Abstract
Growth hormone (GH) is widely prescribed for children with short stature across a range of growth disorders. We describe the variability of responses seen in conditions approved for GH therapy. Although responses in different growth disorders are satisfactory, evidence is increasing for an unacceptably high rate of poor or unsatisfactory response (i.e., not leading to significant catch-up growth) in terms of change in height standard deviation score and height velocity. Consequently, there is a need to define a poor response and to prevent or correct it by optimizing treatment regimens. This review discusses the optimal investigation of the child who is a candidate for GH therapy so that a diagnosis-based guide to therapy and dosage can be made. The relevant parameters in the evaluation of growth response are described together with the definitions of a poor response.
Keywords: Growth, growth hormone therapy, growth response, height, recombinant human insulin-like growth-factor-1 therapy, short stature
INTRODUCTION
The management of short stature comprises many challenges, not least the options of appropriate hormonal therapies and their administration in regimens that are most beneficial. GH therapy is licensed by the European Medicines Agency (EMA) for treatment of GH deficiency (GHD), Turner syndrome (TS), short stature related to birth size small for gestational age (SGA), Prader–Willi syndrome, short stature homeobox-containing (SHOX) deficiency and chronic renal insufficiency. In the USA, the Food and Drugs Administration (FDA) has in addition approved GH therapy for idiopathic short stature (ISS) and Noonan syndrome.
Experience with GH therapy, both before and following approval has demonstrated good and satisfactory growth responses in each of the licensed indications. However, as experience with different treatment regimens accumulates, it is clear from reports of GH treatment that individual 1st year height responses vary considerably even with individualized treatment regimens.[1] Poor short-term response is also translated into an unsatisfactory gain in adult height.
This review discusses the published responses to GH therapy [Table 1] concentrating on factors, which influence the response during the 1st year of treatment. We also describe the identification and management of poor or unsatisfactory growth responses in children with licensed indications for GH therapy. We discuss the investigation of short stature aimed at establishing a diagnosis, the parameters of response, factors predicting response, the problem of compliance and finally, the management of the poorly responding patient.
Table 1.
Variability of responses to growth hormone therapy in children with diagnoses in the continuum of growth disorders
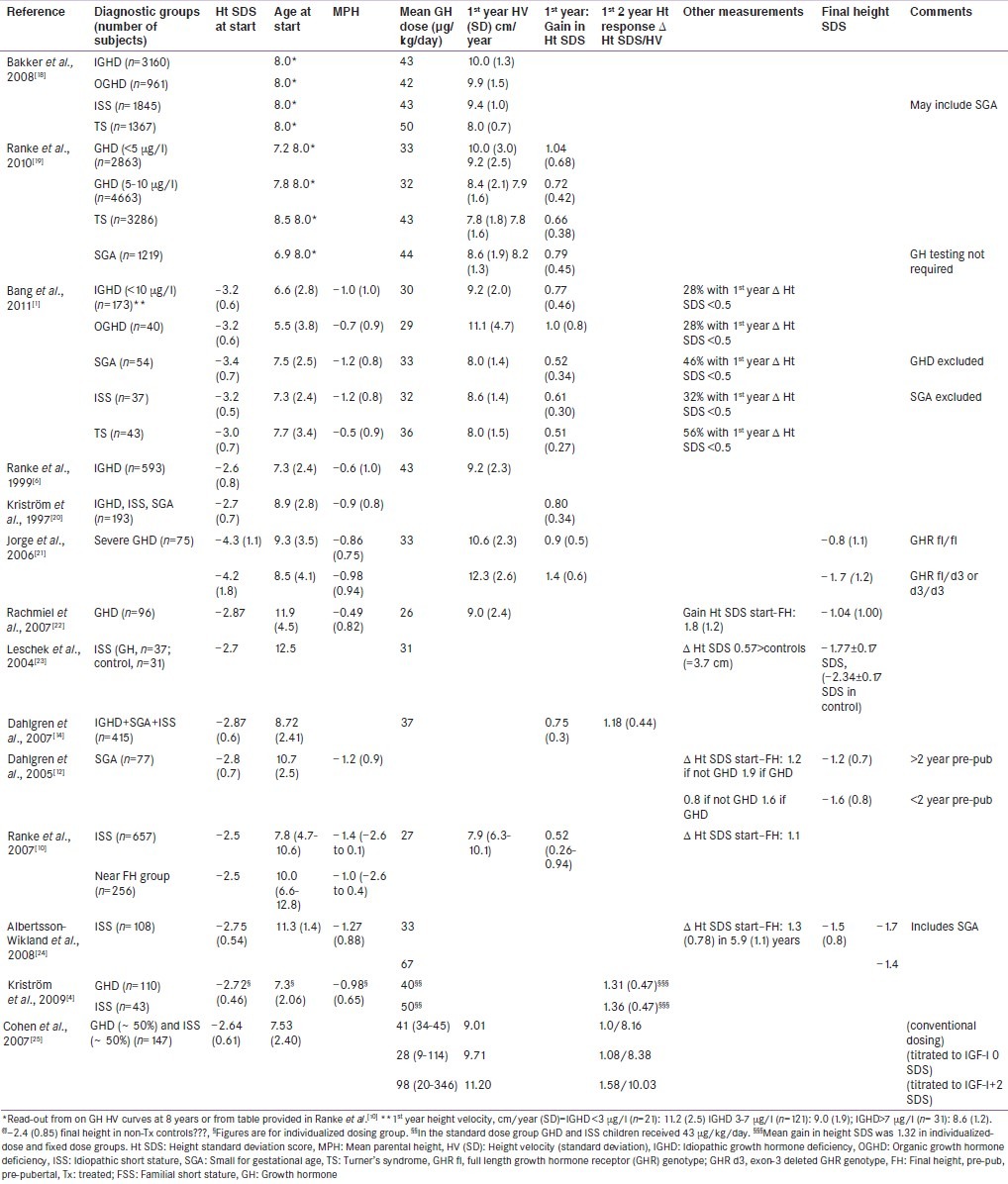
The continuum of growth disorders
