Abstract
Purpose of review
This article reviews current data on pathophysiologic mechanisms by which sleep-disordered breathing during pregnancy may cause harm, and explores biological pathways for associated adverse maternal and fetal outcomes, especially pregnancy-induced hypertension and gestational diabetes.
Recent findings
Accumulating data indicate that snoring and sleep apnea during pregnancy are likely to increase the risk for gestational hypertension and preeclampsia. Several new studies have observed that sleep-disordered breathing and short sleep duration also increase the risk of gestational diabetes, similar to observations in the general population. There are varying levels of emerging evidence for potential mechanisms, including oxidative stress, increased sympathetic activity and inflammation, adipokine levels and insulin resistance, linking sleep-disordered breathing events during pregnancy to adverse outcomes.
Summary
Sleep-disordered breathing and adverse maternal–fetal outcomes such as preeclampsia and gestational diabetes share a number of mechanistic pathways, and growing data in pregnant women indicate that snoring and sleep apnea increase the risk of these and other complications for both the mother and the fetus. Nevertheless, direct evidence of the pathophysiologic mechanisms by which sleep-disordered breathing during pregnancy exerts negative effects remains sparse.
Keywords: gestational diabetes, gestational hypertension, preeclampsia, sleep apnea, sleep-disordered breathing
Introduction
Sleep-disordered breathing (SDB) is a common sleep disturbance among pregnant women. The term SDB refers to the spectrum of breathing disorders during sleep, ranging from uncomplicated snoring to the most severe forms of SDB, obstructive sleep apnea (OSA) and the obesity–hypoventilation syndrome [1]. These breathing disturbances are characterized by repeated episodes of partial or complete upper airway obstruction during sleep and can result in disruption of normal ventilation, intermittent hypoxemia, and arousals from sleep [2,3]. Physiologic and hormonal changes occur during pregnancy that increase the likelihood of developing SDB and magnify its effects, including gestational weight gain, pregnancy-associated nasopharyngeal edema, decreased functional reserve capacity and increased arousals from sleep [4,5] (a review of diagnosis and management of pregnancy-associated SDB is beyond the scope of this paper, but is covered by these reviews).
During the third trimester, when gestational SDB is most likely to occur, the prevalence of habitual snoring has been estimated to be 10–27% of pregnant women [5]. The prevalence of OSA in pregnancy has not been systematically evaluated. There is growing evidence that SDB is associated with adverse pregnancy outcomes, especially pregnancy-induced hypertension and gestational diabetes [6–8]. This article reviews current data on pathophysiologic mechanisms by which SDB during pregnancy may cause harm, and explores biological pathways for associated adverse maternal and fetal outcomes.
Potential mechanisms for adverse perinatal outcomes
In the nonpregnant population, SDB and short sleep duration are strongly associated with diabetes [9,10], hypertension [11–15], and cardiovascular disease [16–18]. Similarly, in pregnant women, evidence suggests that snoring, sleep apnea and short sleep duration are likely to increase adverse outcomes, including gestational diabetes and preeclampsia [19–22]. Among pregnant women with OSA, chronic intermittent hypoxia and sleep fragmentation with sleep loss are thought to be key factors [3,9,19,23,24]. The primary affected domains include sympathetic activity, oxidative stress and inflammation, adipokines, and the hypothalamic–pituitary–adrenal (HPA) axis [3,23,25,26]. Most adverse pregnancy outcomes, aside from early pregnancy loss, emerge in the third trimester. Thus, sleep disturbances during the first two trimesters may potentially disrupt placental and fetal development.
Oxidative stress owing to intermittent hypoxia
During sleep, individuals with SDB experience recurrent episodes of intermittent hypoxia, alternating between hypoxia and reoxygenation. In animal studies, intermittent hypoxia leads to oxidative stress, inflammation, and reductions in antioxidant levels (reviewed by Lavie, [27]). Increased oxidative stress caused by intermittent hypoxia appears to play an important role in the mechanism for insulin resistance and thus, potentially, in the onset of gestational diabetes. For instance, mice exposed to intermittent hypoxia demonstrated increased pancreatic beta cell proliferation and cell death owing to oxidative stress [28].
There is also increasing evidence that preeclampsia is associated with increased oxidative stress owing to ischemia–reperfusion events and with reduced antioxidant defences [29]. During hypoxia/reperfusion, the initial response is an increase in the generation of reactive oxygen species. When the generation of reactive oxygen species generation exceeds antioxidant capacity, oxidative stress damages cells and tissues [27,30,31]. It is possible that, among pregnant women with SDB, intermittent hypoxia and abnormal sympathovagal balance contribute to the development of preeclampsia [30].
