Abstract
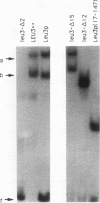
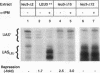
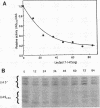
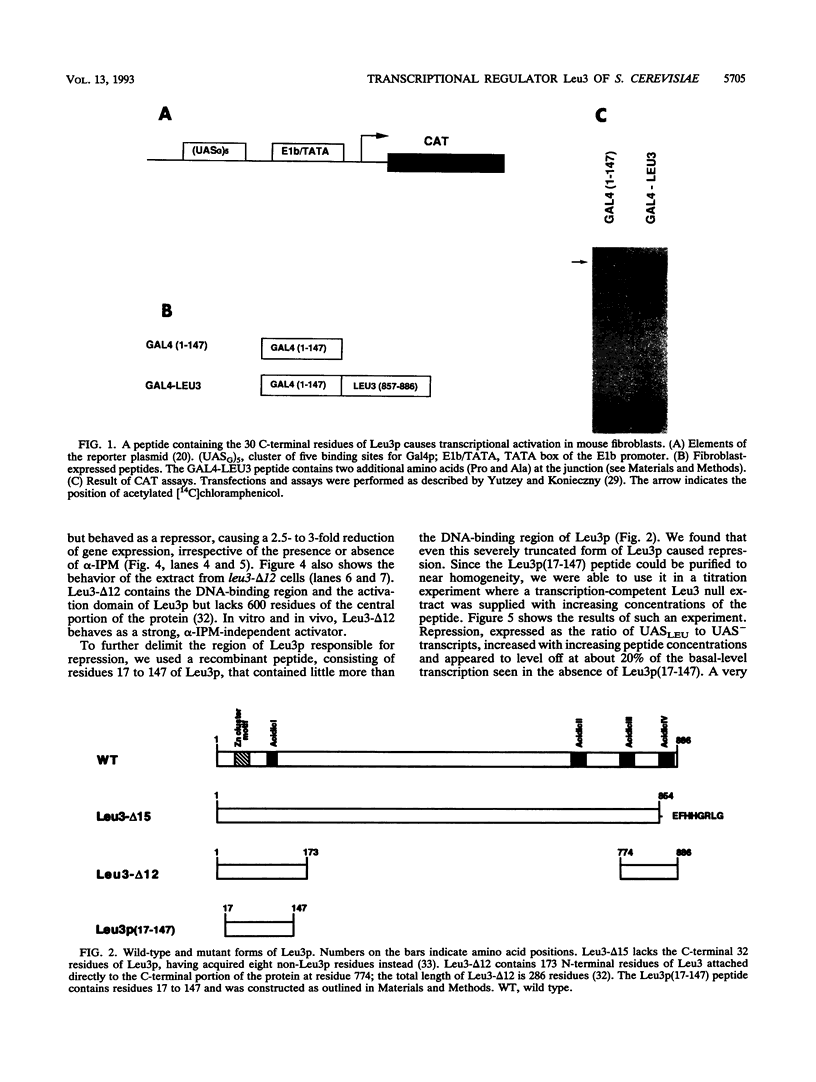
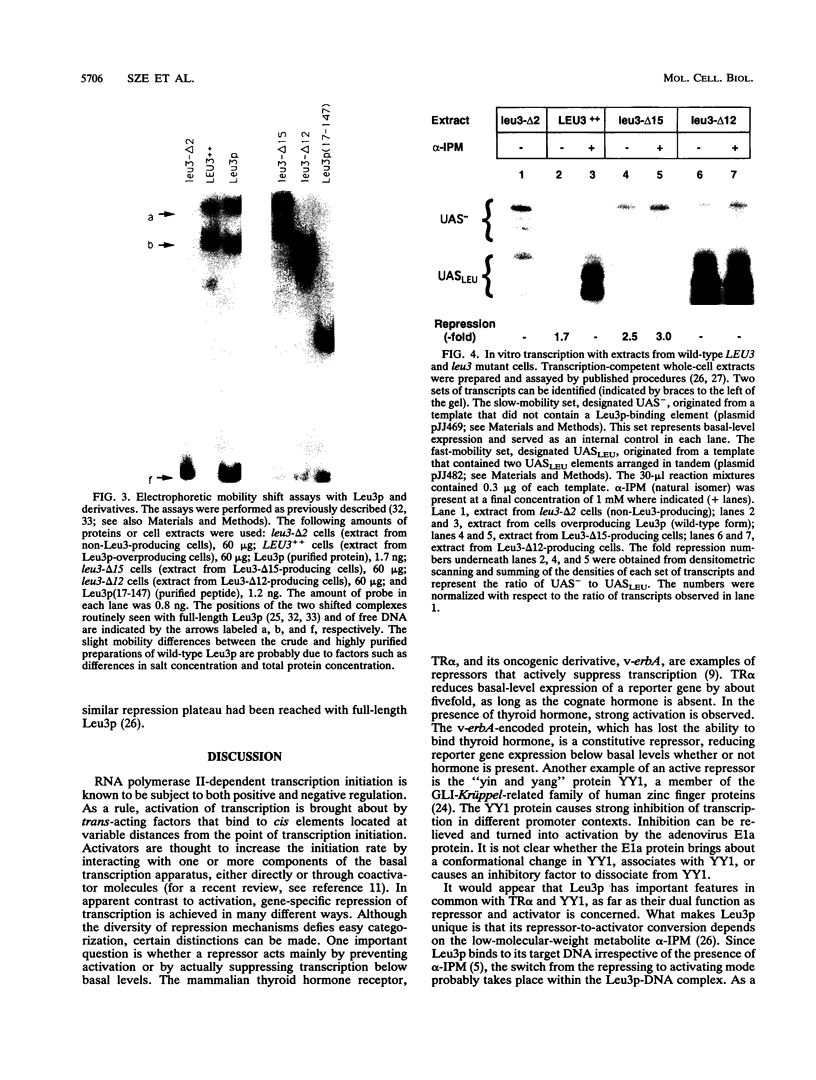
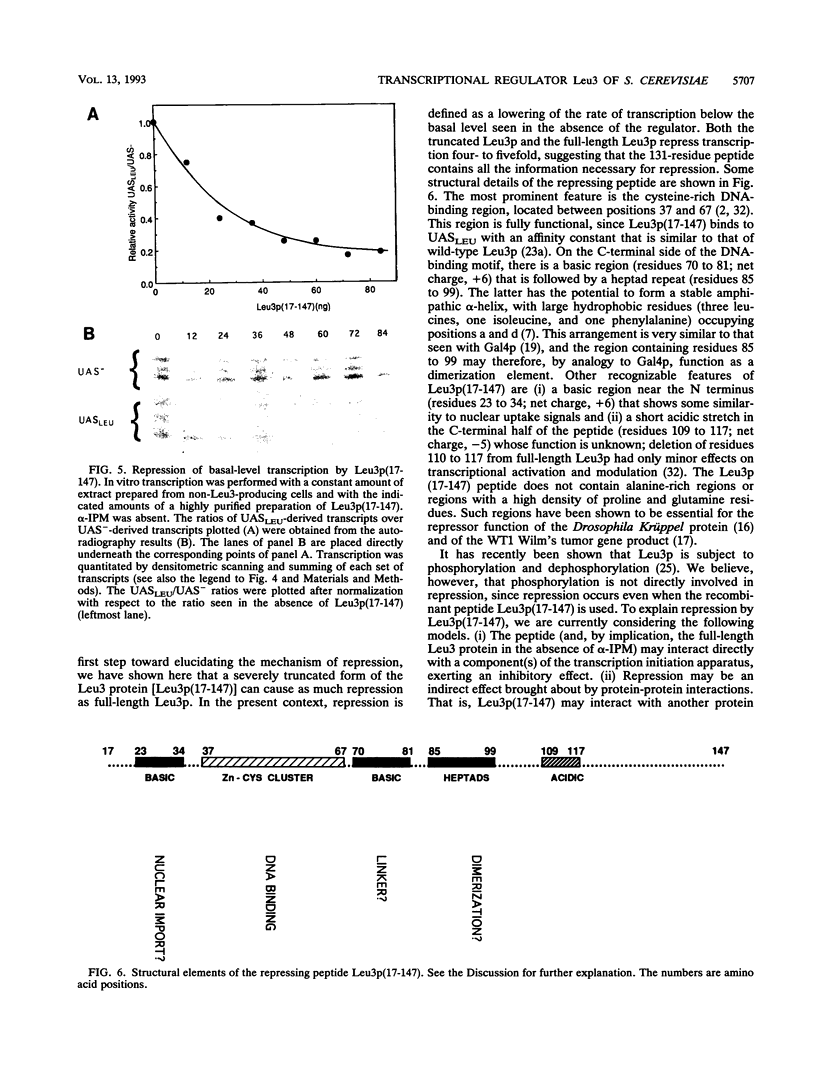
The Leu3 protein of Saccharomyces cerevisiae binds to specific DNA sequences present in the 5' noncoding region of at least five RNA polymerase II-transcribed genes. Leu3 functions as a transcriptional activator only when the metabolic intermediate alpha-isopropylmalate is also present. In the absence of alpha-isopropylmalate, Leu3 causes transcription to be repressed below basal levels. We show here that different portions of the Leu3 protein are responsible for activation and repression. Fusion of the 30 C-terminal residues of Leu3 to the DNA-binding domain of the Gal4 protein created a strong cross-species activator, demonstrating that the short C-terminal region is not only required but also sufficient for transcriptional activation. Using a recently developed Leu3-responsive in vitro transcription assay as a test system for repression (J. Sze, M. Woontner, J. Jaehning, and G. B. Kohlhaw, Science 258:1143-1145, 1992), we show that mutant forms of the Leu3 protein that lack the activation domain still function as repressors. The shortest repressor thus identified had only about 15% of the mass of the full-length Leu3 protein and was centered on the DNA-binding region of Leu3. Implications of this finding for the mechanism of repression are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai Y. L., Kohlhaw G. B. Manipulation of the 'zinc cluster' region of transcriptional activator LEU3 by site-directed mutagenesis. Nucleic Acids Res. 1991 Nov 11;19(21):5991–5997. doi: 10.1093/nar/19.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisco P. R., Cunningham T. S., Kohlhaw G. B. Cloning, disruption and chromosomal mapping of yeast LEU3, a putative regulatory gene. Genetics. 1987 Jan;115(1):91–99. doi: 10.1093/genetics/115.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisco P. R., Kohlhaw G. B. Regulation of yeast LEU2. Total deletion of regulatory gene LEU3 unmasks GCN4-dependent basal level expression of LEU2. J Biol Chem. 1990 Jul 15;265(20):11667–11675. [PubMed] [Google Scholar]