Growth disorders exist across a continuum ranging from extreme GH sensitivity to GH resistance.[2] An inherent component of the continuum is the variation in responsiveness to GH therapy. It is now well recognised that children with severe GHD are highly responsive to GH replacement[3] and patients with less severe or questionable GHD, responds lesser. In recent reports of GH responses, there were no differences in response between subjects with less severe GHD and those with ‘normal’ GH secretion labelled as having ISS.[1] A strict distinction cannot be drawn between GHD and ISS and as the continuum of responsiveness to GH varies across and within diagnostic groups, the relevance of relating a sufficient response to a specific diagnosis can be questioned.
Clinical assessment and investigations aimed at identification of a primary diagnosis
Clinical assessment and investigation are important, because, the choice of therapy and dosage should be related to the primary diagnosis. The predicted response depends on a number of variables identified at initial assessment.
History and physical examination
The history and physical examinations are essential and attention should be paid to premature and/or SGA (low birth weight or birth length) birth. The presence of chronic disease should be considered and dysmorphic features should be documented. Parental growth and adult heights are relevant and known to be related to the response to GH.
Hormonal status
The identification of genetic defects in the GH–insulin-like growth factor (IGF)-axis has underlined the importance of endocrine assessment, including determination of serum insulin-like growth factor-I (IGF-I) and GH secretion. Severe classical GHD should be diagnosed early in patients with neonatal symptoms of hypoglycemia and prolonged jaundice, a characteristic growth pattern and possible additional pituitary hormone deficiencies. GHD in these children and in those with less severe idiopathic GHD (IGHD) can be confirmed by a low IGF-I concentration and GH provocative testing with a GH cut-off set at 7 or 10 μg/l.[3]
However, this cut-off is artificial and leads to a separation between IGHD and ISS that lacks physiological evidence as indicated by similar responsiveness to GH treatment.[1] Furthermore, approximately in 30% of short children born, SGA may have low stimulated GH concentrations. Poor reproducibility and a high incidence of false subnormal responses to different pharmacological stimuli are further limitations of GH stimulation tests.[3] The difficulties in discriminating between IGHD and ISS or SGA were clarified by studies, which showed that 85% of IGHD patients with two stimulated peak GH values <10 μg/l and normal pituitary magnetic resonance imaging (MRI) had values of GH >10 μg/l when re-tested 1-6 months later.[4]
Serum IGF-I levels are largely GH-dependent, but also influenced by age, pubertal development, malnutrition, chronic inflammation or hepatic diseases. Evaluation of spontaneous nocturnal GH secretion is used in a small number of centers and may have higher predictive value for the response to GH treatment, although this remains to be confirmed.
