SUMMARY
An Aequorin-based Film Adhesive Seedling (FAS) Ca2+ recording system was developed for monitoring Ca2+ in response to various stimuli in Arabidopsis. This system revealed stimulus- and tissue-specific Ca2+ signatures in seedlings with a simple, sensitive, and robust Ca2+ recording.
Key words: Aequorin, Case12, abiotic stress, calcium, Arabidopsis.
Abstract
Calcium ion is a versatile second messenger for diverse cell signaling in response to developmental and environmental cues. The specificity of Ca2+-mediated signaling is defined by stimulus-elicited Ca2+ signature and downstream decoding processes. Here, an Aequorin-based luminescence recording system was developed for monitoring Ca2+ in response to various stimuli in Arabidopsis. With the simple, highly sensitive, and robust Ca2+ recording, this system revealed stimulus- and tissue-specific Ca2+ signatures in seedlings. Cellular Ca2+ dynamics and relationship to Aequorin-based Ca2+ recording were explored using a GFP-based Ca2+ indicator, which suggested that a synchronous cellular Ca2+ signal is responsible for cold-induced Ca2+ response in seedlings, whereas asynchronous Ca2+ oscillation contributes to osmotic stress-induced Ca2+ increase in seedlings. The optimized recording system would be a powerful tool for the identification and characterization of novel components in Ca2+-mediated stress-signaling pathways.
INTRODUCTION
Calcium ion has been adopted as a primary signal element during evolution. To respond to changing developmental and environmental cues, plant cells rapidly change cytosolic free Ca2+ concentration in time and space. The spatiotemporal patterning of cellular Ca2+ dynamics has been formulated as a concept of Ca2+ signature. Ca2+ signature, namely the alterations in amplitude, duration, frequency, and spatial distribution of Ca2+ signal, encodes information of the type and the strength of the stimuli (Knight et al., 1996; Webb et al., 1996; McAinsh and Hetherington, 1998; Trewavas and Malho, 1998). Such Ca2+-encoded stimulus-specific information is decoded by downstream effectors. Most downstream effectors are Ca2+ sensing proteins, which bind to Ca2+ to initiate or regulate biochemical processes and ultimately translate information into specific end responses (Harper et al., 2004; Kim et al., 2009; Luan, 2009; DeFalco et al., 2010; Kudla et al., 2010). Therefore, the specificity of stimulus–response coupling is believed to be achieved by stimulus-defined Ca2+ encoding and decoding processes.
Ca2+ signaling regulates diverse cellular responses to abiotic and biotic stresses, plant hormones, light, and mechanical stimulation (Kudla et al., 2010). However, it remains unknown how plant cells generate stimulus-specific Ca2+ signals. A number of Ca2+-signaling components have been identified in plant cells that comprise Ca2+-signaling toolkits (Harper, 2001; McAinsh and Pittman, 2009; Kudla et al., 2010). Such cellular Ca2+-signaling toolkits enable the cell to use both extracellular and internal sources of Ca2+. However, in plant cells, most Ca2+ channels that control the entry of extracellular Ca2+ or the release of Ca2+ from internal store are still largely unidentified. So far, three families of proteins have been demonstrated to have Ca2+-influx activity. Ligand-gated receptors, cyclic nucleotide-gated channels (CNGCs), and glutamate receptor-like channels (GLRs) have been shown to possess Ca2+-influx activities. CNGCs function in defense signaling and thermosensing (Ali et al., 2007; Ma and Berkowitz, 2011; Finka et al., 2012), whereas GLRs are proposed to be responsible for selected amino acid-triggered Ca2+ influx, functioning in pollen tube growth (Michard et al., 2011), stomatal closure (Cho et al., 2009), root development (Li et al., 2006; Qi et al., 2006), and defense signaling (Kwaaitaal et al., 2011; Vatsa et al., 2011). Unlike CNGCs and GLRs, two pore channel (TPC1) is a vacuolar Ca2+ release channel, regulating ABA-sensitive germination and stomatal opening (Peiter et al., 2005; Islam et al., 2010; Hedrich and Marten, 2011). In addition to the Ca2+-influx system mentioned above, electrophysiological studies have shown Ca2+ currents when plasma membrane is hyperpolarized or depolarized, indicating the existence of voltage-gated Ca2+ channels in plants. In addition, the ligands 1,4,5-trisphosphate and cyclic ADP-ribose likely trigger Ca2+ release from internal stores through ligand receptor-operated channels in plants (Allen et al., 1995; Martinec et al., 2000; Navazio et al., 2000; Lemtiri-Chlieh et al., 2003), although the molecular identities of these channels have yet to be identified. On the other hand, most Ca2+ pumps and exchangers that rapidly transport cytosolic Ca2+ back to the extracellular space or internal membrane stores have been well characterized. Two types of Ca2+ pumps and exchangers, P-type Ca2+ ATPase (ECAs, ER-type Ca2+ ATPase and ACAs, autoinhibited Ca2+ ATPase) and Ca2+ exchanger (CAXs), are located in the plasma membrane and different organelles (Sze et al., 2000; Mäser et al., 2001; Shigaki and Hirschi, 2006). They bring Ca2+ to the resting level and are important in shaping Ca2+ signatures (McAinsh and Pittman, 2009).
Unveiling the mechanism by which plant cells initiate stimulus-specific Ca2+ signals is crucial to understanding how plants perceive and transduce signals to adapt to various stresses. A precise measurement of cellular Ca2+ dynamics is a prerequisite for identifying genes and defining their functions in Ca2+ signaling. So far, the measurements of Ca2+ dynamics at the cellular or whole plant level are performed in various plant species using different Ca2+ indicators, including ratiometric fluorescent dyes and genetically encoded Ca2+ indicators (Campbell et al., 1996; Knight et al., 1996, 1997; Cessna et al., 1998; Gong et al., 1998; Mori et al., 1998; Blume et al., 2000; Allen et al., 2001; Kosuta et al., 2008; Monshausen et al., 2009; Krebs et al., 2011; Munemasa et al., 2011; Ranf et al., 2012). However, stimuli-specific Ca2+ signatures have not been systematically defined in Arabidopsis at the level of either the single cell or the whole plant. Furthermore, the identification of the components, especially Ca2+-influx channels and their regulators in Ca2+-signaling toolkits, necessitate well-designed genetic screens in Arabidopsis.
To bridge these gaps, we developed an Aequorin-based FAS Ca2+ recording system that allows a systematic comparison of Ca2+ dynamics in response to stimuli of various strengths and types, including abiotic and biotic stresses, plant hormones, and amino acids in Arabidopsis seedlings. Using the GFP-based Ca2+ indicator Case12, we demonstrated cellular Ca2+ dynamics in response to cold and osmotic stresses, and provided experimental evidence that the whole seedling or organ-level Ca2+ patterns reflect combined single-cell Ca2+ dynamics of populations of cells. We further demonstrated that the FAS recording was able to detect alterations of Ca2+ signals by Ca2+ channel blockers. As a result, FAS recording could be a powerful system for the isolation of mutants with altered stimulus-specific Ca2+ signals, which may help reveal the molecular identities of Ca2+-signaling components that are important in abiotic stress-signaling pathways.
