Abstract
Although the main regulators of leukocyte trafficking are chemokines, another family of chemotactic agents is cyclophilins. Intracellular cyclophilins function as peptidyl-protyl cis-trans isomerases and are targets of the immunosuppressive drug, cyclosporine A (CsA). Cyclophilins can also be secreted in response to stress factors, with elevated levels of extracellular cyclophilins detected in several inflammatory diseases. Extracellular cyclophilins are known to have potent chemotactic properties, suggesting they might contribute to inflammatory responses by recruiting leukocytes into tissues. The objective of the current study was to determine the impact of blocking cyclophilin activity using a cell-impermeable derivative of CsA, MM218, to specifically target extracellular pools of cyclophilins. We show that treatment with this compound in a mouse model of allergic lung inflammation: 1) demonstrates up to 80% reduction in inflammation, 2) directly inhibits the recruitment of antigen-specific CD4+ T cells, and 3) works equally well when delivered at 100-fold lower doses to the airways. Our findings suggest that cell-impermeable analogs of CsA can effectively reduce inflammatory responses by targeting leukocyte recruitment mediated by extracellular cyclophilins. Specifically blocking the extracellular function(s) of cyclophilins may provide a novel approach for inhibiting the recruitment of one of the principal immune regulators of allergic lung inflammation, antigen-specific CD4+ T cells, into inflamed airways and lungs.
Introduction
Chemokines are critical factors responsible for the recruitment and migration of leukocytes from the circulation into inflamed tissues. Several different members of the CC chemokine family have been described as playing critical roles in allergic lung inflammation and pathology by initiating and promoting the recruitment of eosinophils and T lymphocytes to lung tissues and airways following allergen challenge (1). In an effort to reduce allergic disease pathology, many studies have focused on intervention techniques that specifically target chemokines or chemokine receptors on the cell-surface of pro-inflammatory leukocyte subsets. This approach is made difficult due to the multiple contributing factors that are released, the redundancy of chemokines, and the promiscuous nature of associations between chemokines and chemokine receptors.
Cyclophilins are ubiquitously distributed intracellular proteins found in all eukaryotic cells. This family of proteins is best known to function in protein folding as peptidyl-prolyl cis-trans isomerases (PPIases) (2). However, there is also evidence that cyclophilins function intracellularly in several other capacities, including signal transduction, protein trafficking and chaperoning, apoptosis, and human immunodeficiency virus (HIV) infection (3–5). Cyclophilins can also be secreted (6, 7) and have been demonstrated to function in an extracellular capacity as potent chemoattractants for various leukocyte subsets (8–10), via their PPIase active site (9). High levels of extracellular cyclophilins, notably cyclophilins A (CypA) and B (CypB), have been observed in patients with ongoing inflammatory diseases. For example, elevated levels of extracellular cyclophilins have been detected in the synovial fluid of patients with rheumatoid arthritis (11), in tissue from individuals with vascular smooth muscle cell disease (12), and in the serum of patients with severe sepsis (13). Following from these observations, we have previously proposed that the presence of extracellular cyclophilins in inflamed tissues may contribute to inflammatory processes via their capacity to induce leukocyte chemoattraction (14).
