Abstract
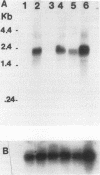
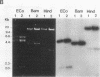
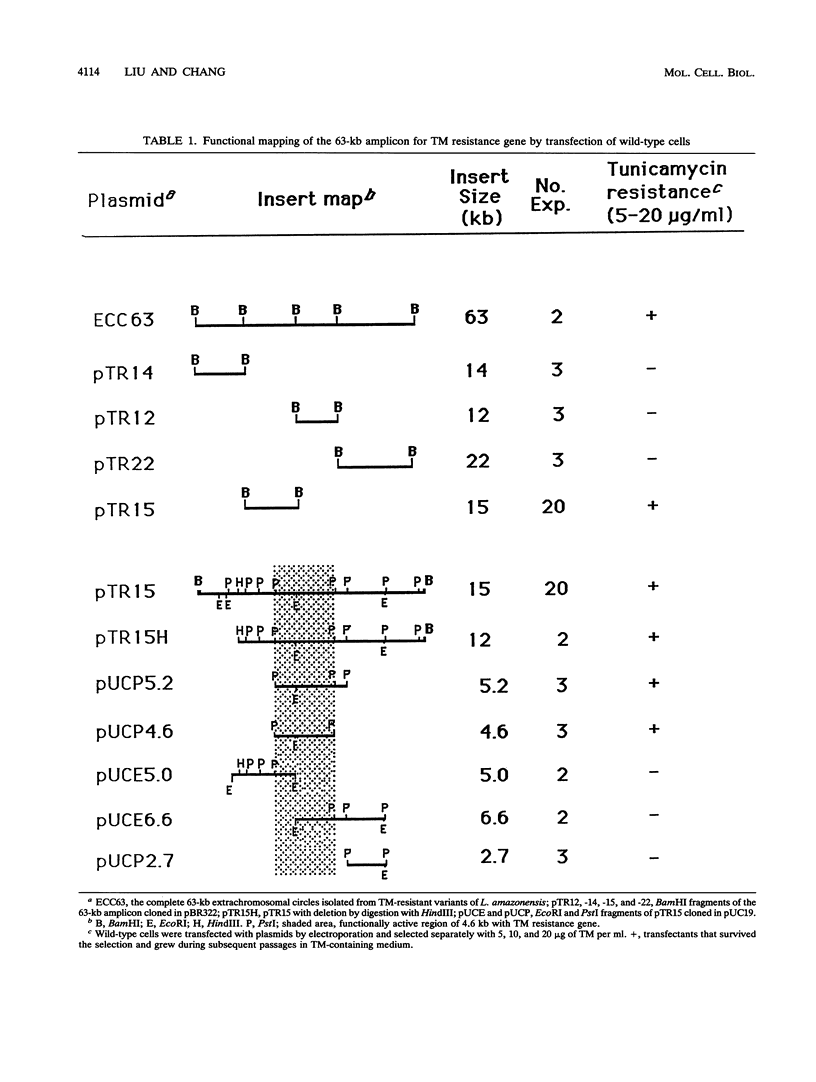
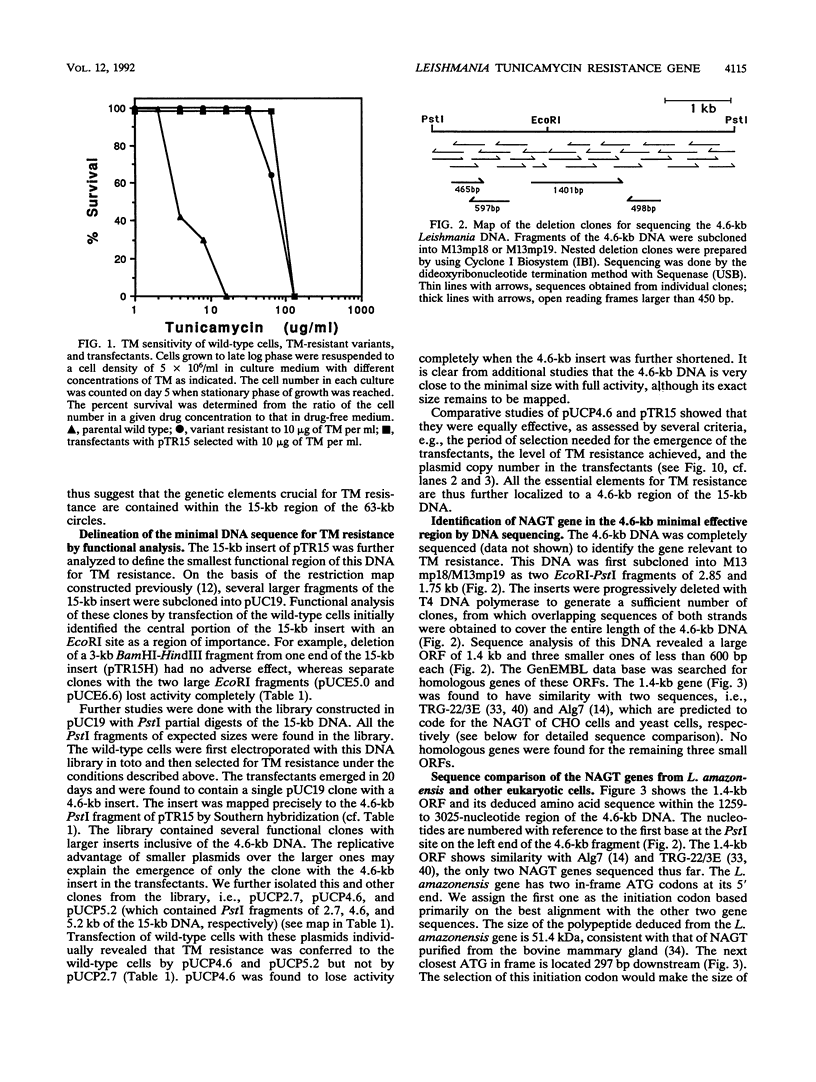
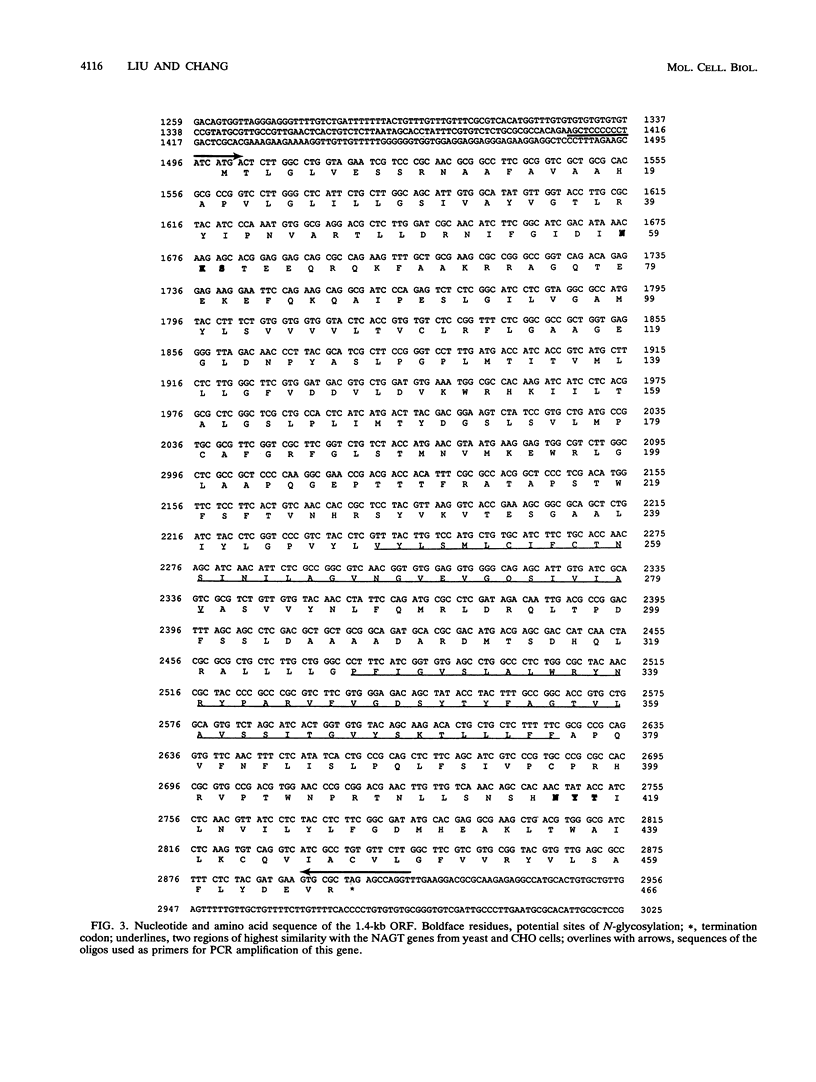
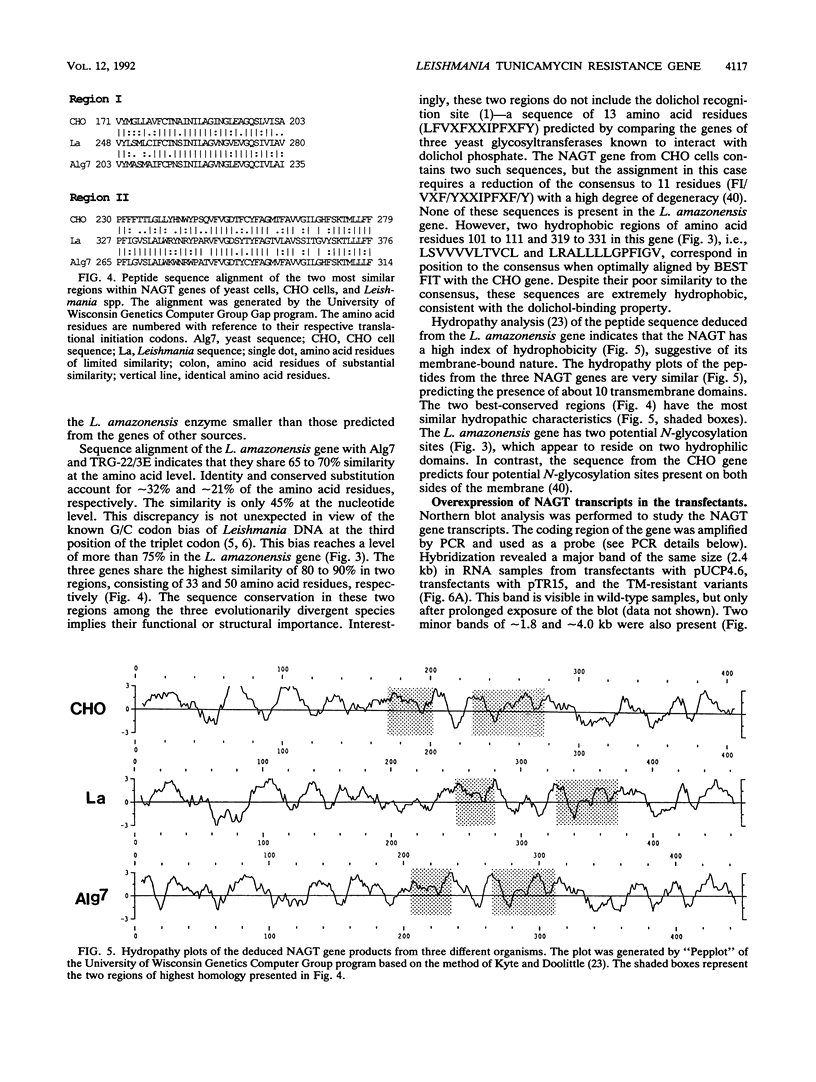
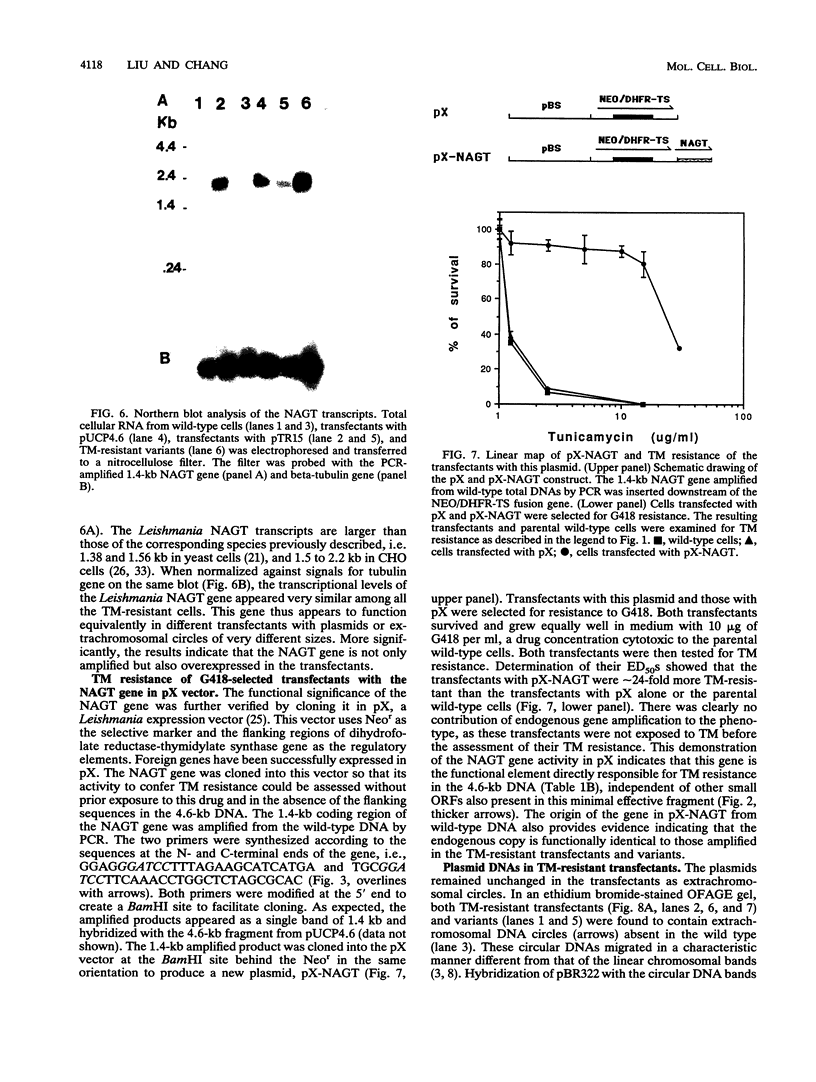
Tunicamycin (TM)-resistant Leishmania amazonensis has been found previously to contain amplified chromosomal DNA, existing exclusively as extrachromosomal circles of 63 kb. Fragments of this DNA cloned into plasmids were functionally analyzed by transfection of wild-type cells. A clone with a 15-kb fragment of the 63-kb circle was initially found to confer TM resistance. A library of the 15-kb fragment was then prepared and used in toto to transfect wild-type cells. The transfectants that emerged after selection were found to contain a plasmid with an insert of 4.6 kb. Evidence from deletion experiments suggests that this is the minimal transfection-effective fragment. Sequencing of the 4.6-kb DNA revealed 1.4-kb homolog of N-acetylglucosamine-1-phosphate transferase genes. The L. amazonensis gene is similar to those from two other sources in their deduced peptide sequence by 65 to 70% and in hydropathic characteristics. The L. amazonensis gene is amplified by more than 128-fold over the wild type and overproduces a major transcript of 2.4 kb in all transfectants. The endogenous copy of this