RESULTS
Aequorin-Based FAS Recording for Tracking Spatiotemporal Ca2+ Signals in Arabidopsis Seedlings
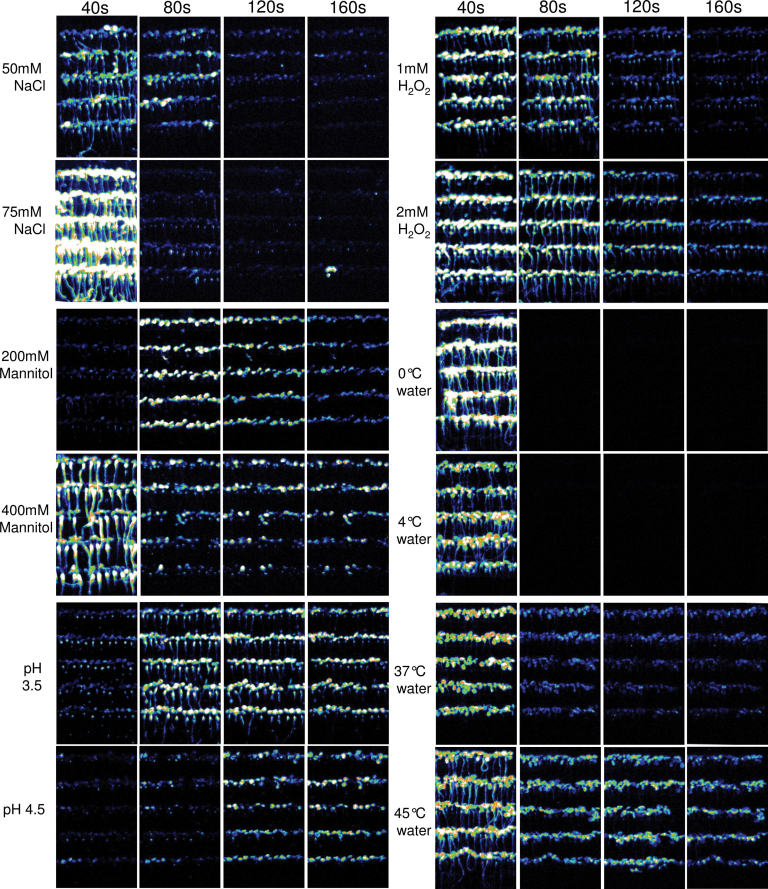
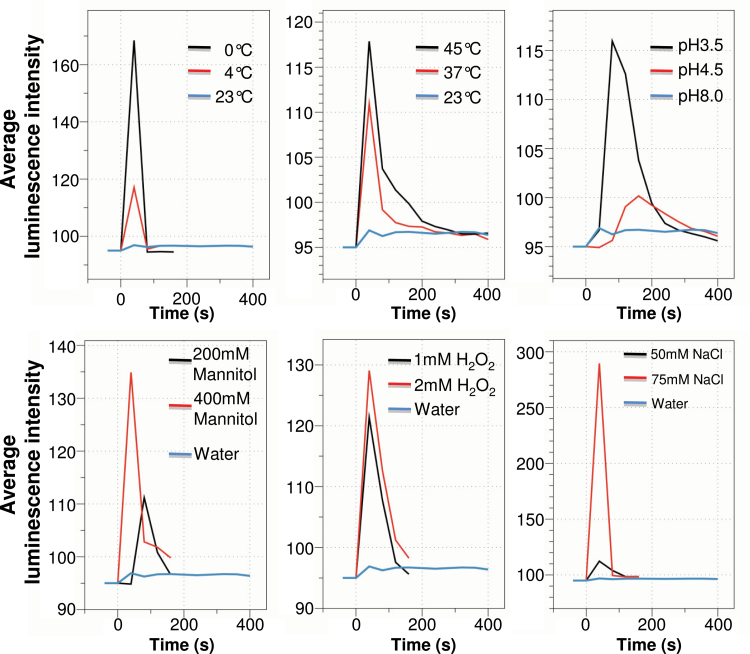
In order to simultaneously monitor tissue-specific Ca2+ responses in multiple Arabidopsis seedlings, we developed an Aequorin-based FAS luminescence recording system. We used transgenic Arabidopsis plants expressing cytosolic Aequorin, referred to as the Aeq or Aeq wild-type plants. Aeq seedlings were grown vertically on MS agar in a square Petri dish at a density of about 100 seedlings per plate until 7 d old, and were then transferred onto an adhesive-backed film. A sheet of FAS was incubated with h-CTZ for the reconstitution of Aequorin. Stimuli-induced Ca2+ signals in FAS were recorded using a photon-counting camera (Supplemental Figure 1). Sequential luminescence images over the time course are shown in Figure 1, which demonstrate spatiotemporal dynamic changes of Ca2+ in response to different abiotic stress stimuli. The free cytosolic Ca2+ level in the cell is correlated with the luminescence intensity emitted from Aequorin (Gilroy et al., 1989). A comparison of the amplitude and duration of Ca2+ increase is made based on the average photon density, namely total photon density divided by the area of region of interest (ROI). As shown in Figures 1 and 2, abiotic stress stimuli-induced Ca2+ signals vary greatly in terms of the speed of signal initiation, and signal amplitude and duration, in addition to differences in tissue distribution patterns. In all cases, the amplitude, but not always the duration, of Ca2+ signals was correlated with the strength of the stimulus. For example, the 0°C water-induced Ca2+ amplitude was 1.4-fold higher than that induced by 4°C water. However, the duration of Ca2+ increase was the same under 0°C or 4°C, and both Ca2+ signals show only one sharp spike. In contrast, heat stress triggered a longer duration of Ca2+ increase, although the Ca2+ amplitudes were very similar to that triggered by 4°C water. As shown in Figures 1 and 2, seedlings responded differently to different levels of acidic pH. A delayed initiation of Ca2+ signals was observed in response to both pH 3.5 and pH 4.5 acidic stimuli, but the stronger acidic stimulus (pH 3.5) initiated an earlier and greater Ca2+ increase than the more moderate acidic stimulus (pH 4.5). In this study, mannitol, hydrogen peroxide, and sodium chloride were used for mimicking osmotic, oxidative, and salt stress stimuli, respectively. The concentrations used in this study were much lower than those used in previously published studies, which indicates a high sensitivity of our FAS recording. The lowest concentrations tested here for mannitol, NaCl, and hydrogen peroxide were 200, 50, and 1mM, in which Ca2+ signals were clearly detectable, although the amplitudes were 2.5-, 1.4-, and 1.06-fold lower than those triggered by 400, 75, and 2mM NaCl, mannitol, and H2O2, respectively. The results indicated that the Ca2+ amplitudes in whole seedlings were correlated with the strengths of osmotic, oxidative, and salt stresses (Figure 2). Interestingly, various tissue patterns of Ca2+ dynamics were observed, and the tissue patterns were not only related to the type, but also to the strength, of the stress stimuli. Moderate cold and heat stresses, such as 4°C and 37°C water, triggered strong Ca2+ signals in leaves, but they triggered only weak Ca2+ signals in roots. In contrast, severe cold and heat stress, such as 0°C and 45°C water, triggered strong Ca2+ signals in roots, in addition to the leaves. Such tissue-specific patterns were even more pronounced in the seedlings responding to the osmotic stress agent mannitol, in which Ca2+ signal in roots was not even detectable in response to 200mM mannitol (Figure 1). The speed of initiation of Ca2+ signal was also correlated with the strength of the stimuli, as exemplified by delayed responses to 200mM mannitol and pH 4.5, compared to the responses to 400 mM mannitol and pH 3.5 medium, respectively.