CypA is well established as the primary intracellular protein target for the immunosuppressive drug, Cyclosporine A (CsA) (15), which is widely administered to patients to prevent allograft rejection. When bound to CypA, the CsA acts to inhibit the T cell receptor (TCR)-dependent activation pathway and production of IL-2 in T lymphocytes. Importantly, CsA has been shown to inhibit the enzymatic activity of many mammalian cyclophilins in the nanomolar range of Ki values (16–18). Therefore, any function of cyclophilins requiring the catalytic site, including chemotaxis, is blocked by CsA. In the current studies we exploited the CsA/CypA interaction and utilized analogs of CsA to target cyclophilin activity and investigate the potential to reduce inflammatory responses in vivo in a mouse model of allergic lung inflammation. Importantly, we made use of CsA analogs that were non-immunosuppressive so as not to affect T cell activation. The non-immunosuppressive cell-permeable analog, NIM811(MeIle4-cyclosporine), differs from CsA by a group replacement at position 4 that prevents NIM811/CypA complexes from binding to and inhibiting calcineurin, although a high affinity interaction with active residues of cyclophilin is maintained (19). Our laboratory has previously made use of NIM811 to investigate the contribution of cyclophilins to leukocyte recruitment in a mouse model of acute lung injury. We demonstrated that intervention with NIM811 significantly reduced the number of neutrophils accumulating in lungs following intranasal delivery of LPS (10). In recent studies we reported the generation of a new cell-impermeable analog of CsA, MM218 (20). This compound contains a highly charged moiety that prohibits its passage through the plasma membrane, thereby preventing it from interacting with intracellular cyclophilins and mediating intracellular immunosuppressive activity. The latter was confirmed by in vitro T cell activation experiments (20). Compared to NIM811, this novel compound enabled us to specifically target the extracellular pool of cyclophilins, and confirm the contribution of extracellular cyclophilins alone to leukocyte recruitment during inflammatory responses. The current studies demonstrate that this cell-impermeable analog of CsA is indeed highly effective at inhibiting leukocyte recruitment, along with other inflammatory parameters, in an experimental model of acute allergic asthma. Such findings suggest these types of analogs might be considered as new therapeutic agents for reducing inflammatory diseases associated with leukocyte recruitment into tissues.
Materials and methods
Mouse model of allergic lung inflammation
All experiments were conducted under the approval of The George Washington University Institutional Animal Care and Use Committee. A previously described (21) mouse model of acute allergic lung inflammation was used for most studies. Briefly, BALB/c mice (purchased from the National Cancer Institute) were primed i.p. with 100 μg OVA protein mixed 1:1 with Imject alum and then challenged with 50 μg OVA in PBS by intranasal (i.n.) delivery over 4 consecutive days (days 7–10). Most analyses were conducted two days later (day 12). For experiments in which airway chemokines were measured, the mice were sacrificed 6 hours after the third OVA challenge (day 9). For the kinetics studies, groups of mice were sacrificed every other day from day 6 to day 16 after priming.
Inducing allergic lung inflammation by adoptive transfer of OVA-specific CD4+ T cells
DO11.10 mice carrying a transgenic (Tg) TCR specific for pOVA323–339 were purchased from The Jackson Laboratory and were maintained on BALB/c mice. Female progeny were used as the source of OVA-specific CD4+ T cells for adoptive transfer into BALB/c recipients. Populations of OVA-specific TH2 CD4+ T cells were generated in vitro as described by previous investigators (22) using 5 μg/ml pOVA323–339 (GenScript, Piscataway, NJ), 15 ng/ml IL-4 (PeproTech, Rocky Hill, NJ), 25 U/ml IL-2 (Roche Diagnostics, Indianapolis, IN), and 2.5 μg/ml anti-IFN-γ (eBioscience, San Diego, CA). After 3 days of culture, 3 × 106 TH2-differentiated CD4+ T cells were delivered via tail vein injection to female BALB/c mice and 24 hours after transfer (day 1) the mice were given i.n. challenges with 50 μg OVA over four consecutive days. Analyses were conducted 48 hours after the final challenge.
In vivo drug intervention
NIM811 CsA (19) was generously provided by Novartis Institute for Biomedical Research, Inc. (Cambridge, MA) and MM218 CsA (20) was synthesized at the Max Planck Research Unit for Enzymology of Protein Folding (Halle, Germany). Based on preliminary dosing studies, OVA primed/challenged mice were given 200 μg doses of MM218 (0.67 mM in PBS), NIM811 (1.66 mM in diluent), or diluent (16% EtOH in 15% Cremophor EL) by i.p. injection on days 7, 9 and 11 of the model regimen, and days 1, 3, and 5 of the transfer regimen.
Tissue isolation and processing
Immediately following sacrifice, blood was collected by cardiac puncture, allowed to clot for several hours at room temperature, and the serum separated by centrifugation. For the analysis of circulating leukocytes, blood was collected in heparin and the red blood cells lysed using ammonium chloride buffer. Bronchoalveolar lavage (BAL) was performed after blood collection to isolate airway cells and fluid. The fluid and cells were then separated by centrifugation and used for different analyses. Intact lungs were perfused with 20 ml cold PBS, finely chopped, and crushed through a metal strainer to generate single-cell suspensions. Spleen and lymph node cell suspensions were generated by crushing the individual organs between glass slides followed by filtration through nylon mesh.
