptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61(11):S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- 53.Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A 2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25(45):10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglia G, Busceti CL, Molinaro G, et al. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigrostriatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24(4):828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Vernon AC, Palmer S, Datla KP, Zbarsky V, Croucher MJ, Dexter DT. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur J Neurosci. 2005;22(7):1799–1806. doi: 10.1111/j.1460-9568.2005.04362.x. Evidence of neuroprotective effects for antagonism of group I or agonism of group II or group III mGlu receptors (mGluRs) [DOI] [PubMed] [Google Scholar]
- 56.Masilamoni GJ, Bogenpohl JW, Alagille D, et al. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134(7):2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Leonibus E, Manago F, Giordani F, et al. Metabotropic glutamate receptors 5 blockade reverses spatial memory deficits in a mouse model of Parkinson’s disease. Neuropsychopharmacology. 2009;34(3):729–738. doi: 10.1038/npp.2008.129. [DOI] [PubMed] [Google Scholar]
- 58▪.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. Review discussing the potential of mGluR modulation as a treatment for anxiety and depression. [DOI] [PubMed] [Google Scholar]
- 59.Samadi P, Gregoire L, Morissette M, et al. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2008;29(7):1040–1051. doi: 10.1016/j.neurobiolaging.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F. Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts L-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis. 2008;29(1):161–168. doi: 10.1016/j.nbd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor Type 5 attenuates L-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101(2):483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 62.Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330(1):227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poli S. Antiparkinsonian and anti-dyskinetic effects of dipraglurant (ADX48621), a novel mGluR5 negative allosteric modulator in clinical development. Presented at: the 7th International Meeting on Metabotropic Glutamate Receptors; Taormina, Sicily, Italy. 2–7 October 2011. [Google Scholar]
- 64.Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II receptor agonist LY354740 in rats. Neurosci Lett. 1997;229(3):161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 65.Cozzi A, Attucci S, Peruginelli F, et al. Type 2 metabotropic glutamate (mGlu) receptors tonically inhibit transmitter release in rat caudate nucleus: in vivo studies with (2S,1S′,2′S,3′R)-2-(2′-carboxy-3′-phenylcyclopropyl)glycine, a new potent and selective antagonist. Eur J Neurosci. 1997;9(7):1350–1355. doi: 10.1111/j.1460-9568.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 66.Johnson KA, Niswender CM, Conn PJ, Xiang Z. Activation of group II metabotropic glutamate receptors induces long-term depression of excitatory synaptic transmission in the substantia nigra pars reticulata. Neurosci Lett. 2011;504(2):102–106. doi: 10.1016/j.neulet.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley SR, Marino MJ, Wittmann M, et al. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia nigra pars reticulata. J Neurosci. 2000;20(9):3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konieczny J, Ossowska K, Wolfarth S, Pilc A. LY354740, a group II metabotropic glutamate receptor agonist with potential antiparkinsonian properties in rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358(4):500–502. doi: 10.1007/pl00005284. [DOI] [PubMed] [Google Scholar]
- 69.Murray TK, Messenger MJ, Ward MA, et al. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav. 2002;73(2):455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 70.Picconi B, Pisani A, Centonze D, et al. Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatement. Brain. 2002;125(12):2635–2645. doi: 10.1093/brain/awf269. [DOI] [PubMed] [Google Scholar]
- 71.Wittmann M, Marino MJ, Conn PJ. Dopamine modulates the function of group II and group III metabotropic glutamate receptors in the substantia nigra pars reticulata. J Pharmacol Exper Ther. 2002;302(2):433–441. doi: 10.1124/jpet.102.033266. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, Kitai ST, Xiang Z. Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta. J Neurosci Res. 2005;82(6):778–787. doi: 10.1002/jnr.20694. [DOI] [PubMed] [Google Scholar]
- 73.Battaglia G, Busceti CL, Pontarelli F, et al. Protective role of group-2 metabotropic glutamate receptors against nigrostriatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45(2):155–166. doi: 10.1016/s0028-3908(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 74.Matarredona ER, Santiago M, Venero JL, Cano J, Machado A. Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J Neurochem. 2001;76(2):351–360. doi: 10.1046/j.1471-4159.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- 75.Venero JL, Santiago M, Tomas-Camardiel M, Matarredona ER, Cano J, Machado A. DCG-IV but not other group II metabotropic receptor agonists induces microglial BDNF mRNA expression in the rat striatum. Correlation with neuronal injury. Neuroscience. 2002;113(4):857–869. doi: 10.1016/s0306-4522(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 76.Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-β. J Neurosci. 1998;18(23):9594–9600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruno V, Sureda FX, Storto M, et al. The neuroprotective activity of group-2 metabotropic glutamate receptors requires new protein synthesis and involves a glial-neuronal signaling. J Neurosci. 