- Chasman D. I., Leatherwood J., Carey M., Ptashne M., Kornberg R. D. Activation of yeast polymerase II transcription by herpesvirus VP16 and GAL4 derivatives in vitro. Mol Cell Biol. 1989 Nov;9(11):4746–4749. doi: 10.1128/mcb.9.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Friden P., Schimmel P. LEU3 of Saccharomyces cerevisiae activates multiple genes for branched-chain amino acid biosynthesis by binding to a common decanucleotide core sequence. Mol Cell Biol. 1988 Jul;8(7):2690–2697. doi: 10.1128/mcb.8.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Tjian R. Eukaryotic coactivators associated with the TATA box binding protein. Curr Opin Genet Dev. 1992 Apr;2(2):236–242. doi: 10.1016/s0959-437x(05)80279-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Z., Sherman F. Two types of TATA elements for the CYC1 gene of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991 Feb;11(2):666–676. doi: 10.1128/mcb.11.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht J. D., Grossel M. J., Figge J., Hansen U. M. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Marmorstein R., Carey M., Ptashne M., Harrison S. C. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408–414. doi: 10.1038/356408a0. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Lillie J. W., Green M. R. Evidence for interaction of different eukaryotic transcriptional activators with distinct cellular targets. Nature. 1990 Jul 12;346(6280):147–152. doi: 10.1038/346147a0. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Chou J., MacKrell A. J., Casadaban M. J., Steiner D. F. Expression of a preproinsulin-beta-galactosidase gene fusion in mammalian cells. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5198–5202. doi: 10.1073/pnas.80.17.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthun M. R., Mangus D. A., Jaehning J. A. The EGD1 product, a yeast homolog of human BTF3, may be involved in GAL4 DNA binding. Mol Cell Biol. 1992 Dec;12(12):5683–5689. doi: 10.1128/mcb.12.12.