Abstract
Hyperglycemia promotes auto-oxidation of glucose to form free radicals. The generation of free radicals beyond the scavenging abilities of endogenous antioxidant defenses results in macro- and microvascular dysfunction.
Antioxidants such as N-acetylcysteine, vitamin C and α-lipoic acid are effective in reducing diabetic complications, indicating that it may be beneficial either by ingestion of natural antioxidants or through dietary supplementation. However, while antioxidants are proving essential tools in the investigation of oxidant stress-related diabetic pathologies and despite the obvious potential merit of a replacement style therapy, the safety and efficacy of antioxidant supplementation in any future treatment, remains to be established
Keywords: Antioxidants, diabetes mellitus, free radicals
INTRODUCTION
Diabetes is a chronic metabolic disorder with a rapidly increasing prevalence[1] highlighting the importance of continued research and the need for novel methods to both prevent and treat this pandemic. Although obesity and physical inactivity are known to be major risk factors for type 2 diabetes (T2DM), recent evidence suggests that oxidative stress may contribute to the pathogenesis of T2DM by increasing insulin resistance or impairing insulin secretion.[2]
While diabetes management has largely focused on control of hyperglycemia, the rising burden of this disease is mainly correlated to its vascular complications. This is reflected by a 4-fold increase in the incidence of coronary artery disease, a 10-fold increase in peripheral vascular disease, and a 3- to 4-fold higher mortality rate with as much as 75% of diabetics ultimately dying from vascular disease.[3] Oxidative stress may play a role in the pathophysiology of diabetes and cardiovascular disease. Consequently, the question of whether antioxidants could have a beneficial effect on reducing the risk of these conditions, especially cardiovascular disease, has been intensively investigated, but the results remain inconclusive. If antioxidants play a protective role in the pathophysiology of diabetes and cardiovascular disease, understanding the physiological status of antioxidant concentrations among people at high risk for developing these conditions, such as people with the metabolic syndrome, is of interest.[4]
DIABETES AND THE ENDOTHELIUM
Endothelial cells line the internal lumen of all the vasculature and serve as an interface between circulating blood and vascular smooth muscle cells (VSMC). Apart from being the key participant during the process of angiogenesis, these dynamic structures can actively regulate basal vascular tone and vascular reactivity in physiological and pathological conditions. The balance between the vasodilatation and vasoconstriction is maintained by the endothelium, and the disruption of this balance leads to endothelial dysfunction and causes damage to the arterial wall. Endothelial cell-derived factors also are critical mediators of VSMC growth and inflammation. Loss of function/regulation of function of the endothelium (endothelial dysfunction) in a basal state or after activation may be a critical and initiating factor in the development of diabetic micro- and macrovascular disease.[3]
The metabolic disturbances of diabetes, like hyperlipidemia, hyperinsulinemia, and hyperglycemia, act as “triggers” eventually causing endothelial dysfunction through the influence of different “mediator” molecules. Several lines of evidence point to the fact that “oxidative stress” caused by these metabolic changes plays a key role in endothelial dysfunction[5] [Figure 1].
Figure 1.
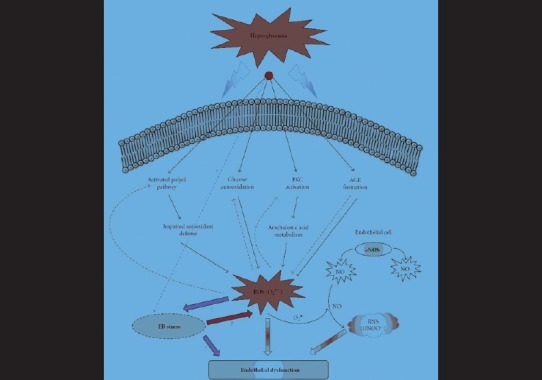
Hyperglycemia-induced oxidative stress and endothelium dysfunction (Adapted from Basha B, Samuel SM, Triggle CR, and Ding H. Endothelial Dysfunction in Diabetes Mellitus: Possible Involvement of Endoplasmic Reticulum Stress? Experimental Diabetes Research 2012; 2012: 481840)
DIABETES AND OXIDATIVE STRESS
A number of complications arise as a consequence of macro and microvascular complications that result from diabetes; these deficits have a central role in the tissue-damaging effects of chronic hyperglycemia. Since endothelial cells (as well as renal mesangial and Schwann cells) are unable to limit glucose transport as well as other cells do, they are more vulnerable to the toxic effects of hyperglycemia.