Radiological assessment
MRI of the hypothalamic–pituitary region must be performed when GHD is diagnosed, to exclude an organic cause.[3] Skeletal survey is indicated for body disproportion and an X-ray of the left hand and wrist for bone age, although not diagnostic, may be relevant to management.
Relevant parameters in the evaluation of growth hormone response
A number of factors are key determinants of the pattern of response to GH treatment[5] Growth hormone dose and growth response during the 1st year of GH therapy are strong predictors of final height outcome.[6] In pre-pubertal GH-treated children with IGHD, a 1st-year height increase of 0.5 standard deviation score (SDS) corresponds to an average final height gain of approximately 1.0 SDS.
Increase in height SDS and height velocity
Increase in height SDS is perhaps the most relevant parameter for the patient and parents, since deviation in growth relative to peers and the demonstration of how the patient's height will change with therapy is clinically important. Height velocity (HV) is also easy to discuss with the patient and parents. However, the actual increase in cm/year that results in a gain in height compared with peers is dependent on age.
Age-dependency of responses
Increases in height SDS and HV during the 1st year on GH treatment in different diagnoses [Table 1] are strongly age-dependent. This age-dependency is largely explained by the physiology of normal linear growth. Although mean heights at given ages differ in different populations, the width of 1.0 height SDS is relatively stable across populations. Several clinical trials and post-marketing registries report data on mean (±SD) or median (percentiles) 1st-year height responses or gain in final height in GH-treated children [Table 1]. These studies consistently report better responses when treatment is started at an early age – the number of years of pre-pubertal GH treatment strongly predicting final height.[7]
Prediction of response to growth hormone therapy
Over 10 years ago, the relative inflexibility of GH treatment regimens and the simplicity of the modalities used to derive them, led to the introduction of mathematical models aimed at predicting growth responses in individual patients.[5,6] Such models attempt to account for the definable variability of responsiveness so that clinicians can adapt GH doses to individual patients.[6] Prediction models for the 1st-year HV as well as the total height gains were published, for patients with GHD[6] TS,[8] or SGA[9] and for patients with varying degrees of GH secretion or ISS.[10]
Based on multiple regression analyses, these models have identified factors that correlate with growth. For example, chronological age, GH peak during provocative tests, dose of GH, birth-weight SDS and height SDS minus target height SDS are key variables associated with the 1st-year HV.[6] Biochemical variables such as the baseline IGF-I and leptin have added to the prediction of response. Prediction models derived from the large Pfizer International Growth Database (KIGS) database explain approximately 60% of the variability of response to GH therapy in patients with GHD and 40% in subjects with ISS.[5]
Management of the 1st year on GH therapy
Decision to treat and expected response
Before the start of GH treatment, the parents and child should be fully informed about the probable pathophysiology of growth retardation, the rationale for GH therapy and the evidence-based expected growth response. This information should reflect the large variability in response among individuals inherent in the continuum in GH sensitivity. Likely duration of therapy and the level of response at which discontinuation of treatment will be decided must also be discussed. The decision to start and stop treatment should be made in consultation with the patient and parents. Interestingly, data in the KIGS database on why GH treatment is being stopped do not include “poor response” as an option. A recent consensus statement on the use of GH in ISS emphasized the importance of discouraging the expectation that taller stature will improve quality of life.[11]