Figure 1.
Abiotic Stress-Induced Spatiotemporal Patterns of Ca2+ Signals in Arabidopsis Seedlings.
Time course of luminescence images of FAS. Sequential images were collected using a photon-counting camera with an interval of 40-s photon-counting integrations upon application of stimuli. The type and strength of abiotic stress stimuli are indicated on the left of each panel, and time points are indicated on the top of each panel. The display range is 100–200 for all images.
Figure 2.
Temporal Patterns of Abiotic Stress Stimulus-Induced Ca2+ Signal of the Whole Seedlings.
Stimulus-induced Ca2+ increases are illustrated by the averaged photon intensity over time. The total photon was counted for 160 s for cold (0°C and 4°C water), oxidative (1 and 2 mM H2O2), osmotic (200 and 400 mM mannitol), and salt (50 and 75 mM NaCl) stress stimuli, and 400 s for heat (37°C and 45°C water), acidic (pH 3.5 and 4.5) stress stimuli, and control (room temperature water, pH 7.6).
Taken together, the Aequorin-based FAS recording system provided a platform for a highly sensitive and robust Ca2+ signal recording. Such Ca2+ recording revealed diverse abiotic stress stimuli-specific Ca2+ spatiotemporal patterns of Ca2+ signals.
A GFP-Based Ca2+ Indicator for Monitoring Cellular Ca2+ Dynamics
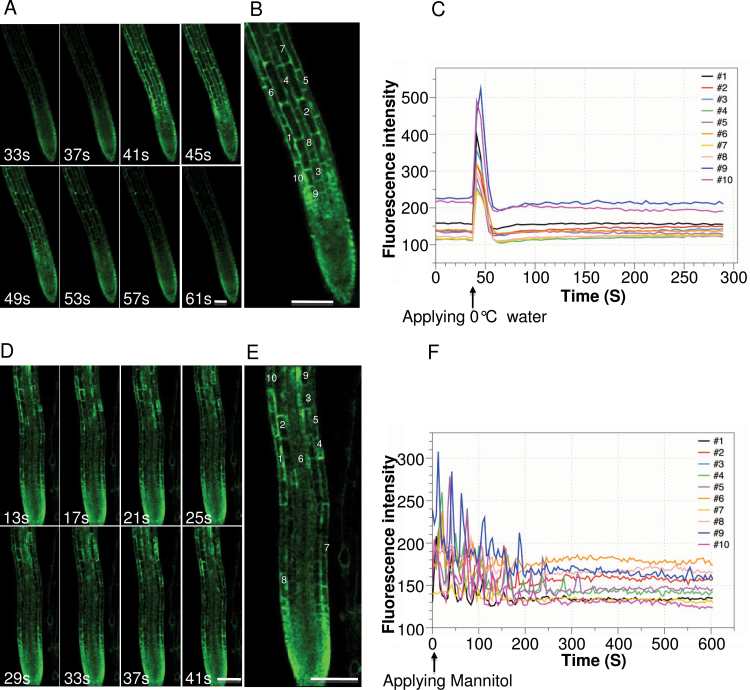
Aequorin-recorded Ca2+ signal is a readout of populations of cells. It is unclear how Aequorin-recorded Ca2+ signature in whole seedlings or tissues of seedlings is related to cellular Ca2+ dynamics. To understand cellular Ca2+ dynamics underlying the FAS-recorded Ca2+ signature, we used a genetically encoded Ca2+ indicator, Case12, for tracking cellular Ca2+ dynamic changes in response to cold and osmotic stresses. Case12 is a GFP-based Ca2+ indicator, for which changes in fluorescent intensity are directly correlated with changes in Ca2+ concentration (Souslova et al., 2007; Zhu et al., 2010). In this study, we generated transgenic Arabidopsis plants constitutively expressing Case12 in the cytosol. Ca2+ signals in the root cells were recorded upon application of 0°C water or 400mM mannitol under a confocal microscope. As shown in Figure 3A and 3B, 0°C water triggered synchronous Ca2+ spikes in root cells (Supplemental Movie 1). The amplitude and duration of 0°C water-induced Ca2+ spikes were various among single cells, and the average amplitude and duration were 218.54±53.67 and 19.27±1.98 s, respectively. In contrast, as shown in Figure 3C and 3D, mannitol-induced Ca2+ oscillations in most cells (Supplemental Movie 2) have an average frequency of 39.95±15.07 s. The average amplitude and duration of mannitol-induced Ca2+ spike were 60.44±21.66 and 17.28±1.54 s, respectively, which were lower than that of 0°C water-induced Ca2+ spikes. It appears that the amplitudes and frequencies varied while the durations were similar among cold and mannitol-induced Ca2+ spikes. Overall, there were great differences in cold and osmotic stress stimuli-induced single-cell Ca2+ dynamics. Our results suggest that Aequorin-based FAS-recorded Ca2+ signals in response to cold and osmotic stress stimuli reflect distinct cellular Ca2+ dynamics under the different stimuli. Synchronous Ca2+ spikes contributed to cold induced overall Ca2+ increase in the root, whereas osmotic stress stimulus induced overall Ca2+ changes in the root were attributed to asynchronous Ca2+ oscillations in individual root cells.
Figure 3.
Cellular Ca2+ Dynamics in Arabidopsis Root Cells.
(A) Selected time series of images of 0°C water treated root of 5-day-old CaFP300 seedling. Time points are indicated on the bottom of each panel.
(B) Enlarged image of root cells showing a Ca2+ response to 0°C water. The responsive cells are indicated by the numbers.
(C) Changes in fluorescence intensity of the single cells responding to 0°C water over the time course. Colored lines tagged by the numbers represent corresponding cells in the root as indicated in (B).
(D) Selected time series of images of 400mM mannitol treated root of 5-day-old CaFP300 seedlings. Time points are indicated on the bottom of each panel.
(E) Enlarged image of root cells showing a Ca2+ response to 400mM mannitol. The responsive cells are indicated by the numbers.