Flow cytometric analysis and tissue histology
For flow cytometric analysis, BAL and lung tissue cells were treated with ammonium chloride lysis buffer, counted, and then stained with various combinations of APC-anti-GR-1 (neutrophils), FITC-anti-CD8 (CD8+ T cells), PE-anti-CD11c (alveolar monocytes), and PE-Cy5-anti-mouse CD4 combined with FITC-anti-mouse CD62L to identify effector/memory CD4+ T cells (CD4+/CD62L-). Cells from the transfer studies were co-stained with PE-Cy5 anti-mouse CD4 and biotinylated-KJ1-26 followed by FITC-avidin. All antibodies were purchased from eBioscience. Eosinophils were identified by FSC/SSC distribution and confirmed by cytospin analysis. For lung histology, whole lungs were first perfused with PBS and then infused with 1 ml cold PLP fixative as previously described (21). Fixed lungs were embedded in paraffin and 5 μm tissue sections stained with hematoxylin and eosin (H&E) or Periodic acid-Schiff (PAS) by Histoserv, Inc. (Gaithersburg, MD).
Western blot analysis
Equivalent volumes (20 μl) of BAL fluid from individual mice were separated under reducing conditions using 4–12% Bis-Tris SDS-PAGE gels, followed by blotting and detection using rabbit anti-human CypA (U.S. Biological, Swampscott, MA) and anti-human CypB (ABR, Rockford, IL) antibodies as previously described (21). Densitometric analysis was conducted using ImageQuant software.
Measuring cytokines, chemokines, and OVA-specific IgE
To measure airway cytokines BAL fluids were pooled within treatment groups and then concentrated 8x using 3-kDa cut-off Centricon columns (Millipore, Billerica, MA). To test cytokine production by OVA-specific CD4+ T cells, suspensions of mononuclear cells were generated from spleens isolated from individual groups of mice. The cells were then cultured at 4 × 106 cells/ml for 4 days with different doses of OVA protein (0–100 μg/ml) to induce cytokine production. Levels of cytokines in BAL fluid and culture supernatants were measured using mouse IL-5 and IL-13 ELISA kits (eBioscience). Levels of eotaxin/CCL11, TARC/CCL17, and MDC/CCL22 were measured in individual BAL samples using ELISA kits (R&D Systems, Minneapolis, MN). Serum from individual animals was analyzed for the presence of OVA-specific IgE using an ELISA kit purchased from MD Bioproducts (St. Paul, MN).
Measuring airway hyperreactivity
On day 12 of the model regimen, mice were anesthetized by 300 μl i.p. injection of ketamine (10 mg/ml) and xylazine (1 mg/ml) and a tracheostomy tube was inserted. Measurements of airway resistance and compliance were conducted using the FinePointe RC system (Buxco Research Systems, Wilmington, NC). Mice were sequentially challenged with aerosolized PBS (baseline) followed by increasing doses of methacholine ranging from 1.56 – 25 mg/ml. Maximum resistance and average compliance values were recorded during a 3-minute period following each challenge.
Chemotaxis assays
Activated CD4+ T cells were generated from total spleen cells isolated from C57BL/6 mice (purchased from NCI) by overnight stimulation with 10 μg/ml concanavalin A. Purified CD4+ T cells were set up in 48-well Boyden chemotaxis chambers (Neuroprobe, Gaithersburg, MD) as previously described (21). Positive and negative controls included 1 ng/ml RANTES (PeproTech) and medium alone (RPMI 1640 + 1% BSA fraction V), respectively. Recombinant cyclophilins were optimized for use at 100 ng/ml for CypA (Calbiochem, San Diego, CA) and 200 ng/ml for CypB (purified in house). For blocking studies, 2 μM MM218 was added to the upper and lower wells of the chamber. A chemotactic index was generated by dividing the number of migrated cells in each well by the average number of cells that had migrated to medium alone.
Statistical analyses
Statistically significant differences between groups were determined using the Student’s t-test for all studies.