Sympathetic activity
Upper airway obstruction during sleep results in recurrent brief awakenings or microarousals that reduce slow wave and total sleep time and increase sympathetic activation, with effects carrying over into daytime [32,33]. Such increases in sympathetic activity may be responsible, at least in part, for acute blood pressure (BP) changes and glucose intolerance seen in patients with OSA [34]. Intermittent hypoxia in early gestation can also contribute to increased sympathetic activity [15,19,35]. As women with preeclampsia have been observed to have elevated levels of sympathetic activity [36], these data suggest that women with SDB and intermittent hypoxia during pregnancy may be at increased risk for preeclampsia.
Gestational hypertension and preeclampsia are characterized by vasoconstriction, which is due to increased sympathetic nerve activity. Sympathetically mediated vasoconstriction of skeletal muscle is markedly greater in preeclamptic women compared with normotensive pregnant women [36]. SDB events leading to increased sympathetic activity could, therefore, be a mechanism for vasoconstriction in some pregnant women.
Inflammation
Intermittent hypoxia induces increased oxidative stress and elevated levels of proinflammatory cytokines [37]. Once inflammation occurs, it can trigger further oxidative stress [38,39]. In an animal model, both oxygen desaturations and respiratory efforts contributed to systemic inflammation; hypoxia–reoxygenation also induced endothelial dysfunction [40].
Habitual poor sleep quality or shortened sleep duration owing to gestational SDB may also contribute to increased inflammation. Poor sleep quality and continuity were associated with elevated C-reactive protein levels in healthy young women even after controlling for covariates [41•]. Furthermore, higher levels of circulating tumor necrosis factor α (TNF-α) were observed in mothers with subjective sleep complaints during the third trimester [42]. Short sleep duration and poor sleep efficiency, common features of sleep in patients with SDB, have been associated with higher levels of interleukin-6 (IL-6), but not TNF-α, in mid and late pregnancy [43]. This is important because IL-6 has been reported to be involved in the pathogenesis of insulin resistance and type 2 diabetes [44].
Increased inflammation has been associated with adverse pregnancy outcomes [45–48], and increased IL-6, TNF-α, C-reactive protein levels and leukocyte counts are reported in gestational diabetes mellitus (GDM) [49], preeclampsia [50], intrauterine growth retardation [51], and preterm delivery [52]. Thus, the inflammatory response induced by SDB could provide a biological pathway between SDB and adverse pregnancy outcomes.
Changes in the HPA axis
Hypoxia and sleep fragmentation caused by SDB can lead to increased secretion of plasma cortisol [25,26] and abnormalities in insulin and glucose metabolism [53,54]. Inflammatory responses are also regulated by the HPA axis through cortisol secretion. Proinflammatory cytokines can trigger the HPA axis to produce more cortisol [55], shutting down the inflammatory response [56,57]. Continual cortisol secretion decreases glucocorticoid receptor sensitivity, weakening cortisol’s suppressive effects [56]. Thus, intermittent hypoxia, disrupted sleep and inflammation can lead to chronic HPA activation, causing decreased glucocorticoid sensitivity and increased inflammatory responses [19].
Changes in adipokines
OSA potentially affects adipokine levels through oxidative stress [58,59], inflammation [60], and sympathetic activation [61]. Abnormalities in levels of leptin, an adipokine that regulates body weight through the control of appetite and energy expenditure, have recently been associated with adverse effects on weight control, cardiovascular health and glucose regulation [3]. However, in a study of pregnant women with severe preeclampsia, leptin levels were highly correlated with BMI, but not with the subsequent onset of preeclampsia [62]. Thus, although intermittent hypoxia may affect leptin levels in pregnant women with SDB, leptin may not play a direct role in the pathogenesis of preeclampsia, and its association with obesity must be considered.
Adiponectin is an active polypeptide hormone with insulin-sensitizing, antiatherogenic, and anti-inflammatory properties that is secreted by adipose tissue [63]. Oxidative stress, TNF-α, and IL-6 all reduce adiponectin production, and levels are lower in patients with OSA, GDM, hypertension, coronary artery disease, and type 2 diabetes compared with normal individuals [63,64]. Although plasma adiponectin is an independent determinant of homeostasis model assessment of insulin resistance in OSA patients, it is more closely related to obesity than to sleep apnea [63]. Whether adiponectin levels are lower among pregnant women with OSA than normal pregnant women and how this affects women’s risk for GDM bears further investigation.
Insulin resistance
Insulin resistance has been reported to contribute to the onset of both diabetes and hypertension [10–12]. It is associated with short sleep duration and can occur in the setting of SDB due to increased sympathetic nervous activity [65,66]. Insulin resistance increases in normal pregnancy [64]. This physiological insulin resistance is not accompanied by a rise in overall sympathetic activity, but there is evidence of moderate sympathetic overactivity in muscle and the heart [67]. In women with GDM, studies in skeletal muscle and adipose tissue demonstrate additional defects in insulin signaling [64].
