Abstract
Thyroid disorders are common in pregnancy and the most common disorder is subclinical hypothyroidism. Due to the complex hormonal changes during pregnancy, it is important to remember that thyroxine requirements are higher in pregnancy. According to recent American Thyroid Association (ATA) guidelines, the recommended reference ranges for TSH are 0.1 to 2.5 mIU/L in the first trimester, 0.2 to 3.0 mIU/L in the second trimester, and 0.3 to 3.0 mIU/L in the third trimester. Maternal hypothyroidism is an easily treatable condition that has been associated with increased risk of low birth weight, fetal distress, and impaired neuropsychological development. Hyperthyroidism in pregnancy is less common as conception is a problem. Majority of them are due to Graves’ disease, though gestational hyperthyroidism is to be excluded. Preferred drug is propylthiouracil (PTU) with the target to maintain free T4 in upper normal range. Doses can be reduced in third trimester due to the immune-suppressant effects of pregnancy. Early and effective treatment of thyroid disorder ensures a safe pregnancy with minimal maternal and neonatal complications.
Keywords: Thyroid disorders, pregnancy, hypothyroidism
INTRODUCTION
Thyroid disorders are encountered frequently during pregnancy and the postpartum period. Thyroid disease is the second most common endocrine condition encountered in women of childbearing age after diabetes. Most of these conditions are treatable, and may affect mother and fetus adversely if they are not evaluated and managed appropriately.
Pregnancy is a time of complex hormonal changes. In women with normal thyroid function, there is an increase in thyroxine (T4) and triiodothyronine (T3) production, which results in inhibition of thyroid-stimulating hormone (TSH) in the first trimester of pregnancy, due to a high human chorionic gonadotropin (hCG) level that stimulates the TSH receptor because of partial structural similarity[1,2] [Figure 1]. A large plasma volume and thus an altered distribution of thyroid hormone, increased thyroid hormone metabolism, increased renal clearance of iodide, and higher levels of hepatic production of thyroxine-binding globulin (TBG) in the hyperestrogenic state of pregnancy are responsible for higher thyroxine requirements in pregnancy.[3] It is very important to remember that biochemical thyroid function should be free thyroid hormone, as total hormone will mislead showing more than normal value when the patient is euthyroid.
Figure 1.
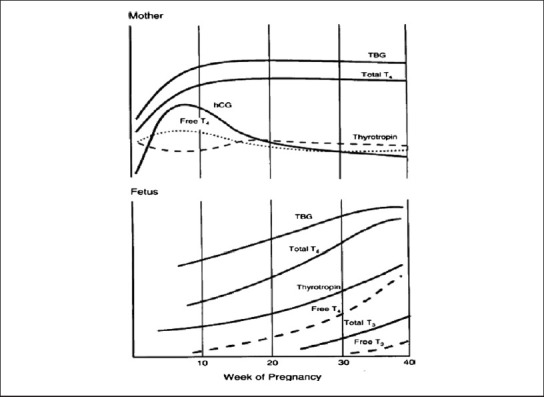
Thyroid hormone profile in the mother and fetus during pregnancy
Pregnancy and Hypothyroidism
Clinical or subclinical thyroid disorders are usually detected during pre-conceptional counseling or in women who have just conceived and have done tests for thyroid function. According to recent American Thyroid Association (ATA) guidelines, if laboratory-dependent, trimester-specific ranges for TSH are not available, the recommended reference ranges for TSH are 0.1 to 2.5 mIU/L in the first trimester, 0.2 to 3.0 mIU/L in the second trimester, and 0.3 to 3.0 mIU/L in the third trimester.[4]
The typical case is a women in the first trimester of pregnancy who is referred with a high TSH value with the free thyroid hormones in the low normal range or with frank hypothyroidism and in certain instances with biochemical euthyroxinemia with TPO antibody positivity. The presence of thyroid antibody, even in euthyroid patients, has been shown to be associated with increased number of miscarriage, perinatal death, and postpartum dysfunction, low motor and intellectual development in the offspring.[5] But, till now, biochemical normal thyroid test with positive antibody do not make a case for thyroxine supplement. We need more data before we start recommending therapy in such a situation.