Results
Extracellular cyclophilins are produced throughout effector cell recruitment phase
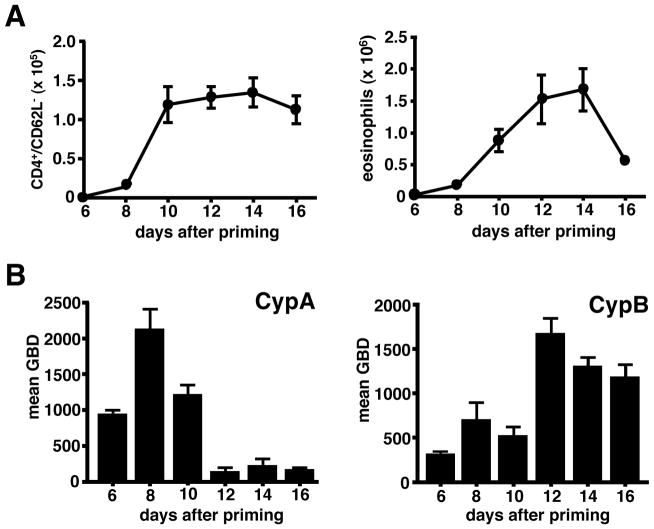
To determine the optimal timing for targeting cyclophilin activity in our model of lung inflammation, we initially established the kinetics of production of extracellular cyclophilins A and B, the two most abundant members of the cyclophilin family, relative to the kinetics of leukocyte recruitment within lung airways and tissues. Mice were sacrificed every other day from day 6 (one day prior to first i.n. OVA challenge) to day 16 (six days after final i.n. OVA challenge) of our regimen and airway and lung tissue cells were analyzed by flow cytometry. In previous (21) and current (Supp. Fig. 1) studies we have observed that this OVA priming/challenge regimen induces an inflammatory response dominated by an influx of eosinophils and CD4+ T cells, with minimal recruitment of other effector cell types including CD8+ T cells and neutrophils. Thus, our leukocyte recruitment analyses were focused on monitoring changes in eosinophils and effector/memory CD4+ T cell numbers due to their dominant pathological contribution in our model. Figure 1A shows significant recruitment of both cell subsets following serial challenge with OVA protein, with peak recruitment between days 12 and 14. When analyzing the BAL fluid from these mice, we observed that levels of both extracellular CypA and CypB increased over the course of leukocyte recruitment (Fig. 1B). While the kinetics of production varied between the two cyclophilins, elevated levels were present throughout the time course of leukocyte recruitment.
FIGURE 1. Extracellular cyclophilins are generated throughout leukocyte recruitment phase of allergic lung inflammation.
Groups of OVA primed/challenged mice were sacrificed every other day starting at day 6 through day 16 of the inflammatory response. (A) Numbers of effector/memory CD4+ T cells and eosinophils were determined in BAL fluid of individual mice by flow cytometric analysis. Data show mean ± SE cell numbers, with n=10 mice per time point. (B) Western blot analysis was conducted on cleared fluid from individual BAL samples to determine levels of extracellular CypA and CypB. Data show mean ± SE gel band density (GBD) for each time point as determined by densitometric analysis. These data are representative of 3 independent experiments.