1997;17(6):1891–1897. doi: 10.1523/JNEUROSCI.17-06-01891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122(3):727–737. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 79.Valenti O, Marino MJ, Wittmann M, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23(18):7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Macinnes N, Duty S. Group III metabotropic glutamate receptors act as hetero-receptors modulating evoked GABA release in the globus pallidus in vivo. Eur J Pharmacol. 2008;580(1–2):95–99. doi: 10.1016/j.ejphar.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 81.MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br J Pharmacol. 2004;141(1):15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neuroscience. 2007;145(2):611–620. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulate. J Neurophysiol. 2001;85(5):1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- 84.Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J Neurosci. 2007;27(25):6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sibille P, Lopez S, Brabet I, et al. Synthesis and biological evaluation of 1-amino-2-phosphono methylcyclopropanecarboxylic acids, new group III metabotropic glutamate receptor agonists. J Med Chem. 2007;50(15):3585–3595. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- 86.Marino MJ, Williams DL, O’Brien JA, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. PNAS. 2003;100(23):13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪.Battaglia G, Busceti CL, Molinaro G, et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci. 2006;26(27):7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. First demonstration that systemically active mGluR4-positive allosteric modulators can be neuroprotective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuomo D, Martella G, Barabino E, et al. Metabotropic glutamate receptor subtype 4 selectively modulates both glutamate and GABA transmission in the striatum: implications for Parkinson’s disease treatment. J Neurochem. 2009;109(4):1096–1105. doi: 10.1111/j.1471-4159.2009.06036.x. [DOI] [PubMed] [Google Scholar]
- 89.Beurrier C, Lopez S, Revy D, et al. Electophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23(10):3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- 90.Maj M, Buno V, Dragic Z, et al. (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45(7):895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 91.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪.Niswender CM, Johnson KA, Weaver CD, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74(5):1345–1358. doi: 10.1124/mol.108.049551. Shows characterization of novel mGluR4-positive allosteric modulators which are highly selective and are active in animal models of PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones C, Bubser M, Thompson A, et al. The mGlu4 positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine A2A antagonist in preclinical rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.187443. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams R, Johnson KA, Gentry PR, et al. Synthesis and SAR of a novel positive allosteric modulator (PAM) of the metabotropic glutamate receptor 4 (mGluR4) Bioorg Med Chem Lett. 2009;19(17):4967–4970. doi: 10.1016/j.bmcl.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopez S, Turle-Lorenzo N, Johnston TH, et al. Functional interaction between adenosine A2A and group III metabotropic glutamate receptors to reduce parkinsonian symptoms in rats. Neuropharmacology. 2008;55(4):483–490. doi: 10.1016/j.neuropharm.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 96.Greco B, Lopez S, van der Putten H, Flor PJ, Almaric M. Metabotroic glutamate 7 receptor subtype modulates motor symptoms in rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2010;332(3):1064–1071. doi: 10.1124/jpet.109.162115. [DOI] [PubMed] [Google Scholar]
- 97.Broadstock M, Austin PJ, Betts MJ, Duty S. Antiparkinsonian potential of targeting group III metabotropic glutamate receptor subtypes in the rodent substantia nigra pars reticulata. Br J Pharmacol. 2012;165(4b):1034–1045. doi: 10.1111/j.1476-5381.2011.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ossowska K, Konieczny J, Wardas J, et al. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32(2):179–188. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- 99.Valenti O, Marino MJ, Conn PJ. Modulation of excitatory transmission onto midbrain dopaminergic neurons of the rat by activation of group III metabotropic glutamate receptors. Ann NY Acad Sci. 2003;1003:479–480. doi: 10.1196/annals.1300.058. [DOI] [PubMed] [Google Scholar]
- 100.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23(6):2150–2160. doi: 10.1523/JNEUROSCI.23-06-02150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Besong G, Battaglia G, D’Onofrio M, et al. Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J Neurosci. 2002;22(13):5403–5411. doi: 10.1523/JNEUROSCI.22-13-05403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fallarino F, Volpi C, Fazio F, et al. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat Med. 2010;16(8):897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- 103.Vernon AC, Zbarsky V, Datla KP, Dexter DT, Croucher MJ. Selective activation of group III metabotropic glutamate receptors by L-(+)-2-amino-4-phosphonobutryic acid protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J Pharmacol Exp Ther. 2007;320(1):397–409. doi: 10.1124/jpet.106.108159. [DOI] [PubMed] [Google Scholar]
- 104.Vernon AC, Croucher MJ, Dexter DT. Additive neuroprotection by metabotropic glutamate receptor subtype-selective ligands in a rat Parkinson’s model. Neuroreport. 2008;19(4):475–478. doi: 10.1097/WNR.0b013e3282f602df. [DOI] [PubMed] [Google Scholar]