In the nonpregnant state, increased sympathetic nervous system activation owing to arousals is thought to contribute to the increased prevalence of diabetes in patients with SDB [9]. When the sleep of normal volunteers was fragmented for two nights using auditory and mechanical stimuli, a 20–25% decrease in insulin sensitivity and glucose effectiveness (the effect of glucose on its own disposal independent of insulin response) and increases in morning cortisol and sympathetic activity were seen [68••]. Punjabi and Beamer [10] also described lower insulin sensitivity among individuals with OSA compared with nonapneic controls, suggesting that obstructive events are associated with impaired insulin sensitivity and pancreatic beta cell dysfunction. If similar events occur in the gravid state, microarousals and intermittent hypoxemia in pregnant women with sleep disruption could lead to alterations in glucose metabolism that increase the risk of gestational diabetes.
Preeclampsia is a state of increased insulin resistance and sympathetic overactivity. Insulin resistance is associated with microvascular dysfunction, and inflammation has also been noted to be involved in the onset of hypertension, coronary artery disease and preeclampsia [69,70]. As insulin resistance, sympathetic overactivity and inflammation are all commonly observed in SDB [69], snoring or sleep apnea during pregnancy may potentially precipitate or exacerbate events associated with the development of preeclampsia.
Adverse pregnancy outcomes: possible biological pathways
SDB during pregnancy may be associated with adverse pregnancy outcomes via the mechanisms outlined in Fig. 1. Recently, a number of studies have examined the relationship between SDB and gestational diabetes [71••,72••]. Both new and older studies have demonstrated that SDB may increase the risk for the hypertensive disorders of pregnancy [6,73•]. Studies have also examined the relationship between SDB and other adverse outcomes including fetal outcomes and preterm labor [6,74]. Possible biological pathways for the development of these outcomes and supporting evidence of involvement of these mechanisms are reviewed.
Figure 1. Possible pathways for impact of sleep-disordered breathing on maternal and fetal outcomes.
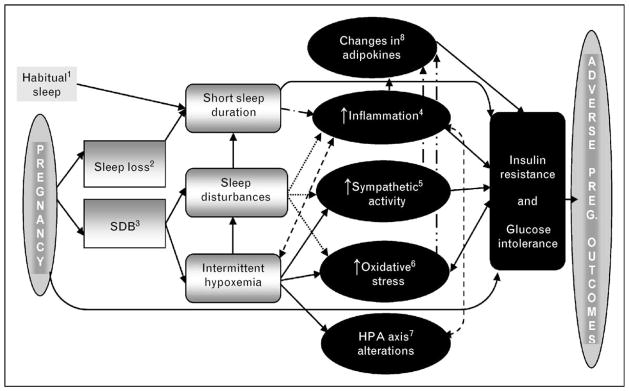
We propose that a number of causal pathways may link sleep during pregnancy with adverse pregnancy outcomes. 1) In the present model, short sleep duration (as a trait factor) may be a predisposing factor for adverse pregnancy outcomes. 2) Sleep loss may contribute to sleep disturbances and reduce sleep duration. 3) SDB can cause repeated arousal from sleep and intermittent hypoxemia. It should be noted that each of these phenomena (2 and 3) is probably not directly related to the various aspects of sleep disturbance, but instead has its own mediators and moderators. For the sake of simplicity, these are not contained in the schematic and include, but are not limited to, pregnancy-related physical changes affecting the respiratory system; pregnancy-related weight gain; and pregnancy-related changes in gonadotrophic hormones. 4) Short sleep duration due to sleep habits or sleep fragmentation and intermittent hypoxemia during pregnancy are associated with increased inflammatory cytokines such as tumor necrosis factor a, interleukin 6 and so on. Other predisposing factors, such as obesity and neuroendocrine changes, may also contribute to sleep disturbances and/or increased inflammation. 5) Arousals and nocturnal hypoxia due to complete or partial upper airway obstruction during sleep are linked with a reduction in both total sleep time and increased sympathetic activation. Elevated sympathetic activity is likely to be directly responsible, at least in part, for the glucose dysregulation and preeclampsia. 6) Increased oxidative stress due to the cyclical nature of hypoxia–reoxygenation has been shown to be an important mechanism for insulin resistance, the onset of diabetes and preeclampsia. 7) Intermittent hypoxia, disrupted sleep and inflammation lead to chronic HPA activation, which, in turn, causes decreased glucocorticoid sensitivity and increased inflammatory responses. 8) Adipokine levels are affected by oxidative stress, inflammation and sympathetic activation seen in SDB and may play a role in the pathogenesis of gestational diabetes mellitus and preeclampsia. These maternal metabolic and hormonal alterations associated with SDB may be implicated in the pathogenesis of adverse maternal and fetal complications. It should be noted that each of these phenomena (4, 5, 6, 7 and 8) is not likely directly related to the various adverse outcomes, but instead has its own mediators and moderators. HPA, hypothalamic–pituitary–adrenal; SDB, sleep-disordered breathing.