Dose of growth hormone
The starting dose of GH depends on the diagnosis of the condition and is usually calculated according to weight or body surface area. The recommended GH dose for each approved indication reflects the responsiveness to GH in the condition being treated. In cases where high GH sensitivity is expected including subjects with extreme GHD or obesity such as craniopharyngioma patients, a lower starting dose is recommended. Although differences in dosing during the 1st year of GH therapy may exist among countries and centres, there is evidence for adherence to the recommended doses within each indication. In some indications such as SGA and ISS, the recommended maximum dose of GH may be used from start of the treatment or the dose may be increased as necessary.[12]
In some situations, such as ISS, concern has been raised that higher GH doses of up to approximately 70 μg/kg/day advance bone age and pubertal progress, but this has not been confirmed. There are no definitive data concerning the long-term safety of doses higher than 50 μg/kg/day in children with ISS. A GH dose of 70 μg/kg/day was approved in the USA for treatment of GHD in puberty, but this regimen is only used by one-third of centres. In December 2010, the EMA issued guidance not to exceed a GH dose of 50 μg/kg/day based on preliminary data from a French post-marketing registry study, now published[13] in addition to data from Belgium, the Netherlands and Sweden.[14]
Monitoring during growth hormone therapy
During the 1st year of GH therapy, children should be seen at 3-6 monthly intervals for assessment of growth, puberty, mood, body composition, and to support compliance with therapy. These visits may be used to judge the response to GH, but growth response cannot be reliably assessed at an interval shorter than 1 year. IGF-I is a short-term biomarker of efficacy as well as a marker for adherence to therapy. It is recommended to consider GH dose reduction if IGF-I is repeatedly above the upper limit of the normal range or +2.5 SDS.[14] Deciding the starting GH dose based on the Swedish prediction model resulted in GH doses from 17 to 100 μg/kg/day but did not decrease the occurrence of serum IGF-I levels above the normal range.[4,15] However, this approach did result in a smaller variability in height responses during the first 2 years of GH therapy compared with conventional dosing.
Compliance with growth hormone therapy and its impact on response
Poor compliance may contribute to the variability in response to GH therapy. In the context of compliance, serum IGF-I is the most commonly used biomarker and its response to treatment is well characterized. Compliance with GH therapy involves daily and sometimes painful injections and physicians prescribing GH have to educate patients and their families about the necessity, context and objectives of the therapy. Individual psychological strain of treatment is also likely to affect adherence, and ethnic and socioeconomic factors and the educational level of families are also relevant. The impact of compliance on outcome to GH treatment has been studied showing that non-adherence impaired the growth response.[16] It remains unclear whether adherence differs between the various indications for GH treatment. Most studies of adherence with GH may not show an accurate picture of the attitudes of the patients and their families. Informed consent and shorter intervals between patient visits as practised in GH treatment studies may improve motivation and reinforce long-term adherence.
Management strategies for children with poor response to growth hormone therapy
As a general rule, the response to GH therapy should be assessed following 12 months of therapy [Figure 1] Guidelines for the identification and management of the patient who shows a poor response to GH therapy have recently been published.[17]. If a patient demonstrates a poor response, further evaluation of the diagnosis and indication for therapy is necessary. Several options for further management can then be considered. Repeated IGF-I measurements after 3 and 6 months of GH therapy may be used for GH dose titration.
Figure 1.