gene was amplified by polymerase chain reaction from the wild type and cloned into pX-NEO, a Leishmania expression vector. Amplification of this plasmid in the transfectants by selection with G418 simultaneously made them resistant to TM. Evidence provided thus indicates that the 1.4-kb DNA is an N-acetylglucosamine-1-phosphate transferase gene whose amplification is responsible for TM resistance in Leishmania variants and transfectants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright C. F., Orlean P., Robbins P. W. A 13-amino acid peptide in three yeast glycosyltransferases may be involved in dolichol recognition. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7366–7369. doi: 10.1073/pnas.86.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- Button L. L., McMaster W. R. Molecular cloning of the major surface antigen of leishmania. J Exp Med. 1988 Feb 1;167(2):724–729. doi: 10.1084/jem.167.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Chaudhuri G., Chang K. P. A simple method for isolation of extrachromosomal circular DNA in unicellular eukaryotes. Nucleic Acids Res. 1988 Mar 25;16(5):2341–2341. doi: 10.1093/nar/16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Cruz A., Coburn C. M., Beverley S. M. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke S., Chaudhuri G., Kink J. A., Chang K. P. DNA amplification in tunicamycin-resistant Leishmania mexicana. Multicopies of a single 63-kilobase supercoiled molecule and their expression. J Biol Chem. 1988 Mar 5;263(7):3418–3424. [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Hartog K. O., Bishop B. Genomic sequence coding for tunicamycin resistance in yeast. Nucleic Acids Res. 1987 Apr 24;15(8):3627–3627. doi: 10.1093/nar/15.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K., Peng Y., Pithawalla R., Detke S., Chang K. P. Tunicamycin-resistant variants from five species of Leishmania contain amplified DNA in extrachromosomal circles of different sizes with a transcriptionally active homologous region. Mol Biochem Parasitol. 1991 Feb;44(2):233–243. doi: 10.1016/0166-6851(91)90009-u. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. Biological and biochemical characterization of tunicamycin-resistant Leishmania mexicana: mechanism of drug resistance and virulence. Infect Immun. 1987 Jul;55(7):1692–1700. doi: 10.1128/iai.55.7.1692-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. N-glycosylation as a biochemical basis for virulence in Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):181–190. doi: 10.1016/0166-6851(88)90037-0. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. Tunicamycin-resistant Leishmania mexicana amazonensis: expression of virulence associated with an increased activity of N-acetylglucosaminyltransferase and amplification of its presumptive gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1253–1257. doi: 10.1073/pnas.84.