Cell-permeable CsA analog (NIM811) significantly reduces leukocyte numbers in lung tissue and airways of allergic mice
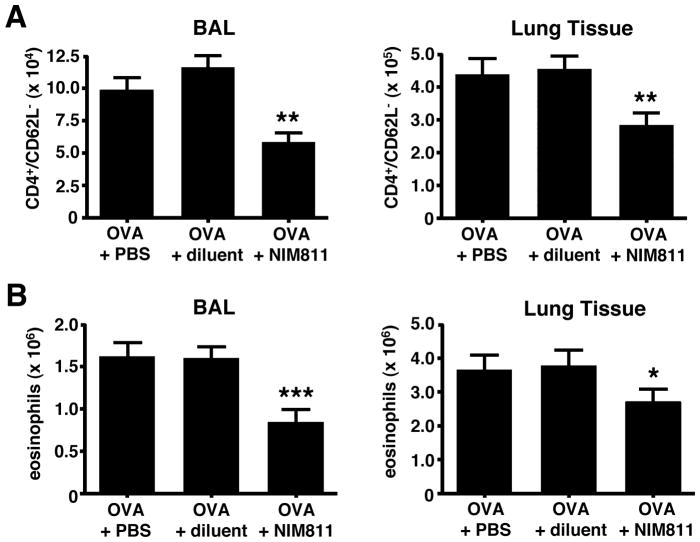
We next investigated the contribution of cyclophilins to acute allergic lung inflammation by administering non-immunosuppressive analogs of CsA to inhibit cyclophilin activity. Since extracellular cyclophilins were present throughout the time course of leukocyte recruitment (Fig. 1B), we spanned our intervention regimens throughout the airway allergen challenge phase, with drug treatment given on days 7, 9, and 11 of the regimen. We initially tested the cell-permeable analog, NIM811 (19), which we had previously shown was highly effective at reducing the influx of neutrophils in a mouse model of LPS-induced acute lung inflammation (10). In the current model of allergic lung inflammation, intervention with NIM811 significantly reduced (29–50%) the number of effector/memory CD4+ T cells and eosinophils both in lung tissues and airways (Fig. 2). Importantly, no changes in cell numbers or cell distribution were observed in the blood, spleen, or peripheral lymph nodes of NIM811 treated mice (Supp. Fig. 2A), ruling out the likelihood of systemic cytotoxic effects. Taken together, these data suggest that leukocyte recruitment to lung tissues and airways can be significantly reduced in experimental acute allergic lung inflammation by blocking the activity of cyclophilins with a non-immunosuppressive analog of CsA.
FIGURE 2. NIM811 intervention reduces leukocyte numbers in the lung tissue and airways of allergic mice.
Mice were primed/challenged with OVA, with some groups treated i.p. with NIM811, diluent alone, or untreated, on days 7, 9 and 11 of the regimen. (A) NIM811 reduces the number of effector/memory CD4+ T cells in the lung tissue (38%) and airway BAL (50%). (B) NIM811 reduces the number of eosinophils in the lung tissue (29%) and airway BAL (48%). Data show mean ± SE cell numbers for each group (n=12 mice per group) with a Student’s t-test analysis used to determine statistical significance. *** = P<0.001, ** = P<0.01, and * = P<0.05 levels of statistical difference comparing OVA + NIM811 to OVA + diluent groups. These data are representative of 3 independent experiments.
Cell-impermeable CsA analog (MM218) also significantly reduces leukocyte numbers in lung tissue and airways of allergic mice
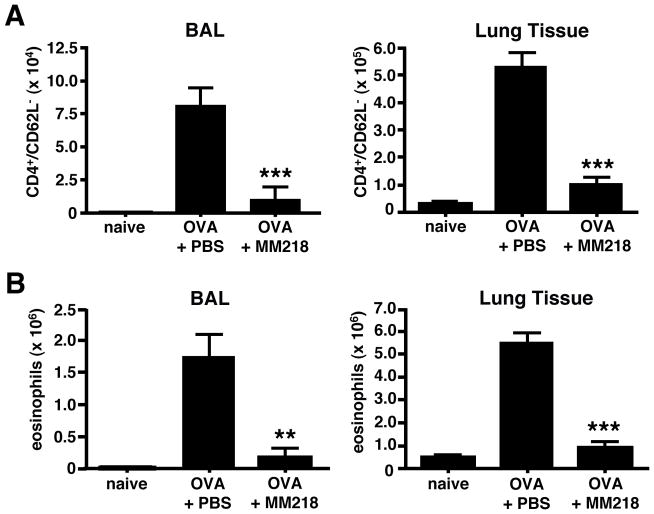
To focus on the contribution of extracellular cyclophilins during allergic lung inflammation, we next tested the capacity of a recently described cell-impermeable analog of CsA, MM218 (20), to reduce leukocyte recruitment in our mouse model. MM218 was found to reduce the number of effector/memory CD4+ T cells and eosinophils in lung tissues and airways of allergic mice by a highly significant 75–88% (Fig. 3). This major reduction in inflammatory infiltrates was supported by lung tissue sections stained with H&E to detect inflammatory infiltrates from untreated versus MM218-treated mice (Fig. 4). As with NIM811, analysis of leukocyte numbers in the spleen, peripheral lymph nodes, and blood demonstrated no evidence that treatment with MM218 had induced systemic cytotoxicity (Supp. Fig. 2B). Further evidence that MM218 does not mediate any significant toxic effects is provided by our recently published studies showing no impact of the drug on in vitro T cell activation (20). To rule out the possibility that MM218 treatment might be acting by inhibiting the production of other known eosinophil and T cell attracting chemokines, levels of three airway chemokines associated with leukocyte recruitment in allergic airway inflammation (eotaxin/CCL11, MDC/CCL22 and TARC/CCL17) were compared in BAL fluid from MM218 treated versus untreated mice. Since these chemokines are secreted acutely following airway challenge, measurements were conducted on BAL fluid collected 6 hours after OVA challenge. While numbers of airway eosinophils and effector CD4+ T cells were reduced by 56–65%, levels of all three chemokines were comparable between treatment groups, with no significant reductions observed in the MM218 treated mice (Supp. Fig. 3). The possibility remains that chemokines other than those measured in the current study may have been impacted, however eotaxin, MDC and TARC are considered to be major contributors to leukocyte recruitment in allergic responses (1).