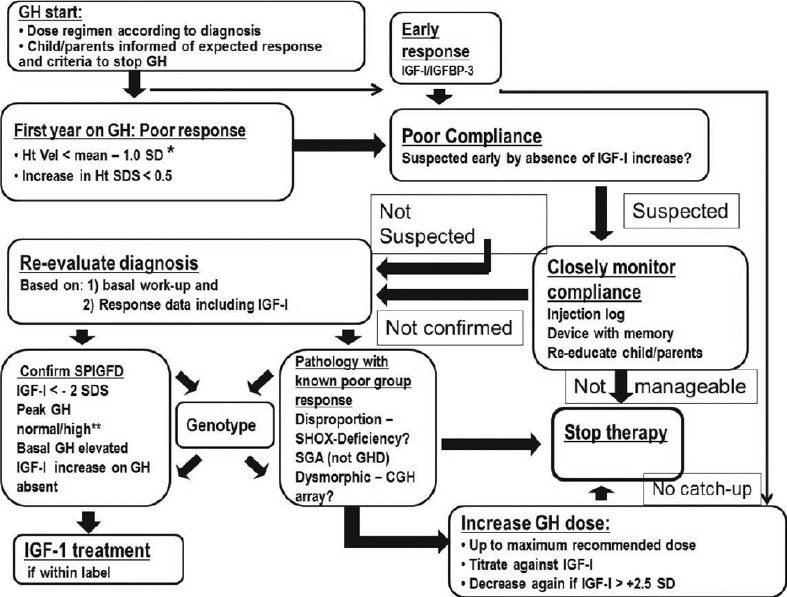
Algorithm for management of poor response to growth harmone.*Height velocity < mean –1.0 standard deviation equals an increase in height standard deviation score of 0.4 for severe idiopathic growth hormone deficiency (IGHD) and 0.3 for other diagnoses. Use reference for severe IGHD according to Ranke[10] for any diagnosis or if other diagnosis-specific references are used then consider using a more strict cut-off.**Consider that GH stimulation tests were falsely low which is the case in the majority of ‘IGHD’ patients without magnetic resonance imaging abnormalities. CGH array: Comparative genomic hybridization array, SHOX deficiency: Short stature homeobox-containing deficiency, SDS: Standard deviation score, SGA: Small for gestational age, SPIGFD: Severe primary insulin-like growth factor deficiency
CONCLUSIONS
The range of growth disorders treated with growth-promoting therapy is large; these disorders vary in their phenotypic, biochemical and molecular characteristics. Consequently, variability of responses in terms of short- and long-term change in height following treatment with GH is to be expected. Some components of this variability can now be predicted and, therefore, prevented by individualization of therapy. However, the reasons for others remain obscure and it has to be accepted that not all growth disorders are amenable to effective therapeutic management. Recognition of the likely variability of GH responses is important. In particular, the recognition of the poor response needs to be prioritized.
Footnotes
Source of Support: Peter Bang has received honoraria from Ipsen, Merck- Serono, Pfizer and Novo Nordisk and grant support from Ipsen, Novo Nordisk and Sanofi-Aventis. Martin Savage has received honoraria from Ipsen, Pfizer, and Merck Serono
Conflict of Interest: None declared
REFERENCES
- 1.Bang P, Bjerknes R, Dahlgren J, Dunkel L, Gustafsson J, Juul A, et al. A comparison of different definitions of growth response in short prepubertal children treated with growth hormone. Horm Res Paediatr. 2011;75:335–45. doi: 10.1159/000322878. [DOI] [PubMed] [Google Scholar]
- 2.Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone-IGF-I axis defects causing short stature: Diagnostic and therapeutic challenges. Clin Endocrinol (Oxf) 2010;72:721–8. doi: 10.1111/j.1365-2265.2009.03775.x. [DOI] [PubMed] [Google Scholar]
- 3.Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85:3990–3. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 4.Kriström B, Aronson AS, Dahlgren J, Gustafsson J, Halldin M, Ivarsson SA, et al. Growth hormone (GH) dosing during catch-up growth guided by individual responsiveness decreases growth response variability in prepubertal children with GH deficiency or idiopathic short stature. J Clin Endocrinol Metab. 2009;94:483–90. doi: 10.1210/jc.2008-1503. [DOI] [PubMed] [Google Scholar]
- 5.Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009;19:1–11. doi: 10.1016/j.ghir.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutefield W, Albertsson-Wikland K, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84:1174–83. doi: 10.1210/jcem.84.4.5634. [DOI] [PubMed] [Google Scholar]
- 7.Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: Analysis of a large international database. J Clin Endocrinol Metab. 2006;91:2047–54. doi: 10.1210/jc.2005-2284. [DOI] [PubMed] [Google Scholar]
- 8.Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, et al. Prediction of long-term response to recombinant human growth hormone in Turner syndrome: Development and validation of mathematical models. KIGS International Board. Kabi International Growth Study. J Clin Endocrinol Metab. 2000;85:4212–8. doi: 10.1210/jcem.85.11.6976. [DOI] [PubMed] [Google Scholar]