Glucose dysregulation
Glucose dysregulation, which spans a continuum from milder abnormal glucose tolerance to GDM [75,76•,77], has been estimated to occur in 1–14% of pregnancies [75,77–79]. GDM and even mild maternal hyperglycemia without GDM are associated with increased risks for maternal and fetal complications such as preeclampsia and fetal growth retardation, and future diseases such as type 2 diabetes, obesity and cardiovascular disease in both mothers and children [76•,78–81].
Numerous epidemiological, clinical and experimental studies in the general population have reported that snoring and OSA are associated with increased risk of type 2 diabetes, glucose intolerance and insulin resistance independent of obesity and other risk factors [10,65,68••,82–84]. Preliminary studies have examined whether there is an analogous relationship in pregnancy between SDB and glucose intolerance or gestational diabetes. Several survey studies have reported that pregnant snorers are 2–7 times more likely to develop gestational diabetes than nonsnoring women [71••,72••,73•]. Additionally, we recently demonstrated that moderate OSA (Apnea–Hypopnea Index>15) is associated with maternal hyperglycemia [22]. These findings suggest that OSA and self-reported habitual snoring are associated with an increased risk of gestational diabetes. However, the underlying mechanism is not clear.
Findings from studies in the general population show that poor-quality sleep and sleep loss can also increase the risk of obesity and diabetes by impairing glucose regulation and the neuroendocrine control of appetite [3,65]. Preliminary studies in pregnant women have observed that short sleep duration (≤4 vs. >9 h or <7 vs. >7 h) is associated with increased risk of GDM independent of other risk factors [71••,72••]. We recently observed that napping, which can reflect nocturnal sleep loss, is associated with higher maternal glucose levels [22].
These data suggest that hypoxia and sleep loss due to SDB may play a role in the development of gestational diabetes. Hypothetically, disturbed sleep/wake cycles or sleep at unusual times may also cause hormones that regulate glucose metabolism and appetite to fluctuate excessively, disrupting the sympathetic–parasympathetic balance.
Among the potential mechanisms for the relationship between SDB and GDM, at present, there is only limited evidence of a direct relationship between GDM and oxidative stress. The release from placental tissue of 8-isoprostane, a biomarker for oxidative stress [85], is greater in women with GDM compared with healthy pregnant women [86] and the response of placental tissue to oxidative stress in women with GDM is reduced [87]. Release of 8-isoprostane from incubated placenta, adipose, and skeletal muscle tissues is also higher in women with GDM compared with pregnant controls [88].
Recent studies demonstrate that low circulating levels of the insulin-sensitizing protein adiponectin and higher serum concentrations of the inflammatory biomarker C-reactive protein may be associated with a higher risk of GDM [89–92]. Lower levels of adiponectin, Th1 cytokines, and inflammatory cytokines such as IL-6, IL-10 and TNF-α have been observed in mothers with GDM compared with controls [93] and, as reviewed above, in individuals with obstructive sleep apnea. However, the relationship between SDB, cytokines and GDM in pregnant women has not been elucidated.
In summary, the limited available data suggest that the mechanisms of injury associated with SDB, including oxidative stress, inflammation and sympathetic activation, are also important in the pathogenesis of GDM. The contribution of specific oxidative stress and inflammatory pathways to alterations in glucose metabolism in pregnancy remains to be investigated.
Pregnancy-induced hypertension
Pregnancy-induced hypertension is usually defined as repeated BP recordings of more than 140/90 mmHg, diagnosed after 20 weeks’ gestation in previously normotensive women [94]. When unaccompanied by proteinuria, this condition is called gestational hypertension, but if it includes proteinuria it is called preeclampsia [94]. Pregnancy-induced hypertension is associated with maternal and neonatal morbidity and mortality, including future hypertension, metabolic syndrome, stroke, and premature cardiovascular death [95].
Snoring during pregnancy has been independently associated with an increased risk of developing pregnancy-induced hypertension [6,73•,96•,97,98]. Women with preeclampsia have also been observed to have smaller upper airways [7], which are associated with SDB and may be due to pharyngeal edema.
The pathophysiology of preeclampsia is multifactorial. Oxidative stress is likely a key factor. Endothelial dysfunction or inappropriate endothelial cell activation is the most common clinical manifestation in preeclampsia [99]. Reactive oxygen species, which have been implicated in preeclampsia [100], have been observed in some but not all studies to be released during intermittent hypoxia events [27,101] and are likely involved in endothelial cell activation [102]. Both SDB and endothelial dysfunction occur more frequently in women with preeclampsia than those with uncomplicated pregnancies [8], suggesting that intermittent hypoxia may cause oxidative stress, leading to endothelial dysfunction as is seen in preeclampsia.