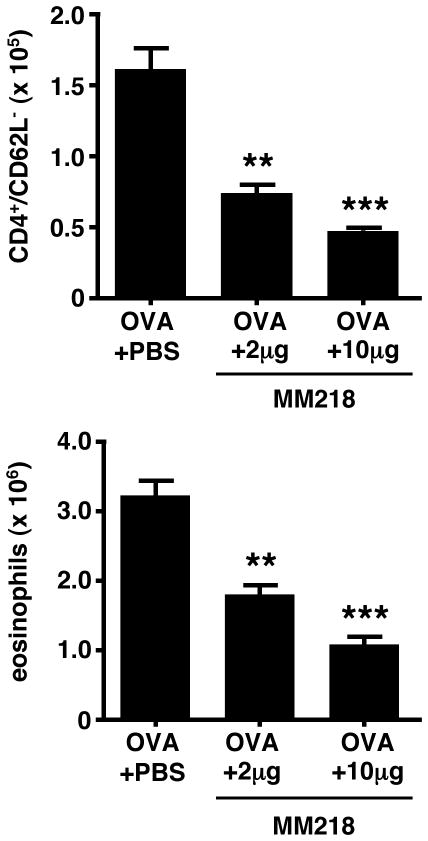
FIGURE 3. MM218 intervention significantly reduces leukocyte numbers in the lung tissue and airways of allergic mice.
Mice were primed/challenged with OVA, with some groups treated i.p. with MM218 or PBS on days 7, 9 and 11 of the regimen. Cells were also collected from naïve BALB/c mice to serve as a baseline. (A) MM218 significantly reduces the number of effector/memory CD4+ T cells in the lung tissue (75%) and airway BAL (88%). (B) MM218 reduces the number of eosinophils in the lung tissue (79%) and airway BAL (88%). Data show mean cell numbers for each group (n=6) ± SE with a Student’s t-test analysis used to determine statistical significance. *** = P<0.001 and ** = P<0.01 levels of statistical difference comparing OVA + MM218 to OVA + PBS groups. These data are representative of 6 independent experiments.
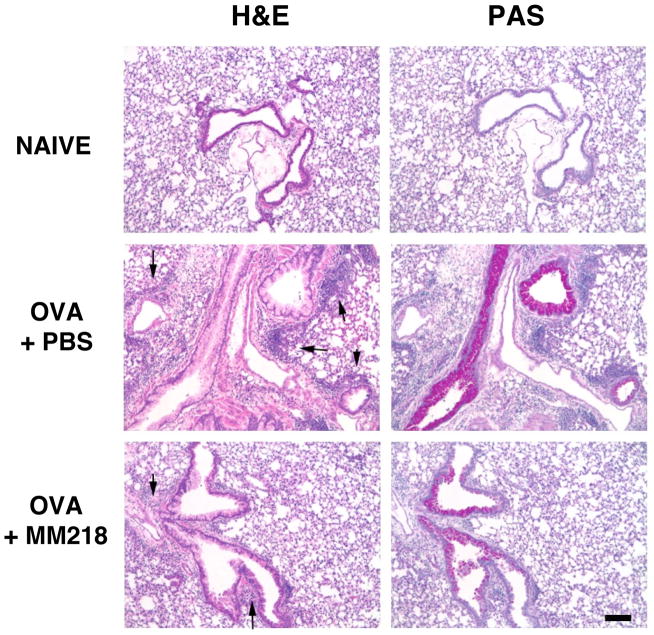
FIGURE 4. MM218 intervention reduces inflammation in the lung tissue of allergic mice.