(F) Changes in fluorescence intensity of the single cells responding to 400mM mannitol over the time course. Colored lines represent corresponding cells in the root as indicated in (E).
FAS Recording for Monitoring Ca2+ Response of Arabidopsis Seedlings to Hormones, Pathogen Elicitor, and Amino Acids
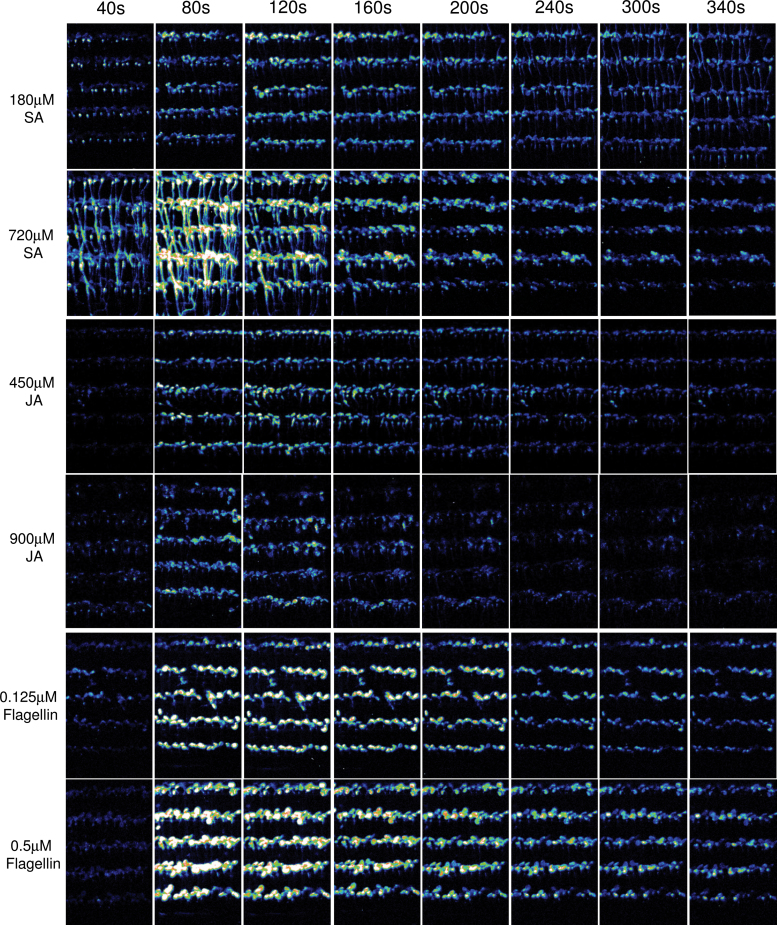
Aiming at adapting the FAS system for a simple, highly sensitive, and robust approach for recording Ca2+ response to any given stimuli, we further evaluated the use of the FAS system in recording Ca2+ signals in response to a number of stimuli, including the plant hormones salicylic acid (SA), Jasmonic acid (JA), Abscisic acid (ABA), and brassinosteriod (BR), the pathogen elicitor flagellin, and the amino acids L-glutamate, L-glycine, L-cystine, L-alanine, L-serine, and L-aspargine. As shown in Figure 4, 180 µM SA and 450 µM JA, the lowest concentrations tested in this study, induced Ca2+ increases that could be visualized by FAS recording. The highest amplitudes of SA- and JA-induced Ca2+ signals occurred in the second or third 40 s, depending on the concentration of SA and JA. High concentrations of SA triggered earlier and higher Ca2+ response than low concentrations of SA, indicating that the initiation and amplitude, but not the duration, of SA-induced Ca2+ were closely related to SA concentration. Interestingly, it appeared that the roots were more sensitive to SA, which was evident by the initial strong and prolonged Ca2+ signals induced by SA in roots. In contrast, JA-induced Ca2+ signals were relative weak, and were detected mostly in leaves. No significant differences in the initiation and amplitude of Ca2+ responses were observed in response to low and high concentrations of JA. ABA- and BR-induced Ca2+ signals could not be detected in 7-day-old seedlings at the highest concentration of 20 µM ABA and 2 µM BR tested in this study (data not shown). However, prolonged Ca2+ increases were observed in the leaves of 14-day-old seedlings in response to 7 µM ABA and 1 µM BR. The highest amplitudes varied greatly among the leaves of 14-day-old seedlings in response to SA, JA, ABA, and BR, as shown in Supplemental Figure 2.
Figure 4.
Spatiotemporal Ca2+ Responses of Arabidopsis Seedlings to Plant Hormones and Pathogen Elicitor.
Sequential integrated luminescence images of FAS over the time course upon application of SA, JA, and flagellin. The type and concentration of the stimuli are indicated on the left of each panel, and time points are indicated on the top of each panel. The display range is 100–200 for all images.
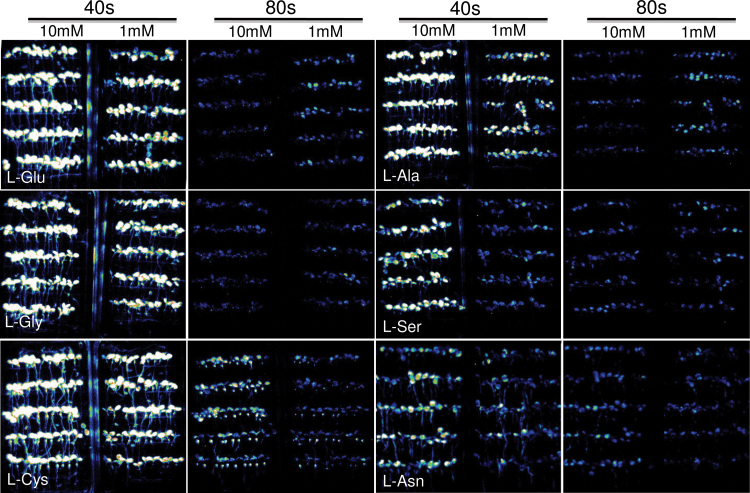
Similarly, FAS recording revealed that flagellin induced prolonged Ca2+ increases that were mostly detected in the leaves, showing a tissue-specific sensing of the pathogen elicitor (Figure 4). The lowest concentration of flagellin used in this study was 0.125 µM, which still induced clearly detectable Ca2+ increases. Flagellin at 0.5 µM induced a Ca2+ signal with a higher amplitude and longer duration compared to that induced by 0.125 µM flagellin. Six amino acids have been considered to be agonists of GRL3.3-mediated Ca2+ influx (Qi et al., 2006). We visualized Ca2+ response to these six amino acids using FAS recording. As shown in Figure 5, all six amino acids induced strong Ca2+ responses during the first 40 s, and the amplitudes were correlated with the concentrations. The highest Ca2+ amplitude was observed for L-cystine, and the lowest one was for L-asparigine (Table1). The order of Ca2+ amplitude induced by each amino acid was very similar to that reported previously with the same concentration using a luminometer which is supposed to be more sensitive than imaging (Qi et al., 2006). The results suggest that our FAS Ca2+ recording is highly sensitive and reliable.