The increased levels of proinflammatory cytokines that occur in SDB are another important potential contributor to the causal pathway in preeclampsia. The oxidative stress associated with SDB can increase inflammatory cytokines and activation of endothelial cells, leading to endothelial dysfunction and cardiovascular disease [103]. Similarly, the normal maternal inflammatory response to pregnancy [95] could be exacerbated by SDB, predisposing pregnant women to preeclampsia. Plasma concentrations of cytokines including C-reactive protein, IL-6, TNF-α, and 8-isoprostane have all been found to be significantly higher in women with preeclampsia than in healthy pregnant women [104•]. Inflammatory cytokines such as TNF-α are also important in the pathogenesis of preeclampsia, as they interfere with trophoblast implantation [105,106], which has been observed in preeclamptic pregnancies [107]. However, whether levels of inflammatory cytokines are higher in women with SDB during pregnancy and are associated with a greater risk of preeclampsia independent of other risk factors is unknown.
Preeclampsia is characterized by a marked increase in peripheral vascular resistance, leading to elevated BP [95]. Among pregnant women with SDB, increased sympathetic vasoconstrictor activity owing to hypoxemia and arousals during sleep may cause increases in peripheral vascular reactivity. Such increases, in turn, could lead to elevations in systemic arterial BP and reductions in maternal cardiac output accompanying compromises in uteroplacental blood flow, as is observed in preeclampsia [108–110].
Fetal complications
Maternal sleep is important for fetal well-being, because uteroplacental blood flow and the secretion of neurohormones, especially growth hormones, are at their peak during sleep [109,111]. Women residents, working long hours compared with controls, and presumably sleeping less, had higher rates of preterm labor (11 vs. 6%) and preeclampsia (8.8 vs. 3.5%). Residents who worked 100 h or more per week during the first trimester also had a 9.8% risk of preterm delivery, compared with 4.6% for residents who worked less [112]. In case series and mostly small studies, SDB has been associated with increased risks of premature delivery, intrauterine growth restriction, lower infant Apgar scores and even infant mortality [4,6,21,109,113••].
The maternal hormonal and metabolic alterations observed in SDB can negatively impact the uterine environment. Pregnant women with SDB can develop hypoxemia during sleep owing to decreased cardiorespiratory reserve. Even small declines in maternal oxygenation can endanger oxygen delivery to the fetus. Furthermore, any cause of maternal hypercapnia leads quickly to fetal respiratory acidosis. Fetal heart decelerations and accompanying acidosis have been recorded during maternal apneic episodes with oxygen desaturation [4,114,115]. Nevertheless, although lower infant Apgar scores and higher rates of intrauterine growth restriction have been observed among snorers compared with nonsnorers [6], other studies found no association between maternal snoring and infant birth weight [116,117].
In an animal model, reductions in fetal growth associated with maternal intermittent hypoxia were readily reversible after birth [118]. However, the pups demonstrated sustained postnatal alterations in respiratory control, leading to speculation that similar changes in humans could place them at risk for conditions such as sudden infant death syndrome. Studies in humans have not tested this hypothesis.
Fewer fetal movements have been observed in preeclamptic women with SDB, though they were compared with women with normal pregnancies rather than other preeclamptic women [74]. Case studies have reported that, when women with SDB received treatment before the third trimester, they delivered infants with normal birth weight and high Apgar scores [4,98,119]. However, causality has not been established.
Other pregnancy complications
In a retrospective cohort study, women with OSA were more likely to have preterm and cesarean deliveries, probably owing to maternal comorbidities [113••]. Snoring women have also been reported to be more likely to require an emergency cesarean delivery than nonsnorers [73•,120].
Conclusion
In summary, many of the pathways via which SDB causes injury leading to increased risks for hypertension, cardiovascular disease and diabetes mellitus in the general population have also been implicated in the pathogenesis of preeclampsia, GDM and other adverse outcomes in the pregnant population. Although evidence of increased risks for preeclampsia and GDM among pregnant women is accumulating, data that explicitly examine the impact of gestational SDB on these mechanistic pathways –oxidative stress, increased sympathetic activity, inflammation, change in the HPA axis, adipokines and insulin resistance – and links them to pregnancy outcomes remain sparse. The need exists for additional studies to promote an improved understanding of how SDB during pregnancy promotes adverse maternal–fetal outcomes, the magnitude of these effects, and interventions that effectively mitigate increased risk.