Maternal hypothyroidism has been associated with increased risk of low birth weight, fetal distress, and impaired neuropsychological development. Haddow and colleagues described a 7-point IQ deficit in 7- to 9-year-old children born to untreated hypothyroid women when compared with age-matched children born to euthyroid women of less than 85, compared with 5% of controls.[6]
All women with overt and subclinical hypothyroidism should be treated irrespective of thyroid peroxidase (TPO) antibody positivity with LT4 during pregnancy to maintain serum TSH in the trimester-specific goal range. It has been recommended to check serum TSH every four weeks during pregnancy so that appropriate dose adjustments can be made,[4] but our routine practice is to check every six weeks. The recommended therapy is with oral LT4, which should be taken on an empty stomach (45 minutes before consumption of food, beverages, or other medications). In addition, calcium, iron, and prenatal vitamin supplements should be avoided within four hours of ingestion of LT4, as these can decrease the absorption of thyroxine. In a typical case, the dose requirement goes up as pregnancy advances, as pregnancy is a hypermetabolic condition [Table 1]. Our aim is to keep the free thyroxine value in the upper normal range.[7] We keep on counseling patients on this so that their compliance remains perfect.
Table 1.
Showing the biochemistry and requirement of thyroxine in pregnancy in a patient with Primary Hypothyroidism, Bhattacharyya A et al.[7]
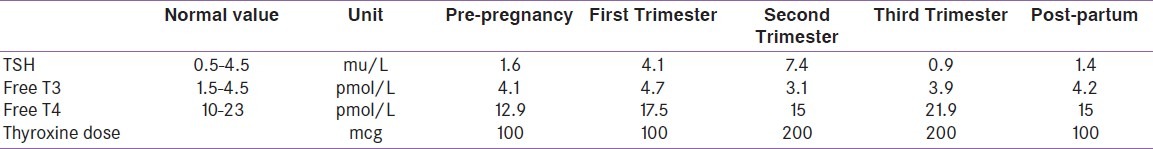
Immediately after delivery, the requirement of the thyroxine drops and women who were taking thyroixine prior to pregnancy will shift to their pre-pregnancy dose and those who started their thyroxine in pregnancy will require half the dose they were taking just before delivery. In women who had started their thyroxine in pregnancy for subclinical hypothyroidism, the medication can be stopped after delivery and thyroid balance re-assessed again after six weeks and decision taken regarding continuation of treatment. Obviously, some of these women goes through post-partum thyroiditis and requires thyroxine replacement for a longer time.
Pregnancy and Hyperthyroidism
Conception is usually more of a problem in untreated hyperthoidism. Majority of the disorders are due to Graves’ disease. In the first trimester, there may be deterioration in control due to reduced absorption of medication secondary to vomiting or to hCG-driven stimulation of TSH receptors. In the third trimesters, typically, treatment doses can be reduced due to the immune-suppressant effects of pregnancy as is seen in other autoimmune conditions like rheumatoid arthritis, systemic lupus etc. The typical ill-effects in pregnancy are accelerated hyperemesis of pregnancy requiring repeated hospital admission for intravenous fluid therapy, repeated miscarriage, poor growth of fetus, premature delivery, pregnancy-induced hypertension, etc. Untreated mother can also develop thyrotoxic crisis, fortunately rare now with an early diagnosis and effective treatment. Other uncommon effects rarely seen now-a-days are stillbirth. Fetal Graves’ disease is very rare and happens due to transplacental transfer of TSH-receptor stimulating antibody.
Hyperthyroidism if diagnosed before conception is best treated before conception in case radioactive iodine is given; current recommendation is not to conceive for at least four months. Neonatal outcome is better with fewer anomalies and there is lower chance of delivering prematurely if euthyroidism is achieved prior to conception, when compared with those women in whom either control is achieved in early pregnancy or later pregnancy, in a stepwise manner. There is chance that they will develop hypothyroidism; we can tackle that with thyroxine replacement as has been detailed earlier. For people diagnosed in the current pregnancy, we straightway start with anti-thyroid medication.
The preferred regimen is titration regimen; preferred medicine is propylthiouracil (PTU). Our aim is to keep free T4 in the upper normal range, sometime TSH can be little lower than normal range, but we concentrate more of free T4 as we know TSH takes time to get settled. Block and replace regimen is not followed in pregnancy as thyroxine does not cross placenta freely but anti-thyroid medications do. The dose of PTU depends on the control, sometime goes even up to 400-800 mg/day. It is to be given every 8th hourly. Liver function tests should be monitored with PTU, as there is a risk of hepatotoxicity. Methimazole is not preferred in the first trimester due to the risk of aplasia cutis and the spectrum of birth defects in pregnancy. Methimazole can be given in the second and third trimesters.[8]
As the pregnancy advances, dose requirement comes down in most of the cases; one-third of pregnant women can actually stop anti-thyroid medication in the third trimester [Table 2]. A significant percentage of these women need to start after delivery for relapse. Our routine practice is to check the thyroid function two weeks after delivery as opposed to six to eight weeks in cases of hypothyroidism. Beta-blockers, if necessary, can be given for a short duration for controlling symptoms. When thyroidectomy is needed for the control of hyperthyroidism, it should be planned in the second trimester of pregnancy; fortunately, this is very rare, and none of the authors can remember a single case they referred to surgeons for uncontrolled hyperthyroidism in pregnancy.