Figure 5.
Spatiotemporal Ca2+ Responses of Arabidopsis Seedlings to Amino Acids.
Integrated luminescence images of FAS at first and second 40 s upon application of amino acids. Amino acids are indicated in the first 40 s integrated images, and the concentration of amino acids and the time points are indicated on the top of each panel.
Table 1.
Ca2+ Response of Arabidopsis Seedlings to Amino Acids.
| Amino acid | L-Glu | L-Gly | L-Cys | |||
|---|---|---|---|---|---|---|
| Time/conc. | 10 mM | 1 mM | 10 mM | 1 mM | 10 mM | 1 mM |
| 40 s | 157.59±16.61 | 120.67±6.32 | 161.21±2.54 | 127.34±4.32 | 177.09±11.07 | 141.44±5.32 |
| 80 s | 93.78±3.41 | 94.45±2.56 | 93.71±2.14 | 93.59±1.02 | 99.99±1.15 | 94.76±2.14 |
| 120 s | 92.79±2.11 | 94.13±1.34 | 92.93±2.33 | 93.06±0.59 | 93.64±1.05 | 93.62±1.12 |
| 160 s | 92.65±1.05 | 94.31±0.89 | 92.83±1.09 | 93.13±0.89 | 92.28±0.75 | 92.68±0.98 |
| Amino acid | L-Ala | L-Ser | L-Asn | |||
|---|---|---|---|---|---|---|
| Time/conc. | 10 mM | 1 mM | 10 mM | 1 mM | 20 mM | 10 mM |
| 40 s | 158.95±8.97 | 102.85±5.43 | 110.39±5.69 | 94.42±1.03 | 101.57±5.33 | 96.26±2.64 |
| 80 s | 93.55±3.59 | 94.59±2.12 | 93.41±2.06 | 93.22±1.12 | 94.22±2.08 | 93.32±3.15 |
| 120 s | 92.87±1.33 | 93.76±1.45 | 92.38±1.08 | 92.70±0.23 | 92.15±1.13 | 92.12±2.08 |
| 160 s | 92.92±0.16 | 94.06±0.32 | 92.20±1.15 | 92.79±0.43 | 91.87±1.08 | 91.67±1.10 |
Note: values are the average photon intensity ± SD of three independent experiments.
FAS Recording Reveals Tissue-Specific Ca2+ Responses to Metal Ions
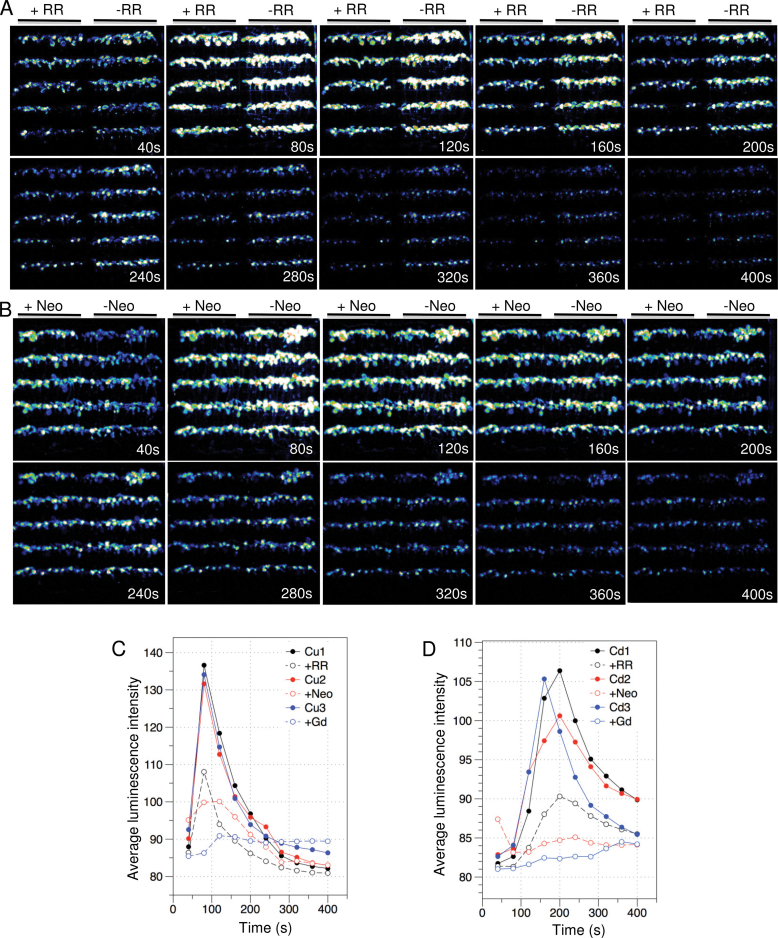
We chose two heavy metal ions, Cu2+ and Cd2+, as the other stress stimuli to examine the spatiotemporal patterns and sources of metal ion-triggered Ca2+ signals. It has been unclear whether these two metal ions can trigger Ca2+ signals in Arabidopsis. Only recently, it has been reported that Cu2+ ion could activate ER Ca2+ channels in the marine algae Ulva compressa to release Ca2+ from the ER (Gonzalez et al., 2010). Ruthenium Red (RR) and neomycin are membrane-permeable Ca2+ channel blockers that predominantly inhibit ryanodine receptors (RYRs)-like intracellular Ca2+ channels and Ca2+ release through IP3-regulated Ca2+ channels, respectively. Gadolinium (Ga3+) has been widely used as a plasma membrane Ca2+ channel blocker to inhibit Ca2+ influx from the extracellular space (Tracy et al., 2008). To examine the effect of blocking Ca2+ entry or release on potential Cu2+- and Cd2+-induced Ca2+ signals, we compared Ga2+, RR, and Neomycin-treated and -untreated FAS recording in response to Cu2+ and Cd2+. Images of Ca2+ channel blocker-untreated and -treated FAS were acquired simultaneously upon application of Cu2+ and Cd2+. As shown in Figure 6 and Supplemental Figure 3, both Cu2+ and Cd2+ induced strong and prolonged Ca2+ increases in leaves, whereas Ca2+ signals in roots were undetectable. Compared to Cu2+, Cd2+ initiated Ca2+ signal later but with a longer duration (Figure 6C and 6D). Application of Ga2+ reduced Cu2+ and Cd2+-induced Ca2+ amplitude by approximately 92% and 94%, respectively, indicating that Cu2+ and Cd2+ trigger Ca2+ increase mostly by activating Ca2+ influx through the plasma membrane. In the case of Cu2+, RR and neomycin reduced Ca2+ amplitude by 49% and 66%, respectively. However, RR almost abolished Ca2+ signals after 280 s (Figure 6A and Supplemental Figure 3A). Similarly, RR and neomycin also greatly inhibited Cd2+-induced Ca2+ signal, reducing the amplitude by 64% and 79%, respectively. We found that neomycin initially enhanced Cu2+ and Cd2+-induced Ca2+ signals at the first 40 s (Figure 6B and Supplemental Figure 3B), which is similar to observations reported previously (Tracy et al., 2008).