Acknowledgments
The authors would like to thank Michael L. Perlis, PhD and Michael A. Grandner, PhD for their helpful suggestions regarding Fig. 1.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Izci B, Lee K. Sleep disturbances during pregnancy. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5. Philadelphia: Saunders; 2010. (forthcoming) [Google Scholar]
- 2.Maski KP, Kothare SV. Searching for marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2009;32:137. doi: 10.1093/sleep/32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnardottir ES, Mackiewicz M, Gislason T, et al. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izci-Balserak B. Sleep-disordered breathing in pregnancy. Int J Sleep Wakefulness. 2008;1:98–108. [Google Scholar]
- 5.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 6.Franklin KA, Holmgren PA, Jonsson F, et al. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–141. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and preeclampsia. Am J Respir Crit Care Med. 2003;167:137–140. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 8.Yinon D, Lowenstein L, Suraya S, et al. Preeclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–333. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 12.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips CL, Cistulli PA. Obstructive sleep apnea and hypertension: epidemiology, mechanisms and treatment effects. Minerva Medica. 2006;97:299–312. [PubMed] [Google Scholar]
- 16.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–415. [PMC free article] [PubMed] [Google Scholar]
- 17.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 18.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 19.Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv. 2009;64:273–280. doi: 10.1097/OGX.0b013e318195160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly G, Razak AR, Hayanga A, et al. Inspiratory flow limitation during sleep in preeclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18:672–676. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C, Palombini L, Poyares D, et al. Preeclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for preeclampsia: preliminary findings. Sleep Med. 2007;9:9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Izci-Balserak B, Ratcliffe S, Pien G. SDB and daytime napping are associated with higher glucose levels in pregnant women. Sleep. 2010;33:A119. (Abstract 0339) [Google Scholar]
- 23.Shaw JE, Punjabi NM, Wilding JP, et al. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Arnaud C, Dematteis M, Pepin JL, et al. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin Immunopathol. 2009;31:113–125. doi: 10.1007/s00281-009-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Follenius M, Brandenberger G, Bandesapt JJ, et al. Nocturnal cortisol release in relation to sleep structure. Sleep. 1992;15:21–27. doi: 10.1093/sleep/15.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L. Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46:783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Roberts JM, Lain KY. Recent insights into the pathogenesis of preeclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 30.Jerath R, Barnes VA, Fadel HE. Mechanism of development of preeclampsia linking breathing disorders to endothelial dysfunction. Med Hypotheses. 2009;73:163–166. doi: 10.1016/j.mehy.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JM, Hubel CA. Oxidative stress in preeclampsia. Am J Obstet Gynecol. 2004;190:1177–1178. doi: 10.1016/j.ajog.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000;119:189–197. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 35.Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-h blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 36.Schobel HP, Fischer T, Heuszer K, et al. Preeclampsia: a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 37.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 38.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- 39.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Nacher M, Farre R, Montserrat JM, et al. Biological consequences of oxygen desaturation and respiratory effort in an acute animal model of obstructive sleep apnea (OSA) Sleep Med. 2009;10:892–897. doi: 10.1016/j.sleep.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 41•.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–354. doi: 10.1016/j.bbi.2008.10.008. Researchers reported that poor sleep quality in young women was associated with higher C-reactive protein levels after controlling for covariates. They suggested that continual poor sleep, from young adulthood to middle age, may increase chronic, low-grade inflammation and the risk for inflammatory-related illnesses in future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reprod Immunol. 2007;73:158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–567. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 45.Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82:1099–1102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 46.Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 47.Holcberg G, Huleihel M, Sapir O, et al. Increased production of tumor necrosis factor-alpha TNF-alpha by IUGR human placentae. Eur J Obstet Gynecol Reprod Biol. 2001;94:69–72. doi: 10.1016/s0301-2115(00)00321-3. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Espinoza J, Goncalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf M, Sauk J, Shah A, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27:21–27. doi: 10.2337/diacare.27.1.21. [DOI] [PubMed] [Google Scholar]
- 50.Teran E, Escudero C, Moya W, et al. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with preeclampsia. Int J Gynaecol Obstet. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 51.Laskowska M, Leszczynska-Gorzelak B, Laskowska K, Oleszczuk J. Evaluation of maternal and umbilical serum TNFalpha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J Matern Fetal Neonatal Med. 2006;19:347–351. doi: 10.1080/14767050600637937. [DOI] [PubMed] [Google Scholar]
- 52.Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006;56:112–118. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 53.Larsen JJ, Hansen JM, Olsen NV, et al. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol. 1997;504 (Pt 1):241–249. doi: 10.1111/j.1469-7793.1997.241bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 55.Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann N Y Acad Sci. 2007;1113:311–324. doi: 10.1196/annals.1391.026. [DOI] [PubMed] [Google Scholar]