Table 2.
Showing the thyroid function and treatment of a patient with Graves’ disease in pregnancy treated by the authors (unpublished)
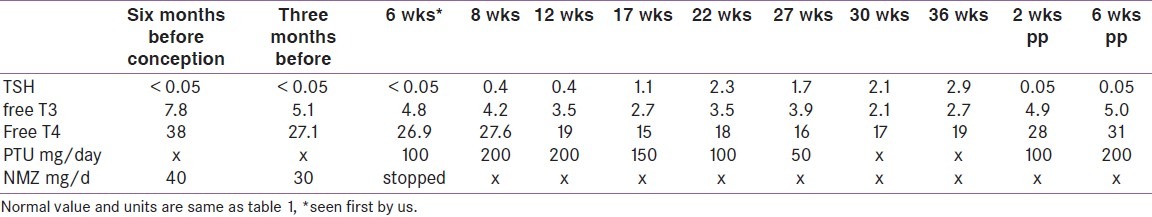
Breast feeding while on anti-thyroid medication remains a sensitive issue; PTU is the preferred medicine as it is more protein-bound and is secreted least in breast milk. Up to 600 mg a day PTU is considered safe; it is recommended to keep an eye on growth of the baby clinically with biochemical test for thyroid function if suspected for growth problem.
Gestational hyperthyroidism or gestational thyrotoxicosis is used when there are symptoms of hyperthyroidism due to the high levels of HCG, which causes thyroid hyperfunction. This condition needs to be differentiated from Graves’ disease, as most of the symptoms are similar to those in pregnancy. Up to 15% of normal pregnancy TSH can be suppressed due to hCG effect; they do not require extra treatment; careful observation is good enough. There is another entity in pregnancy called transient gestational thyroticosis, where free thyroid hormone can be increased, and they require a short course of anti-thyroid medication. Gestational thyrotoxicosis is usually transient and recovers over a period of few weeks. This is essentially a retrospective diagnosis.[9]
Thyroid cancer and pregnancy
Thyroid cancer is the most common endocrine malignancy affecting about 14 of 100000 pregnant women. The types of cancers seen in pregnant women are the same as in non-pregnant women. If the cancer is well-differentiated, surgery can be done in the immediate postpartum period; however, in not well-differentiated cancers, surgery can be done in the second trimester. Post-surgery, radioiodine scan and ablation is absolutely contraindicated during pregnancy and lactation. In women who have been operated and are on suppressive treatment with levothyroxine, dose should be adjusted so as to prevent thyrotoxicosis.[10]
CONCLUSION
Thyroid disorders are common in pregnancy, and the most common disorder is subclinical hypothyroidism. Early and effective treatment of thyroid disorder ensures a safe pregnancy with minimal maternal and neonatal complications.
Footnotes
Source(s) of Support: None
Presentation at a meeting: None
REFERENCES
- 1.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 2.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 3.Krajewski DA, Burman KD. Thyroid disorders in pregnancy. Endocrinol Metab Clin N Am. 2011;40:739–63. doi: 10.1016/j.ecl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matalon ST, Blank M, Ornoy A, Shoenfeld Y. The association between anti-thyroid antibodies andpregnancy loss. Am J Reprod Immunol. 2001;45:72–7. doi: 10.1111/j.8755-8920.2001.450202.x. [DOI] [PubMed] [Google Scholar]
- 6.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya A, Wright JD, Vice PA. Obstetric difficulties due to Graves’ disease. Postgrad Med J. 2002;77:661. doi: 10.1136/pmj.77.912.661. 669-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diav-Citrin O, Ornoy A. Teratogen update: Anti-thyroid drugs-methimazole, carbimazole and propylthiouracil. Teratology. 2002;65:38–44. doi: 10.1002/tera.1096. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin TM, Hershman JM. Hyperthyridism due to inappropriate production of human chorionic gonadotropin. Clin Obstet Gynecol. 1997;40:3244. doi: 10.1097/00003081-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: Results of linkage with California cancer registry. Am J Obstet Gynecol. 2003;189:1128–35. doi: 10.1067/s0002-9378(03)00537-4. [DOI] [PubMed] [Google Scholar]