Figure 6.
Cu2+ and Cd2+-Induced Ca2+ Signals in the Leaves of Arabidopsis Seedlings.
(A, B) Effect of 100 µM RR and 600 µM neomycin on Cu2+-triggered Ca2+ increase. Time series of integrated luminescence images of FAS were collected with an interval of 40-s photon-counting integrations upon application of 10mM CuCl2. Time points are indicated on each image panel. 100 µM RR (A) and 600 µM neomycin (B)-treated and -untreated FAS are indicated on the top of each panel.
(C, D) Temporal pattern of effects of RR, neomycin and Ga3+ on Cu2+ (C) and Cd2+ (D)-induced Ca2+ increases. Changes of the averaged photon intensity over times are indicated by the colored lines with open circles for RR, neomycin, and Ga3+ treatments, and the same colored lines with solid circles are corresponding controls treated with only CuCl2 and CdCl2.
Taken together, our results show that Cu2+ and Cd2+ induced strong Ca2+ signals specifically in leaves. The FAS-recorded spatiotemporal patterns of these two divalent metal ion-induced Ca2+ signals are different from the patterns induced by the other abiotic stress stimuli. It appears that Cu2+ and Cd2+ initiate Ca2+ signals by activating Ca2+ influx across the plasma membrane. Because internal Ca2+ channel blockers also significantly reduced Cu2+- and Cd2+-induced Ca2+ signals, Ca2+ release from internal stores may also play a role in Ca2+ signaling in Arabidopsis.
DISCUSSION
Genetically encoded Ca2+ indicators have been perfected to meet the needs of accurate measurement of Ca2+ dynamics in cells. Aequorin (McCombs and Palmer, 2008), an early version of Ca2+ indicator, contributed to the formulation of the concept of Ca2+ signature in plants (Knight et al., 1991). However, low photon production and less-than-robust measurement have hindered the use of Aequorin in high-resolution Ca2+ imaging and genetic screens in plants (Plieth, 2001). In this study, we revisited Aequorin and developed an Aequorin-based FAS luminescence Ca2+ recording system in Arabidopsis. Using the FAS recording system, we compared various stimuli, including abiotic and biotic stresses, hormones, and amino acids in their induction of Ca2+ responses in Arabidopsis seedlings. The FAS Ca2+ recording revealed tissue-specific Ca2+ signatures that are associated with the type and strength of stimuli. To our knowledge, such a systematic comparison, which is important for the concept of Aequorin-recorded Ca2+ signatures, has not been made in the Arabidopsis model plant previously. The optimized FAS recording system may also enable a high-throughput genetic screen for the identification of potentially novel Ca2+-signaling components in stress responses. Therefore, the FAS system can be a useful tool for studying Ca2+ signaling in Arabidopsis plants.
The non-invasive Case12-based Ca2+ recording revealed the complexity of cellular Ca2+ dynamics in individual living cells. The readout of FAS recording represents the averaged signals from cell populations, which masks the behavior of individual cells. In many cases, such as under osmotic, oxidative, and salt stresses, there are unsynchronized Ca2+ oscillations or transients in individual cells. This cellular heterogeneity in Ca2+ oscillations could be overcome by cold stress. Thus, FAS-recorded Ca2+ signal in cold stress-treated seedlings represent the collective behavior of synchronized Ca2+ oscillations in single cells. The observations imply that different cellular machinery in the cell is responsible for responding to different stimuli. Unsynchronized cellular Ca2+ dynamics could originate from cell-specific Ca2+-signaling toolkits that are regulated by the stimuli, and may also be attributed to cell–cell communication and the accessibility of individual cells to the stimuli. Mathematic simulations of oscillatory behaviors of cell populations suggested that Aequorin-recorded Ca2+ increases results from in-phase oscillations whereas the Ca2+ decrease is due to the out-phase oscillation of cell populations (Dodd et al., 2006; Plieth, 2010; Batistic and Kudla, 2012). However, our results suggest that such models of Ca2+ signaling may not be applicable to diverse stimulus-specific Ca2+ dynamics. In addition, experiments with Ca2+ channel blockers suggested that Aequorin-recorded initial Ca2+ increase is attributed to single-cell Ca2+ amplitudes in which both in-phase or out-phase oscillations might occur. In the present study, we could not correlate the Case12 recording of cellular Ca2+ dynamics with FAS recording of tissue-level Ca2+ dynamics in response to a given stimulus due to a lack of mutants that express both Ca2+ indicators. Nevertheless, we observed the effects of Ca2+ channel blockers on Ca2+ signals at the tissue level, suggesting that FAS-recorded signals are relevant to Ca2+ dynamic changes at the cellular level.
Aequorin-recorded Ca2+ dynamics to a great extent depends on not only the sensitivity of the assay system, but also the state of the plants. For the FAS system, 7-day-old seedlings are ideal for detecting Ca2+ responses of both roots and leaves to most stimuli tested in this study. However, for some stimuli that are known to trigger Ca2+ responses in the leaves, such as the plant hormones ABA and BR, the pathogen elicitor flagellin and heavy metal ions, characterization of Ca2+ responses or screening for the mutants with altered Ca2+ responses would ideally be conducted in older seedlings. The high sensitivity and robustness are also a prerequisite for high-throughput screens. In particular, the high sensitivity of the system allows the use of relatively mild stress stimuli for triggering Ca2+ responses. Severe stress treatments likely cause damage to the cells, leading to more artifacts. For all stimuli tested in this study, the FAS system was able to image Ca2+ responses to the stimuli applied at levels used for Ca2+ reading by luminometers (Knight et al., 1997; Kawano et al., 1998; Tracy et al., 2008; Munemasa et al., 2011; Pan et al., 2012; Ranf et al., 2012), which normally is more sensitive than Ca2+ luminescence imaging. Therefore, the FAS system has advantages over other systems reported previously not only for its highly sensitive Ca2+ recording, but also for its detection of spatial Ca2+ responses.
In conclusion, the Aequorin-based FAS system developed in this study provides a powerful tool for studying Ca2+ signaling in response to various stimuli. Importantly, the FAS system offers a platform for future identification of Ca2+-signaling components in stress-signaling pathways. In combination with other techniques, the FAS system would become a very useful tool for studying the specificity and crosstalk in plant-signaling networks.
METHODS
Plant Material and Growth Conditions
The Arabidopsis thaliana Col-0-expressing cytosolic Aequorin (referred to as Aeq wild-type) was obtained from Dr Marc R. Knight (Knight et al., 1991). Seedlings were grown in the growth chamber conditioned with a standard light–dark cycle of 16 h of light and 8 h of dark at 23°C. Ca2+ indicator Case12 (Souslova et al., 2007) constructed in a binary vector was introduced into Arabidopsis ecotype Col-0 by Agrobacterium-mediated transformation. An independent transgenic line, CaFP300, expressing a high level of Case12 was used in this study.