- 56.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 57.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares AF, Guichardant M, Cozzone D, et al. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–889. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto T, Kihara M, Imai N, et al. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol. 2007;171:1705–1712. doi: 10.2353/ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 61.Lam JC, Xu A, Tam S, et al. Hypoadiponectinemia is related to sympathetic activation and severity of obstructive sleep apnea. Sleep. 2008;31:1721–1727. doi: 10.1093/sleep/31.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salomon LJ, Benattar C, Audibert F, et al. Severe preeclampsia is associated with high inhibin A levels and normal leptin levels at 7 to 13 weeks into pregnancy. Am J Obstet Gynecol. 2003;189:1517–1522. doi: 10.1016/s0002-9378(03)00902-5. [DOI] [PubMed] [Google Scholar]
- 63.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64:12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 64.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50:938–948. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 65.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 66.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 67.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24:131–141. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 68••.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. In this study, sleep fragmentation across all stages of sleep causing electroencephalography microarousals was associated with a 20–25% reduction in glucose effectiveness and insulin sensitivity. Sleep fragmentation also caused alterations in sympathovagal balance and high-morning levels of serum cortisol. This study expands current knowledge by showing that, independent of sleep duration, nonspecific fragmentation of sleep can adversely affect insulin and glucose metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 70.Kaaja R, Tikkanen MJ, Viinikka L, Ylikorkala O. Serum lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynecol. 1995;85:353–356. doi: 10.1016/0029-7844(94)00380-V. [DOI] [PubMed] [Google Scholar]
- 71••.Facco FL, Grobman WA, Kramer J, et al. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142.e1–142.e5. doi: 10.1016/j.ajog.2010.03.041. This prospective cohort study showed that self-reported short sleep duration (sleeping <7 h) and snoring were associated with impaired glucose tolerance and GDM in healthy nulliparous women after controlling for potential confounders. This study is one of the first studies to examine these associations in pregnant women. The prospective design is the strength of this study, but it may be limited by ascertainment bias and misclassification bias, as well as wide confidence intervals in estimation of increased risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Qiu C, Enquobahrie D, Frederick IO, et al. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. This study demonstrated an association between short sleep duration (sleeping ≤4 h compared with those sleeping 9 h per night) and snoring in pregnant women with glucose intolerance and GDM after adjusting for maternal age and race/ethnicity. This is one of the first studies to investigate the association between short sleep duration, snoring and glucose dysregulation in pregnancy. Investigators collected information on sleep duration and snoring in early pregnancy. The results of this study suggest that habitual short sleep duration and snoring precede the clinical diagnosis of GDM. However, habitual sleep duration and snoring were self-reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73 •.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Sleep disordered breathing symptoms in pregnancy and adverse pregnancy and fetal outcomes. Eur Respir J. 2010 doi: 10.1183/09031936.00021810. [Epub ahead of print] This cross-sectional survey of randomly selected women in the immediate post-partum period reported that symptoms of SDB are associated with a higher likelihood of gestational hypertensive disorders, gestational diabetes and unplanned cesarean sections after controlling for confounders including age and BMI. Results from this study illustrate the need for further studies to assess the mechanisms for the association between SDB and adverse pregnancy outcomes. [DOI] [PubMed] [Google Scholar]
- 74.Edwards N, Blyton D, Sullivan C. Fetal movement is suppressed during maternal sleep in preeclampsia. Sleep. 2007;30:A321. (Abstract 0939) [Google Scholar]
- 75.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30 (Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 76•.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181:371–376. doi: 10.1503/cmaj.090569. This large retrospective population-based cohort study found that women with mild glucose intolerance in the absence of gestational diabetes may be at increased risk of subsequent cardiovascular disease. These findings also suggest that the vascular risk among women with gestational diabetes is much higher than among women with mild glucose intolerance during pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201:61.e1–61.e7. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tovar A, Must A, Bermudez OI, et al. The impact of gestational weight gain and diet on abnormal glucose tolerance during pregnancy in Hispanic women. Matern Child Health J. 2009;13:520–530. doi: 10.1007/s10995-008-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–228. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 80.Dang K, Homko C, Reece EA. Factors associated with fetal macrosomia in offspring of gestational diabetic women. J Matern Fetal Med. 2000;9:114–117. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<114::AID-MFM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 81.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 82.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 83.Renko AK, Hiltunen L, Laakso M, et al. The relationship of glucose tolerance to sleep disorders and daytime sleepiness. Diabetes Res Clin Pract. 2005;67:84–91. doi: 10.1016/j.diabres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Yaggi HK, Araujo AB, McKinlay JB, et al. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 85.Carpagnano GF, Karitonov SA, Resta O, et al. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 86.Coughlan MT, Vervaart PP, Permezel M, et al. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 87.Coughlan MT, Permezel M, Georgiou HM, Rice GE. Repression of oxidant-induced nuclear factor-kappaB activity mediates placental cytokine responses in gestational diabetes. J Clin Endocrinol Metab. 2004;89:3585–3594. doi: 10.1210/jc.2003-031953. [DOI] [PubMed] [Google Scholar]
