Abstract
Background:
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of reproductive age. Due to the logistics of diagnosis and lack of consensus on the diagnostic criteria, there are very few prevalence studies in the community. This study was aimed to assess the prevalence of PCOS in women 18-25 years of age, conducted in college girls from Lucknow, North India.
Materials and Methods:
Sample size for the study was calculated as 1052. Girls from 3 different colleges were approached (n = 2150), 1520 (70.7%) agreed to participate. They were asked to fill up a questionnaire asking details of menstrual cycle and features of hyperandrogenism. Hirsutism was self-reported. Responses were verified by a trained research assistant. A probable case was defined as a girl with menstrual irregularity (MI) or hirsutism (H) or both. All the probable cases were invited for detailed examination, hormone estimation, and ovarian ultrasonography.
Results:
Of the 1520 girls, 200 (13.1%) were labeled as probable cases; 175 (87.5%) had MI and 25 (12.5%) had both MI and H. Of the 200 cases, 75 (37.5%) had hormonal evaluation while 11 agreed for ultrasonography. 27 girls had confirmed PCOS. Therefore, if all the 200 girls would have had hormonal evaluation, 56 girls were likely to be confirmed as PCOS, giving a calculated prevalence of 3.7% (95% CI, 2.6–4.4) in this population. The mean age of these PCOS cases was 18.96 ± 1.73 yrs, body mass index was 21.72 ± 5.48 Kg/m2, and waist hip ratio was 0.81 ± 0.08. Only 12% girls had a body mass index ≥ 27.5 Kg/m2, but 44% had waist hip ratio > 0.81, again highlighting that despite low BMI, Indians have more abdominal obesity.
Conclusion:
Calculated prevalence of PCOS in women between the ages of 18-25 years from Lucknow, north India, is 3.7%. Majority of these girls were lean but have abdominal obesity.
Keywords: Body Mass Index (BMI), hirsutism (H), polycystic ovary syndrome (PCOS)
INTRODUCTION
Polycystic ovary syndrome (PCOS) is said to be the commonest endocrine disorder of women of reproductive age with a heterogeneous presentation, which includes hyperandrogenism and ovulatory dysfunction. PCOS usually has a peri-pubertal onset; so, it is a disorder of significant health concern. This necessitates estimation of proportion of women are affected by PCOS in the population.
The prevalence reported in earlier studies varies between 2.2% to 26%. These variations are due to difficulties in hormonal evaluation and lack of consensus on diagnostic criteria. For diagnosis of PCOS, ovarian ultrasonography and blood tests have to be done in the follicular phase. This limits large epidemiological studies in the community. Using different criteria, prevalence has been estimated as 4.0%–11.9% in the community from 3 different countries. There is paucity of data from India.
Insulin resistance is central to the pathogenesis of PCOS, and Indians are known to have high prevalence of insulin resistance, so the prevalence of PCOS may be high in our population.
This study was undertaken with the aim to assess prevalence of PCOS in young women (age group 18-25 years) from North India [Figure 1].
Figure 1.
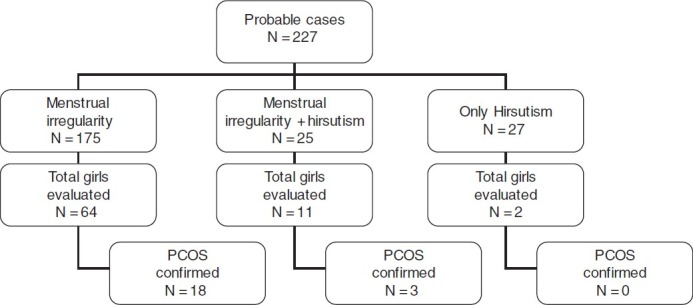
Flow Chart
MATERIALS AND METHODS
Sample size was calculated as 864 with an estimated prevalence of 10%, precision of 2%, and confidence interval of 95%. We added 20% for non-responders making the total number to 1073. We conducted this study in girls’ degree colleges. The girls who volunteered to participate were asked to fill up a questionnaire asking about the details of menstrual history and features of hyperandrogenism. Responses were verified by a trained research assistant.
A girl was labeled as a probable case of PCOS if she had menstrual irregularity or hirsutism (self-reported) or both. Menstrual irregularity was defined as the presence of chronic amenorrhea or usual cycle length of >35 days. All probable cases were called for detailed clinical examination to confirm features of hyperandrogenism, anthropometry, hormone estimation, and ovarian ultrasonography. To define biochemical hyperandrogenism, 90 controls from the same population (girls with regular menstrual cycles and no evidence of hyperandrogenism) were studied. Serum testosterone and free androgen index above the 97th percentile were labeled as hyperandrogenism, values of which were 1.76 nmol/L and 5.22 nmol/L, respectively
The study was approved by the institutional ethics committee. Written informed consent was obtained from participants prior to detailed evaluation.
RESULTS
About 2150 girls were eligible for the study in the 3 colleges. Of these, 1520 (70.7%) girls volunteered to participate in the study. Out of these 1520 girls, 175 (11.5%) had menstrual irregularity, 27 (1.83%) had hirsutism, and 25 (1.6%) had both menstrual irregularity and hirsutism, making a total of 227 probable cases. All of these 227 cases were invited for further evaluation. 67 (38.3%) of 175 girls with menstrual irregularity, 11 of 25 (44%) with menstrual irregularity and hirsutism group, and just 2 of the only hirsutism group came for further evaluation. There was no difference in the girls who came for further evaluation from those who did not. Out of 80 probable cases, who had hormonal evaluation, 5 girls were excluded because they had secondary causes for menstrual dysfunction.