Oxidative stress results from an imbalance between radical- generating and radical-scavenging systems, i.e. increased free radical production or reduced activity of antioxidant defenses or both. Hyperglycemia-induced oxidative stress has also been associated with increased endothelial cellapoptosis in vitro and in vivo.[6] Several studies have shown that diabetes mellitus (types 1 and 2) is accompanied by increased formation of free radicals and decreased antioxidant capacity, leading to oxidative damage of cell components.[7]
Increased free radical production
There are multiple sources of reactive oxygen species (ROS) production in diabetes including those of mitochondrial and non-mitochondrial origins; ROS accelerates the four important molecular mechanisms involved in hyperglycemia-induced oxidative tissue damage. These four pathways are activation of protein kinase C (PKC), increased hexosamine pathway flux, increased advanced glycation end-product (AGE), and increased polyol pathway flux[8] [Figure 1].
Mitochondrial sources
The mitochondrial respiratory chain is a non-enzymatic source of ROS. Almost 10 years have elapsed since Brownlee's concept of the central role of mitochondrial superoxide production in the pathogenesis of diabetic complications. Hyperglycemia-induced generation of free radicals at the mitochondrial level is thought to be the major driver of the vicious cycle of oxidative stress in diabetes.[9] Briefly, he stated that increased intracellular glucose leads to an abundance of electron donors generated during the Kreb's cycle, so driving the inner mitochondrial membrane potential upward—a state that is associated with mitochondrial dysfunction and increased ROS production. The augmented generation of pyruvate via accelerated glycolysis under hyperglycemic conditions is thought to flood the mitochondria and thus generates ROS formation at the level of complex II in the respiratory chain. Reactive oxygen species stimulates oxidation of LDL; ox-LDL is not recognized by the LDL receptor and is subsequently taken up by scavenger receptors in macrophages to form foam cells and so lead to atherosclerotic plaques.[10] The production of ROS is reduced by using either an uncoupler of oxidative phosphorylation or by the over-expression of either uncoupling protein-1 or MnSOD, such that normalizing the levels of mitochondrial ROS with any of these agents will prevent glucose-induced activation of protein kinase C, formation of advanced glycation-end products, sorbitol accumulation, and NF-xB activation. These findings support the feasibility of targeting the triggering role of mitochondrial superoxide production in hyperglycemia-induced tissue damage.[11]
Non-Mitochondrial sources
Non-mitochondrial sources of ROS include: NAD(P)H oxidase, xanthine oxidase, uncoupled eNOS, lipoxygenase, cyclooxygenase, cytochrome P450 enzymes, and other hemoproteins.[12]
Common stimulators of vascular NAD(P)H are angiotensin II, thrombin, platelet-derived growth factor, and tumor necrosis factor-α. Inhibition of NADPH oxidase-dependent production of ROS in diabetes by a variety of PKC inhibitors suggests a regulatory role of PKC in hyperglycemia-induced NADPH oxidase activity. In keeping with this, PKC inhibitors decrease the expression of NADPH oxidase in high glucose-treated endothelial cells.[1]
Xanthine oxidase and xanthine dehydrogenase are collectively referred to as xanthine oxidoreductase. While both these enzymes catalyze the conversion of hypoxanthine toxanthine and then to uric acid, xanthine oxidase reduces oxygen as an electron acceptor while xanthine dehydrogenase can reduce either oxygen or NAD+. Hydroxyl radicals, hydrogen peroxide, and superoxide are byproducts of xanthine oxidase. Even though there is some controversy about the presence of xanthine oxidase in normal endothelial cells, it has been identified as a source of oxidative stress in the pathogenesis of atherosclerosis, ischemia-reperfusion, and diabetes mellitus.[13]
Nitric oxide is produced by inducible and constitutive nitric oxide synthases (NOSs), enzyme systems that incorporate oxygen into L-arginine. If NOS lacks its substrate L-arginine or one of its co-factors (“uncoupled” NOS), NOS produces superoxide instead of nitric oxide. The excessive ROS generation is known to impair endothelial nitric oxide synthase (eNOS) activity and NO production, thereby affecting endothelium-dependent vasodilation.[14]
Lipoxygenase products, especially 12(S)-HETE and 15(S)-HETE, are involved in the pathogenesis of several diseases including diabetes where they have proatherogenic effects and mediate the actions of growth factors and pro-inflammatory cytokines. Elevated levels of glucose induce endothelium-derived vasoconstrictor prostanoids, suggesting a role for cyclooxygenase-2 in diabetic vasculopathies. Further evidence supporting a role for oxidative stress in the induction of COX expression is that the expression of COX enzymes is normalized by glycemic control.[1]
Concentrations of free radicals in the body may also be increased by environmental factors such as cigarette smoke.[15]
Decreased antioxidant defenses
Cells have evolved highly complex enzymatic and non-enzymatic antioxidant systems, which work synergistically, and in combination with each other, to protect the body against free radical-induced damage.