- 88.Lappas M, Permezel M, Rice GE. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:5627–5633. doi: 10.1210/jc.2003-032097. [DOI] [PubMed] [Google Scholar]
- 89.Retnakaran R, Hanley AJ, Raif N, et al. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 90.Williams MA, Qiu C, Muy-Rivera M, et al. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 91.Wolf M, Sandler L, Hsu K, et al. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26:819–824. doi: 10.2337/diacare.26.3.819. [DOI] [PubMed] [Google Scholar]
- 92.Qiu C, Sorensen TK, Luthy DA, Williams MA. A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatr Perinat Epidemiol. 2004;18:377–384. doi: 10.1111/j.1365-3016.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 93.Ategbo JM, Grissa O, Yessoufou A, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiologic Reviews. 1997;19:218–232. doi: 10.1093/oxfordjournals.epirev.a017954. [DOI] [PubMed] [Google Scholar]
- 95.Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 96•.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33:559–565. doi: 10.1183/09031936.00122607. This case–control study using polysomnography to ascertain OSA reported a strong association between gestational hypertension and OSA, after adjustment for confounders such as maternal age, gestational age and BMI in different severity of OSA. [DOI] [PubMed] [Google Scholar]
- 97.Ursavas A, Karadag M, Rodoplu E, et al. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007;74:525–532. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 98.Poyares D, Guilleminault C, Hachul H, et al. Preeclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 99.Cudihy D, Lee RV. The pathophysiology of preeclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 100.Zhang C, Williams MA, Sanchez SE, et al. Plasma concentrations of carotenoids, retinol, and tocopherols in preeclamptic and normotensive pregnant women. Am J Epidemiol. 2001;153:572–580. doi: 10.1093/aje/153.6.572. [DOI] [PubMed] [Google Scholar]
- 101.Grabska-Kobylecka I, Kobylecki A, Bialasiewicz P, et al. No evidence of enhanced oxidant production in blood obtained from patients with obstructive sleep apnea. J Negat Results Biomed. 2008;7:10. doi: 10.1186/1477-5751-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palan PR, Mikhail MS, Romney SL. Placental and serum levels of carotenoids in preeclampsia. Obstet Gynecol. 2001;98:459–462. doi: 10.1016/s0029-7844(01)01437-5. [DOI] [PubMed] [Google Scholar]
- 103.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 2005;23:1059–1075. doi: 10.1016/j.ncl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 104•.Ouyang YQ, Li SJ, Zhang Q, et al. Interactions between inflammatory and oxidative stress in preeclampsia. Hypertens Pregnancy. 2009;28:56–62. doi: 10.1080/10641950802233064. The findings of this study indicate that oxidative stress and inflammatory reaction are strongly associated with preeclampsia. These findings provide a pathway via which oxidative stress induced by SDB may precipitate or exacerbate preeclampsia. [DOI] [PubMed] [Google Scholar]
- 105.Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–444. doi: 10.1055/s-2007-991041. [DOI] [PubMed] [Google Scholar]
- 106.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 107.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 108.Edwards N, Blyton DM, Kirjavainen T, et al. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–257. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 109.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 110.Edwards N, Blyton DM, Kirjavainen TT, Sullivan CE. Hemodynamic responses to obstructive respiratory events during sleep are augmented in women with preeclampsia. Am J Hypertens. 2001;14:1090–1095. doi: 10.1016/s0895-7061(01)02190-2. [DOI] [PubMed] [Google Scholar]
- 111.Blyton DM, Sullivan CE, Edwards N. Lactation is associated with an increase in slow-wave sleep in women. J Sleep Res. 2002;11:297–303. doi: 10.1046/j.1365-2869.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 112.Klebanoff MA, Shiono PH, Rhoads GG. Outcomes of pregnancy in a national sample of resident physicians. N Engl J Med. 1990;323:1040–1045. doi: 10.1056/NEJM199010113231506. [DOI] [PubMed] [Google Scholar]
- 113••.Louis JM, Auckley D, Sokol RJ, et al. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261.e1–261.e5. doi: 10.1016/j.ajog.2009.10.867. This retrospective cohort study observed that women with OSA are at increased risk of developing preterm delivery and preeclampsia. Thus, this study implicates OSA as a potential contributor to maternal and neonatal morbidities. [DOI] [PubMed] [Google Scholar]
- 114.Roush SF, Bell L. Obstructive sleep apnea in pregnancy. J Am Board Fam Pract. 2004;17:292–294. doi: 10.3122/jabfm.17.4.292. [DOI] [PubMed] [Google Scholar]
- 115.Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet. 2008;100:141–146. doi: 10.1016/j.ijgo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Tremblay K, Bullough A, O’Brien L. Habitual snoring is not associated with lower infant birth weight. Sleep. 2009;32:A334. (Abstract 1025) [Google Scholar]
- 117.Hedman C, Pohjasvaara T, Tolonen U, et al. Effects of pregnancy on mothers’ sleep. Sleep Medicine. 2002;3:37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 118.Gozal D, Reeves SR, Row BW, et al. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–1547. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- 119.Hastie SJ, Prowse K, Perks WH, et al. Obstructive sleep apnoea during pregnancy requiring tracheostomy. Aust N Z J Obstet Gynaecol. 1989;29:365–367. doi: 10.1111/j.1479-828x.1989.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 120.Leung PL, Hui DS, Leung TN, et al. Sleep disturbances in Chinese pregnant women. BJOG. 2005;112:1568–1571. doi: 10.1111/j.1471-0528.2005.00737.x. [DOI] [PubMed] [Google Scholar]