Clinical examination for features of hyperandrogenism showed that the girls tended to over-report hirsutism. So, results are further reported on the 200 girls, 175 with menstrual irregularity and 25 with both.
Of the 75 girls with oligo/anovulation, who had further evaluation, 13.3% (10/75) girls had clinically significant hirsutism, 18.9% (14/75) had biochemical hyperandrogenemia. 9.4% (7/75) girls had both clinical as well as biochemical hyperandrogenemia. Only 11 girls agreed for ovarian ultrasonography, and 8 girls were found to have polycystic ovaries.
According to the NIH criteria, 21 girls were confirmed to have PCOS. Calculated percentage will be 3.7% (95% CI, 2.6–4.4) [Table 1–5].
Table 1.
Distribution of girls according to their presentation, completely evaluated, confirmed, probable, and expected number of case
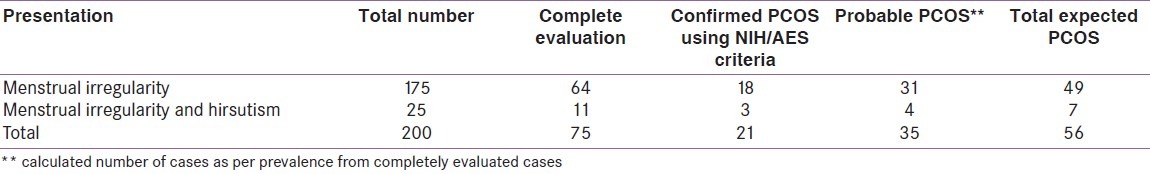
Table 5.
In comparison with other study

Table 2.
Demographic characteristics of PCOS cases and controls
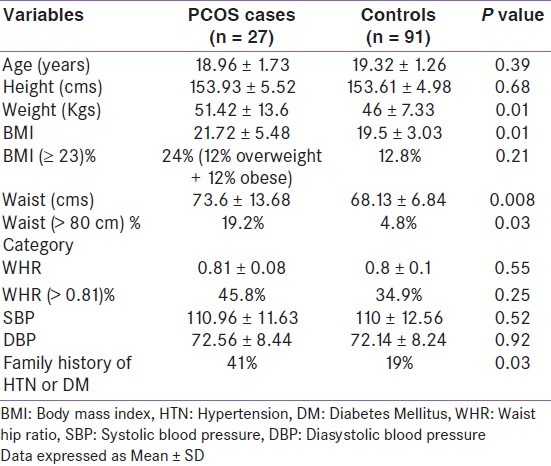
Table 3.
Univariate logistic regression analysis
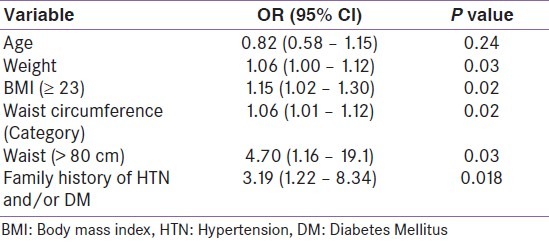
Table 4.
Multivariate Logistic Regression Analysis

DISCUSSION
The community prevalence of PCOS in young women (18-25 years) using the NIH criteria in our study was 3.7% (95% CI–2.6–4.4%), which is less than that reported in earlier studies. Using the NIH criteria, a birth cohort study from Australia has shown prevalence of 8.7%. In this study, the definition of menstrual irregularity was very wide, polymenorrhea (<21 days cycle) or a gap between usual cycle lengths of 4-5 days was taken as oligo-anovulation, while in our study, cycle length >35 days was the criteria.
Using the Rotterdam criteria where ovarian ultrasonography is a component of diagnosis, in the same cohort, prevalence increased to 11.9%. Contrary to this using the Rotterdam criteria, Kumarapeli et al. have reported a lesser prevalence of 6.1% in Sri Lankan population and 6.3% in Chinese population by Yanmin et al, both are community-based studies
Majority of our cases were lean as only 24% had a BMI ≥ 23 Kg/m2, and none was morbidly obese. In contrast, 30-38% subjects are obese in other studies. Obesity can itself cause menstrual irregularity and increase prevalence of PCOS, so this could be a reason for lower prevalence of menstrual irregularity and PCOS in our cohort. In spite of lower prevalence of obesity, waist-hip ratio was abnormal in 44% of PCOS cases, highlighting that Indians have more central obesity, even at low BMI. A significant number of these girls had pre-hypertension; both these factors increasing their long-term cardiovascular disease risk. These abnormal metabolic features are evident, even at the young ages of 18-25 years.
The major limitation of our study was that just 40% of the probable cases came for further follow up and evaluation and a minority agreed for ultrasonography.
CONCLUSION
Prevalence of PCOS in young women (18-25 years) was 3.7%, and majority of them were lean. Even at this young age, these women were at a high risk of metabolic syndrome because of the increased prevalence of abnormal waist-hip ratio and pre-hypertension.
ACKNOWLEDGMENTS
This study was funded by an extramural grant from UP Council of Science and Technology.[6]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of Polycystic Ovary Syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of Polycystic Ovary Syndrome in the Greek Island of Lesbos–Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 4.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. Prevalence of Polycystic Ovary Syndrome in a community sample done under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 5.Ma YM, Li R, Qiao J, Zhang XW, Wang SY, Zhang QF, et al. Charactestics of abnormal menstrual cycles and polycystic ovary syndrome in community and hospital population. Chin Med J. 2010;123:2185–9. [PubMed] [Google Scholar]
- 6.Kumarapeli V, Seneviratne Rde A, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semiurban population in Sri Lanka. Am J Epidemiol. 2008;168:321–8. doi: 10.1093/aje/kwn137. [DOI] [PubMed] [Google Scholar]