There are several lines of evidence to suggest that antioxidant defences may be lower in diabetes. These include reports of reduced plasma/serum total antioxidant status or free radical scavenging activity and increased plasma oxidisability in type 2 diabetics, together with reduced levels of specific antioxidants such as ascorbic acid and vitamin E. In addition, the activities of the antioxidant enzymes catalase, superoxide dismutase, and glutathione peroxidase have been described as reduced in diabetics. A diminution in the endothelial synthesis of NO has also been suggested in type 2 diabetics, which apart from detracting from vascular antioxidant defence, would of course compound any defect in the anti-atherogenic signaling role of NO.[16]
In patients with T2DM, the content of oxidized fatty acids is increased, and the anti-inflammatory and antioxidant activities of HDLs are impaired.[17]
ANTIOXIDANT THERAPY AND DIABETES
The inhibition of intracellular free radical formation would provide a therapeutic strategy to prevent oxidative stress and the related diabetic vascular complications. Antioxidants may act at different levels, inhibiting the formation of ROS or scavenge free radicals, or increase the antioxidants defense enzyme capabilities. Supplementation with antioxidants and/or factors essential to nitric oxide (NO) production may potentially improve endothelial dysfunction in T2DM by re-coupling eNOS and mitochondrial function, as well as decreasing vascular NAD(P)H oxidase activity.[18] However, in the case of macrovascular/microvascular complications, the antioxidant therapy is beneficial together with blood pressure control, management of dyslipidemia, and optimal glucose control.[19]
Generally, the antioxidant pharmacotherapy can be divided in the use of antioxidant enzyme and substrates, biogenic elements, combined drugs, synthetic antioxidants, and drugs with antioxidant activity. There are also a large number of natural cellular defense mechanisms as the naturally existing antioxidant components, which neutralizes free radical damage. The enzymatic antioxidant systems, such as copper, zinc, manganese superoxide dismutase, gluthatione peroxidase, gluthathione reductase, and catalase may remove the ROS directly or sequentially, preventing their excessive accumulation and consequent adverse effects. Non-enzymatic antioxidant systems consist of scavenging molecules that are endogenously produced such as glutathione, ubichinol, and uric acid or derivatives of the diet such as vitamins C and E, carotenoids, lipoic acid, selenium, etc.[20] Exercise training results in an up-regulation of antioxidant defense mechanisms in various tissues, presumably due to increased levels of oxidative stress that occurs during exercise.[1]
Well-established antioxidants derived from the diet are vitamins C, E, A, and carotenoids, which have been studied intensively. In general, exogenous antioxidants can compensate for the lower plasma antioxidant levels often observed in T2DM and in pre-diabetic individuals, whether their diabetes is primarily genetic in origin or due to obesity and a sedentary lifestyle.[21] Vitamin C (ascorbic acid) and vitamin E (tocopherol) have well-described antioxidant properties. Vegetables and fruits have in their natural composition other substances besides these antioxidant vitamins, which guarantees health benefits associated with its consumption. Over the past decade, evidence has been accumulated that plant polyphenols are an important class of defense antioxidants. These compounds are widespread virtually in all plant foods, often at high levels, and include phenols, phenolic acids, and flavonoids.[22]
In a prospective cohort study, vitamin C intake was found to be significantly lower among incident cases of T2DM.[23] In three prospective observational studies, serum α-tocopherol levels were associated with lower risk of type T1DM or T2DM. In another prospective study cohort of more than 4000 non-diabetic subjects over 23 years, vitamin E intake was significantly associated with a reduced risk of T2DM.[2]
However, despite observational studies suggesting an association between antioxidant vitamin intake and reduced cardiovascular risk, this has not been borne out in interventional trials. Studies of the effect of ascorbic acid and tocopherol on endothelial dysfunction in T2DM have yielded mixed results.[18]
Coenzyme Q or ubiquinone may decrease oxidative stress not only by quenching reactive oxidant species but also by ‘recoupling’ mitochondrial oxidative phosphorylation, thereby reducing superoxide production. Alpha-lipoic acid, a critical co-factor for mitochondrial dehydrogenase reactions, is another compound with free radical-scavenging activity.[18] Lipoic acid was found to increase glucose transport in muscle cells in culture by stimulating translocation of GLUT4 from internal pools to the plasma membrane. In cultured adipocytes, treatment with lipoic acid protected the insulin receptor from oxidative damage, maintaining its functional integrity. A placebo-controlled explorative study of patients with T2DM indicated that oral administration of lipoicacid significantly increased insulin-mediated glucose uptake, presumably by modulating insulin sensitivity.[24]