Establishment of Aequorin-Based FAS Luminescence Recording System
For establishing FAS system, Aeq wild-type seeds were planted in a square plate (10 × 10 cm square Petri dish with grid, Fisher Scientific, USA) containing full- strength MS medium, 1% sucrose, and 1.2% agar. Aeq seedlings were grown vertically on the plate at a density of about 100 seedlings per plate and transferred onto a clear adhesive film (Adhesive PCR Plate Seals, Thermo Scientific, USA) on the seventh day after germination. A sheet of FAS (about 100 seedlings) was incubated in the plate containing 15 ml water and 30 µg h-CTZ (coelenterazine, NanoLight Technolgies, Arizona, USA) for 5 h overnight for reconstitution of Aequorin.
Aequorin Luminescence Imaging and Analysis
A sheet of FAS was placed on the stage of the luminescence imaging system (Princeton Instruments, New Jersey, USA) and images were acquired immediately upon application of stimuli. The time gap between applying stimuli and recording image was about 2–4 s. For EMS screening, two integrated images were collected at first and second 40 s. For other experiments, the time series of Ca2+ recording was as indicated in the figures or figure legends. Qualitative analysis of luminescence images was performed using ImageJ. An average photon intensity of stimulus-induced luminescence signals at any given time was defined as the total photon intensity of luminescence signals emitted by FAS divided by the area of ROI. Plant hormones, SA, JA, and ABA were dissolved in 100% ethanol, whereas eBL was dissolved in DMSO. Therefore, a negative control of ethanol or DMSO in the same final concentrations as in the working solution of hormones was included in hormone-induced Ca2+ response. The stocks of 1 mM flagellin and 100 mM amino acid were dissolved in water and pHs of the working solutions of these two stimuli were unadjusted. For pretreatments with Ca2+ channel blockers, the FAS sheets were cut and divided equally into two parts. Half of the FAS sheets were pretreated with 50 µM GaCl3, 100 µM RR, or 600 µM neomycin in h-CTZ incubation solution for 20 min, respectively, while other half of the FAS sheets remained in the h-CTZ incubation solution without adding blockers. Treated and untreated FAS sheets were the assembled into one piece and imaged for 400 s with a 40-s interval of integration upon application of 10 mM CuCl2 or 10 mM CdCl2. For cam mutant analysis, Aeq wild-type and cam mutant seeds were planted on MS agar in the same plate with a similar density, occupying the left or the right half of the plate. Seven-day-old Aeq and cam seedlings were transferred onto an adhesive film and incubated with h-CTZ, and images were acquired for 160 s as described above. Stimuli-induced Ca2+ amplitudes were compared at the time point in which the highest Ca2+ amplitudes were observed. The highest Ca2+ amplitudes were normalized by total photon intensity of luminescence signals emitted from discharged FAS in the solution of 1 M CaCl2 and 20% ethanol.
Confocal Ca2+ Imaging
Five-day-old seedlings of CaFP300 were placed in a customized chamber, which was created by placing a small piece of clay between a coverslip and a slide. A polyethylene tube (0.58 mm diameter, PerkinElmer, USA) was connected to a 1-ml syringe and the other end of the tube was fixed on the slide next to the chamber. Stimulus solution was slowly injected into the chamber just prior to acquiring images. For cold stimulus, CaFP300 seeds were directly planted in a chambered cover glass (Nalge Nunc International, USA) containing a thin layer of MS medium, and grown vertically for 5 d. 0°C water was added into the chamber just prior to acquiring images. Ca2+ imaging was performed using an inverted Nikon A1R confocal laser-scanning microscope with a 20× water immersion lens (numerical aperture 0.75). Time series images were collected at an interval of 4 s with excitation and emission wavelengths of 488 nm and 500–550 nm at a pixel resolution of 512 × 512. Data were exported and processed in DataGraph (Visual Data Tools Inc., Chapel Hill, North Carolina, USA).
SUPPLEMENTARY DATA
Supplementary Data are available at Molecular Plant Online.
FUNDING
This work was supported by National Institutes of Health grant R01GM059138 to J.-K.Z.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to B. Stevenson for technical assistance and Dr Marc R. Knight for providing Aequorin transgenic line. No conflict of interest declared.
REFERENCES
- Ali R., Ma W., Lemtiri-Chlieh F., Tsaltas D., Leng Q., von Bodman S., Berkowitz G.A. (2007). Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 19, 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G.J., Chu S.P., Harrington C.L., Schumacher K., Hoffmann T., Tang Y.Y., Grill E., Schroeder J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 411, 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen G.J., Muir S.R., Sanders D. (1995). Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 268, 735–737 [DOI] [PubMed] [Google Scholar]
- Batistic O., Kudla J. (2012). Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta. 1820, 1283–1293 [DOI] [PubMed] [Google Scholar]
- Blume B., Nurnberger T., Nass N., Scheel D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 12, 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.K., Trewavas A.J., Knight M.R. (1996). Calcium imaging shows differential sensitivity to cooling and communication in luminous transgenic plants. Cell Calcium. 19, 211–218 [DOI] [PubMed] [Google Scholar]
- Cessna S.G., Chandra S., Low P.S. (1998). Hypo-osmotic shock of tobacco cells stimulates Ca2+ fluxes deriving first from external and then internal Ca2+ stores. J. Biol. Chem. 273, 27286–27291 [DOI] [PubMed] [Google Scholar]
- Cho D., Kim S.A., Murata Y., Lee S., Jae S.K., Nam H.G., Kwak J.M. (2009). De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. Plant J. 58, 437–449 [DOI] [PubMed] [Google Scholar]
- DeFalco T.A., Bender K.W., Snedden W.A. (2010). Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 425, 27–40 [DOI] [PubMed] [Google Scholar]
- Dodd A.N., et al. (2006). Time of day modulates low-temperature Ca signals in Arabidopsis . Plant J. 48, 962–973 [DOI] [PubMed] [Google Scholar]
- Finka A., Cuendet A.F., Maathuis F.J., Saidi Y., Goloubinoff P. (2012). Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell. 24, 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Hughes W.A., Trewavas A.J. (1989). A comparison between Quin-2 and Aequorin as indicator of cytoplasmic calcium levels in higher plant cell protoplasts. Plant Physiol. 90, 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., van der Luit A.H., Knight M.R., Trewavas A.J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437 [Google Scholar]
- Gonzalez A., Trebotich J., Vergara E., Medina C., Morales B., Moenne A. (2010). Copper-induced calcium release from ER involves the activation of ryanodine-sensitive and IP(3)-sensitive channels in Ulva compressa. Plant Signal Behav. 5, 1647–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.F. (2001). Dissecting calcium oscillators in plant cells. Trends Plant Sci. 6, 395–397 [DOI] [PubMed] [Google Scholar]