Mice were primed/challenged with OVA, with some groups treated i.p. with MM218 or PBS on days 7, 9 and 11 of the regimen. Whole lungs isolated from naïve, OVA + PBS and OVA + MM218 mice were paraffin-embedded and 6 μm sections cut and stained with H&E or PAS. Images (10X magnification) show tissue areas surrounding bronchioles. Arrows on H&E sections denote inflammatory foci. The magnification bar represents 10 μm. These data are representative of 3 independent experiments.
Cell-impermeable CsA intervention significantly reduces airway mucus and TH2 cytokine levels
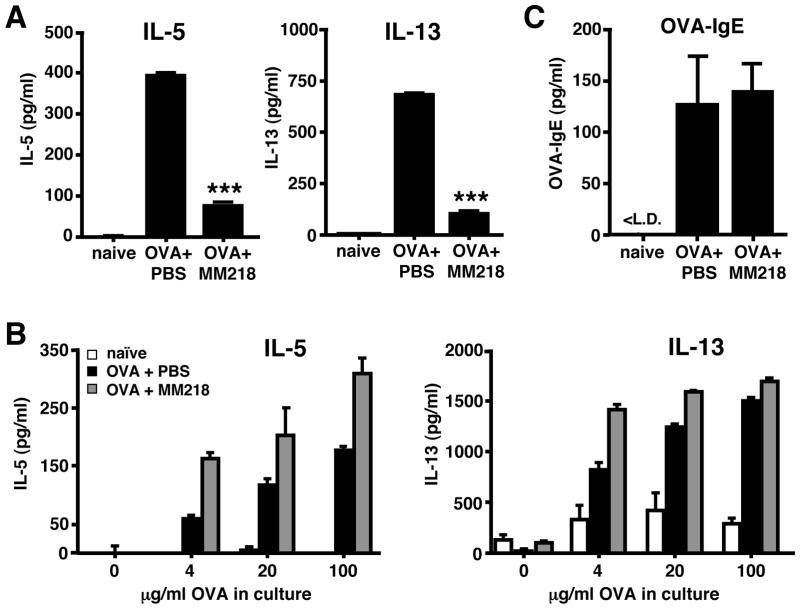
We next investigated whether other immune parameters of inflammation associated with allergic lung inflammation would also be reduced by treatment with MM218. Indeed, lung tissue sections stained with PAS to detect airway mucus hypersecretion demonstrated a marked reduction in the frequency and abundance of staining of PAS-positive airways in MM218 treated versus untreated mice (Fig. 4). BAL fluid from the two groups of mice was also assessed for the presence of airway TH2 cytokines, specifically IL-13 and IL-5. Figure 5A shows that levels of both IL-13 and IL-5 were significantly reduced in the airways of mice treated with MM218 (reduction of 83% and 76%, respectively). These data fit well with the observed reduction in effector/memory CD4+ T cells in lung tissues and airways (Fig. 3), since CD4+ T cells are known to be a major source of the two cytokines. To rule out the possibility that MM218 treatment might be impacting CD4+ T cell priming, thereby reducing the total number of TH2 CD4+ T cells generated in vivo, spleen cells from OVA primed/challenged mice treated, or not treated, with MM218 were isolated and restimulated in vitro with OVA protein to induce cytokine production. As shown in Figure 5B, culture supernatants from the MM218 treated mice showed no reduction in levels of TH2 cytokines, suggesting that the treatment with MM218 did not adversely affect in vivo T cell priming. Moreover, levels of serum OVA-specific IgE were found to be equivalent between MM218 treated and untreated mice (Fig. 5C), providing additional evidence that TH2 T cell priming was not affected by the drug.
FIGURE 5. MM218 intervention reduces TH2 cytokine levels in the airways of allergic mice but does not affect TH2 T cell priming.