In addition to the antioxidants mentioned, a number of commonly used drugs have been reported to have antioxidant activity, in addition to their primary pharmacological property. For example, gemfibrozil, a lipid-lowering fibrate, was previously reported to have antioxidant actions. Anti- hyperlipidemic statins are thought to exert antioxidant effects. In addition, it has been demonstrated that at least a part of the beneficial vascular effects of thiazolidinediones are linked with their antioxidant properties.[25]
CONCLUSIONS
Hyperglycemia, an inevitable consequence of T2DM, is the source of most of the deleterious effects usually associated with this disease. High blood glucose concentrations promote auto-oxidation of glucose to form free radicals. The generation of free radicals beyond the scavenging abilities of endogenous antioxidant defenses results in macro- and microvascular dysfunction and polyneuropathy.[21]
Antioxidants such as N-acetylcysteine, vitamin C, and α-lipoic acid are effective in reducing diabetic complications, indicating that it may be beneficial either by ingestion of natural antioxidants or through dietary supplementation.[20] However, while antioxidants are proving essential tools in the investigation of oxidant stress-related diabetic pathologies and despite the obvious potential merit of a replacement style therapy, the safety and efficacy of antioxidant supplementation in any future treatment remains to be established.[16] Future trials with antioxidants should not be discouraged, but better identification of criteria identifying potential candidates for antioxidant treatment should be studied.[26]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Golbidi S, Badran M, Laher I. Antioxidant and Anti-Inflammatory Effects of Exercise in Diabetic Patients. Exp Diabetes Res. 2012;2012:941868. doi: 10.1155/2012/941868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care. 2004;27:362–6. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 3.Basha B, Samuel SM, Triggle CR, and Ding H. Endothelial Dysfunction in Diabetes Mellitus: Possible Involvement of Endoplasmic Reticulum Stress? Exp Diabetes Res. 2012;2012:481840. doi: 10.1155/2012/481840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–52. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 5.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: A clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 6.Kangralkar VA, Patil SD, Bandivadekar RM. Oxidative Stress and Diabetes: A Review. Int J Pharma Appl. 2012;1:38–45. [Google Scholar]
- 7.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 8.Rolo AP, Palmeira CM. Diabetes and mitochondrialfunction: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 10.Boullier A, Bird DA, Chang MK, Dennis EA, Friedman P, Gillotre-Taylor K, et al. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–22. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 12.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 13.Desco MC, Asensi M, Ḿarquez R, Martínez-Valls J, Vento M, Pallardó FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: Protectionby allopurinol. Diabetes. 2002;51:1118–24. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 14.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 15.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: Current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 16.Laight DW, Carrier MJ, Anggard EE. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc Res. 2000;47:457–64. doi: 10.1016/s0008-6363(00)00054-7. [DOI] [PubMed] [Google Scholar]
- 17.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, et al. Anti-inflammatory and Antioxidant Properties of HDLs Are Impaired in Type 2 Diabetes. Diabetes. 2011;60:2617–23. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton SJ, Chew GT, Watts GF. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4:89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- 19.Jakus V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratisl Lek Listy. 2000;101:541–51. [PubMed] [Google Scholar]
- 20.da Silva SB, Costa JP, Pintado ME, Ferreira DC, Sarmento B. Antioxidants in the Prevention and Treatment of Diabetic Retinopathy - A Review. J Diabetes Metab. 2010;1:111. [Google Scholar]
- 21.Ruhe RC, McDonald RB. Use of Antioxidant Nutrients in the Prevention and Treatment of Type 2 Diabetes. J Am Coll Nutr. 2001;20:363S–9. doi: 10.1080/07315724.2001.10719169. [DOI] [PubMed] [Google Scholar]
- 22.Pietta PG. Flavonoids as Antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 23.Feskens EJ, Virtanen SM, Rasanen L, Tuomilehto J, Stengård J, Pekkanen J, et al. dietary factors determining diabetes and impaired glucosetolerance: A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care. 1995;18:1104–12. doi: 10.2337/diacare.18.8.1104. [DOI] [PubMed] [Google Scholar]
- 24.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, et al. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients withtype-2 diabetes mellitus: A placebo-controlled pilot trial. Radical Bioland Med. 1999;27:309–14. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 25.Karasu C. Glycoxidative Stress and Cardiovascular Complications in Experimentally-Induced Diabetes: Effects of Antioxidant Treatment. Open Cardiovasc Med J. 2010;4:240–56. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Violi F, Loffredo L, Musella L, Marcoccia A. Should antioxidant status be considered in interventional trials with antioxidants? Heart. 2004;90:598–602. doi: 10.1136/hrt.2003.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]