- 9.Ranke MB, Lindberg A, Cowell CT, Wikland KA, Reiter EO, Wilton P, et al. Prediction of response to growth hormone treatment in short children born small for gestational age: Analysis of data from KIGS (Pharmacia International Growth Database) J Clin Endocrinol Metab. 2003;88:125–31. doi: 10.1210/jc.2002-020867. [DOI] [PubMed] [Google Scholar]
- 10.Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res. 2007;68:53–62. doi: 10.1159/000098707. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210–7. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren J, Wikland KA Swedish Study Group for Growth Hormone Treatment. Final height in short children born small for gestational age treated with growth hormone. Pediatr Res. 2005;57:216–22. doi: 10.1203/01.PDR.0000148716.71231.81. [DOI] [PubMed] [Google Scholar]
- 13.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: Preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97:416–25. doi: 10.1210/jc.2011-1995. [DOI] [PubMed] [Google Scholar]
- 14.Sävendahl L, Maes M, Albertsson-Wikland K, Borgström B, Carel JC, Henrard S, et al. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: Preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. 2012;97:E213–7. doi: 10.1210/jc.2011-2882. [DOI] [PubMed] [Google Scholar]
- 15.Dahlgren J, Kriström B, Niklasson A, Nierop AF, Rosberg S, Albertsson-Wikland K. Models predicting the growth response to growth hormone treatment in short children independent of GH status, birth size and gestational age. BMC Med Inform Decis Mak. 2007;7:40. doi: 10.1186/1472-6947-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6:e16223. doi: 10.1371/journal.pone.0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang P, Ahmed SF, Argente J, Backeljauw P, Bettendorf M, Bona G, et al. Identification and management of poor response to growth-promoting therapy in children with short stature. Clin Endocrinol (Oxf) 2012;77:169–81. doi: 10.1111/j.1365-2265.2012.04420.x. [DOI] [PubMed] [Google Scholar]
- 18.Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93:352–7. doi: 10.1210/jc.2007-1581. [DOI] [PubMed] [Google Scholar]
- 19.Ranke MB, Lindberg A. KIGS International Board. Observed and predicted growth responses in prepubertal children with growth disorders: Guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95:1229–37. doi: 10.1210/jc.2009-1471. [DOI] [PubMed] [Google Scholar]
- 20.Jorge AA, Marchisotti FG, Montenegro LR, Carvalho LR, Mendonca BB, Arnhold IJ. Growth hormone (GH) pharmacogenetics: Influence of GH receptor exon 3 retention or deletion on first-year growth response and final height in patients with severe GH deficiency. J Clin Endocrinol Metab. 2006;91:1076–80. doi: 10.1210/jc.2005-2005. [DOI] [PubMed] [Google Scholar]
- 21.Kriström B, Jansson C, Rosberg S, Albertsson-Wikland K. Growth response to growth hormone (GH) treatment relates to serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in short children with various GH secretion capacities. Swedish Study Group for Growth Hormone Treatment. J Clin Endocrinol Metab. 1997;82:2889–98. doi: 10.1210/jcem.82.9.4234. [DOI] [PubMed] [Google Scholar]
- 22.Rachmiel M, Rota V, Atenafu E, Daneman D, Hamilton J. Final height in children with idiopathic growth hormone deficiency treated with a fixed dose of recombinant growth hormone. Horm Res. 2007;68:236–43. doi: 10.1159/000101427. [DOI] [PubMed] [Google Scholar]
- 23.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140–8. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 24.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008;93:4342–50. doi: 10.1210/jc.2008-0707. [DOI] [PubMed] [Google Scholar]
- 25.Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG, et al. Insulin growth factor-based dosing of growth hormone therapy in children: A randomized, controlled study. J Clin Endocrinol Metab. 2007;92:2480–6. doi: 10.1210/jc.2007-0204. [DOI] [PubMed] [Google Scholar]