- Harper J.F., Breton G., Harmon A. (2004). Decoding Ca(2+) signals through plant protein kinases. Annu. Rev. Plant Biol. 55, 263–288 [DOI] [PubMed] [Google Scholar]
- Hedrich R., Marten I. (2011). TPC1–SV channels gain shape. Mol. Plant. 4, 428–441 [DOI] [PubMed] [Google Scholar]
- Islam M.M., Munemasa S., Hossain M.A., Nakamura Y., Mori I.C., Murata Y. (2010). Roles of AtTPC1, vacuolar two pore channel 1 in Arabidopsis stomatal closure. Plant Cell Physiol. 51, 302–311 [DOI] [PubMed] [Google Scholar]
- Kawano T., Sahashi 1 N., Takahashi K., Uozumi N., Muto S. (1998). Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol. 39, 721–730 [Google Scholar]
- Kim M.C., Chung W.S., Yun D.J., Cho M.J. (2009). Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant. 2, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H., Trewavas A.J., Knight M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 8, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H., Trewavas A.J., Knight M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078 [DOI] [PubMed] [Google Scholar]
- Knight M.R., Campbell A.K., Smith S.M., Trewavas A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 352, 524–526 [DOI] [PubMed] [Google Scholar]
- Kosuta S., Hazledine S., Sun J., Miwa H., Morris R.J., Downie J.A., Oldroyd G.E. (2008). Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc. Natl Acad. Sci. U S A. 105, 9823–9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M., Held K., Binder A., Hashimoto K., Den Herder G., Parniske M., Kudla J., Schumacher K. (2011). FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 69, 181–192 [DOI] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell. 22, 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaaitaal M., Huisman R., Maintz J., Reinstädler A., Panstruga R. (2011). Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana . Biochem. J. 440, 355–365 [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F., MacRobbie E.A., Webb A.A., Manison N.F., Brownlee C., Skepper J.N., Chen J., Prestwich G.D., Brearley C.A. (2003). Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc. Natl Acad. Sci. U S A. 100, 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhu S., Song X., Shen Y., Chen H., Yu J., Yi K., Liu Y., Karplus V.J., Wu P., et al. (2006). A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell. 18, 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S. (2009). The CBL–CIPK network in plant calcium signaling. Trends Plant Sci. 14, 37–42 [DOI] [PubMed] [Google Scholar]
- Ma W., Berkowitz G.A. (2011). Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol. 190, 566–572 [DOI] [PubMed] [Google Scholar]
- Martinec J., Feltl T., Scanlon C.H., Lumsden P.J., Machackova I. (2000). Subcellular localization of a high affinity binding site for D-myo-inositol 1,4,5-trisphosphate from Chenopodium rubrum . Plant Physiol. 124, 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P., Thomine S., Schroeder J.I., Ward J.M., Hirschi K., Sze H., Talke I.N., Amtmann A., Maathuis F.J., Sanders D., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiol. 126, 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh M.R., Hetherington A.M. (1998). Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 3, 32–36 [Google Scholar]
- McAinsh M.R., Pittman J.K. (2009). Shaping the calcium signature. New Phytol. 181, 275–294 [DOI] [PubMed] [Google Scholar]
- McCombs J.E., Palmer A.E. (2008). Measuring calcium dynamics in living cells with genetically encodable calcium indicators. Methods. 46, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E., Lima P.T., Borges F., Silva A.C., Portes M.T., Carvalho J.E., Gilliham M., Liu L.H., Obermeyer G., Feijo J.A. (2011). Glutamatereceptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 332, 434–437 [DOI] [PubMed] [Google Scholar]
- Monshausen G.B., Bibikova T.N., Weisenseel M.H., Gilroy S. (2009). Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 21, 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I.C., Iida H., Tsuji F.I., Isobe M., Uozumi N., Muto S. (1998). Salicylic acid induces a cytosolic Ca2+ elevation in yeast. Biosci. Biotechnol. Biochem. 62, 986–989 [DOI] [PubMed] [Google Scholar]
- Munemasa S., Hossain M.A., Nakamura Y., Mori I.C., Murata Y. (2011). The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 155, 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L., Bewell M.A., Siddiqua A., Dickinson G.D., Galione A., Sanders D. (2000). Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl Acad. Sci. U S A. 97, 8693–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Zhao Y., Zheng Y., Liu J., Jiang X., Guo Y. (2012). A high-throughput method for screening Arabidopsis mutants with disordered abiotic stress-induced calcium signal. Journal of Genetics and Genomics. 39, 225–235 [DOI] [PubMed] [Google Scholar]
- Peiter E., Maathuis F.J., Mills L.N., Knight H., Pelloux J., Hetherington A.M., Sanders D. (2005). The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 434, 404–408 [DOI] [PubMed] [Google Scholar]
- Plieth C. (2001). Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma. 218, 1–23 [DOI] [PubMed] [Google Scholar]
- Plieth C. (2010). Signal percolation through plants and the shape of the calcium signature. Plant Signal Behav. 5, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Stephens N.R., Spalding E.P. (2006). Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 142, 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S., Grimmer J., Pöschl Y., Pecher P., Chinchilla D., Scheel D., Lee J. (2012). Defense-related calcium signaling mutants uncovered via a quantitative high-throughput screen in Arabidopsis thaliana . Mol. Plant. 5, 115–130 [DOI] [PubMed] [Google Scholar]
- Shigaki T., Hirschi K.D. (2006). Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol. (Stuttg.). 8, 419–429 [DOI] [PubMed] [Google Scholar]
- Souslova E., Belousov V., Lock J., Stromblad S., Kasparov S., Bolshakov A., Pinelis V., Labas Y., Lukyanov S., Mayr L., et al. (2007). Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol. 7, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Liang F., Hwang I., Curran A.C., Harper J.F. (2000). Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 433–462 [DOI] [PubMed] [Google Scholar]
- Tracy F.E., Gilliham M., Dodd A.N., Webb A.A., Tester M. (2008). NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ. 31, 1063–1073 [DOI] [PubMed] [Google Scholar]
- Trewavas A.J., Malho R. (1998). Ca2+ signalling in plant cells: the big network!. Curr. Opin. Plant Biol. 1, 428–433 [DOI] [PubMed] [Google Scholar]
- Vatsa P., Chiltz A., Bourque S., Wendehenne D., Garcia-Brugger A., Pugin A. (2011). Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie. 93, 2095–2101 [DOI] [PubMed] [Google Scholar]
- Webb A.A.R., McAinsh M.R., Taylor J.E., Hetherington A.M. (1996). Calcium ions as intracellular second messengers in higher plants. In Advances in Botanical Research, Callow, J.A., ed. (London: Academic Press; ), pp. 45–96 [Google Scholar]
- Zhu X., Caplan J., Mamillapalli P., Czymmek K., Dinesh-Kumar S.P. (2010). Function of endoplasmic reticulum calcium ATPase in innate immunity-mediated programmed cell death. EMBO J. 29, 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.