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Robbins P. W. Protein glycosylation in yeast: transcript heterogeneity of the ALG7 gene. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2145–2149. doi: 10.1073/pnas.84.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Tabuki T., Akiyama S., Mifune K., Takatsuki A., Tamura G., Ikehara Y. Isolation and characterization of Chinese hamster ovary cell mutants with altered sensitivity to high doses of tunicamycin. Somatic Cell Genet. 1981 Sep;7(5):507–521. doi: 10.1007/BF01549655. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Zhu X. Y., Khounlo S. Amplification and molecular cloning of the hamster tunicamycin-sensitive N-acetylglucosamine-1-phosphate transferase gene. The hamster and yeast enzymes share a common peptide sequence. J Biol Chem. 1988 Dec 25;263(36):19796–19803. [PubMed] [Google Scholar]
- Liu X., Chang K. P. Extrachromosomal genetic complementation of surface metalloproteinase (gp63)-deficient Leishmania increases their binding to macrophages. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4991–4995. doi: 10.1073/pnas.89.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Hettema E., Wüst D., Fase-Fowler F., Borst P. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 1991 Apr;10(4):1009–1016. doi: 10.1002/j.1460-2075.1991.tb08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Scocca J. R., Krag S. S. Sequence of a cDNA that specifies the uridine diphosphate N-acetyl-D-glucosamine:dolichol phosphate N-acetylglucosamine-1-phosphate transferase from Chinese hamster ovary cells. J Biol Chem. 1990 Nov 25;265(33):20621–20626. [PubMed] [Google Scholar]
- Shailubhai K., Dong-Yu B., Saxena E. S., Vijay I. K. Purification and characterization of UDP-N-acetyl-D-glucosamine:dolichol phosphate N-acetyl-D-glucosamine-1-phosphate transferase involved in the biosynthesis of asparagine-linked glycoproteins in the mammary gland. J Biol Chem. 1988 Nov 5;263(31):15964–15972. [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Sudo T., Onodera K. Isolation and characterization of tunicamycin resistant mutants from Chinese hamster ovary cells. J Cell Physiol. 1979 Oct;101(1):149–156. doi: 10.1002/jcp.1041010117. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Wilson K., Collart F. R., Huberman E., Stringer J. R., Ullman B. Amplification and molecular cloning of the IMP dehydrogenase gene of Leishmania donovani. J Biol Chem. 1991 Jan 25;266(3):1665–1671. [PubMed] [Google Scholar]
- Zhu X. Y., Lehrman M. A. Cloning, sequence, and expression of a cDNA encoding hamster UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1-phosphate transferase. J Biol Chem. 1990 Aug 25;265(24):14250–14255. [PubMed] [Google Scholar]