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M. H., Beltzer J. P., Kohlhaw G. B. Expression of the yeast LEU4 gene is subject to four different modes of control. Arch Biochem Biophys. 1990 Jan;276(1):294–298. doi: 10.1016/0003-9861(90)90041-v. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Sze J. Y., Kohlhaw G. B. Purification and structural characterization of transcriptional regulator Leu3 of yeast. J Biol Chem. 1993 Feb 5;268(4):2505–2512. [PubMed] [Google Scholar]
- Sze J. Y., Woontner M., Jaehning J. A., Kohlhaw G. B. In vitro transcriptional activation by a metabolic intermediate: activation by Leu3 depends on alpha-isopropylmalate. Science. 1992 Nov 13;258(5085):1143–1145. doi: 10.1126/science.1439822. [DOI] [PubMed] [Google Scholar]
- Woontner M., Wade P. A., Bonner J., Jaehning J. A. Transcriptional activation in an improved whole-cell extract from Saccharomyces cerevisiae. Mol Cell Biol. 1991 Sep;11(9):4555–4560. doi: 10.1128/mcb.11.9.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutzey K. E., Kline R. L., Konieczny S. F. An internal regulatory element controls troponin I gene expression. Mol Cell Biol. 1989 Apr;9(4):1397–1405. doi: 10.1128/mcb.9.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutzey K. E., Konieczny S. F. Different E-box regulatory sequences are functionally distinct when placed within the context of the troponin I enhancer. Nucleic Acids Res. 1992 Oct 11;20(19):5105–5113. doi: 10.1093/nar/20.19.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutzey K. E., Rhodes S. J., Konieczny S. F. Differential trans activation associated with the muscle regulatory factors MyoD1, myogenin, and MRF4. Mol Cell Biol. 1990 Aug;10(8):3934–3944. doi: 10.1128/mcb.10.8.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. M., Bai Y. L., Kohlhaw G. B. Yeast regulatory protein LEU3: a structure-function analysis. Nucleic Acids Res. 1990 Jan 25;18(2):291–298. doi: 10.1093/nar/18.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. M., Kohlhaw G. B. Transcriptional activator LEU3 of yeast. Mapping of the transcriptional activation function and significance of activation domain tryptophans. J Biol Chem. 1990 Oct 15;265(29):17409–17412. [PubMed] [Google Scholar]