Mice were primed/challenged with OVA and some groups were treated i.p. with MM218 or PBS on days 7, 9 and 11 of the regimen. (A) BAL fluid was collected and pooled for each group, followed by 8x concentration. Levels of IL-5 and IL-13 cytokines were measured in the resulting fluid by ELISA. Data show the mean ± SE for replicate wells (n=4). Student’s t-test established *** P<0.001 for both cytokines comparing OVA + MM218 to OVA + PBS groups. (B) Spleens were collected from the same groups of mice and cell suspensions were generated and stimulated in vitro with different doses of OVA protein to induce cytokine production. After four days of culture, supernatants were collected and tested for levels IL-5 and IL-13. Data show the mean ± SE for replicate wells (n=4). (C) Serum was collected from individual mice and levels of OVA-specific IgE measured by ELISA. Data shown mean ± SE titers, with n=6 mice per group. These data are representative of 2–3 independent experiments.
Airway hyperreactivity is significantly reduced in allergic mice treated with cell-impermeable CsA
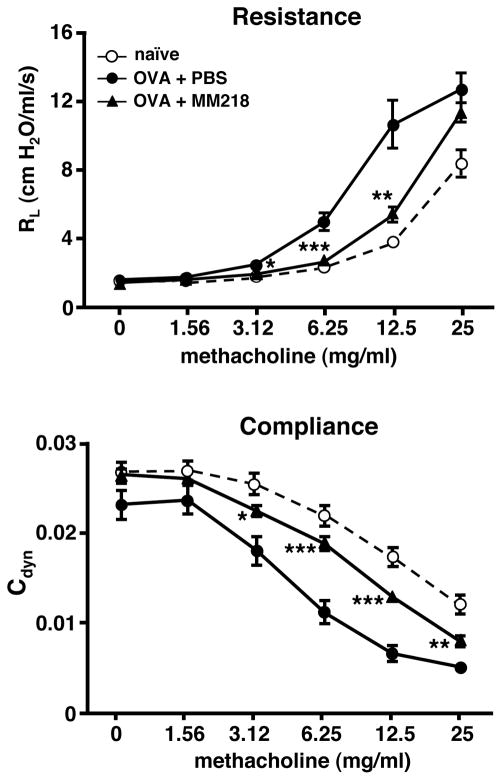
We next investigated whether the observed reductions in leukocyte infiltrates and TH2 cytokines levels mediated by MM218 treatment would also lead to improved lung function. For these studies airway function was assessed by measuring changes in lung resistance (RL) and compliance (Cdyn) in response to increasing doses of inhaled methacholine to induce bronchoconstriction. Measurements were conducted on individual mice from groups that were OVA primed/challenged, with and without MM218 treatment, versus naïve mice. Measurements of airway resistance and compliance for the three groups are shown in Figure 6. As expected, airway resistance was significantly increased and lung compliance was decreased in OVA primed/challenged mice, compared to naïve mice (Fig. 6). Strikingly, mice treated with MM218 showed levels of airway resistance and lung compliance that were almost equivalent to those of naïve mice. Collectively, these data demonstrate the capacity of cell-impermeable CsA to reduce several parameters of allergic lung inflammation associated with leukocyte recruitment, including TH2 cytokine production, and airway hyperreactivity.
FIGURE 6. MM218 intervention reduces airway hyperreactivity in allergic mice.
Mice were primed/challenged with OVA with some groups treated i.p. with MM218 or PBS on days 7, 9 and 11 of the regimen. On day 12, mice were individually anesthetized with an i.p. injection of ketamine/xylazine, a tracheostomy tube inserted, and attached to a respirator. The mice were then sequentially challenged for 2 minutes with increasing doses of methacholine (1.5625 – 25 mg/ml). Measurements of airway resistance and compliance were determined using the Buxco FinePointe RC and associated software. Data show mean ± SE RL and Cdyn measurements at individual doses of methacholine for each group of mice (n=10 per group). Student’s t-test analysis established *** P<0.001, ** P<0.01 and * P<0.05 levels of statistical difference at various doses of methacholine between OVA + MM218 and OVA + PBS groups. These data are representative of 3 independent experiments.
Cell-impermeable CsA inhibits migration of activated CD4+ T cells in vitro and in vivo
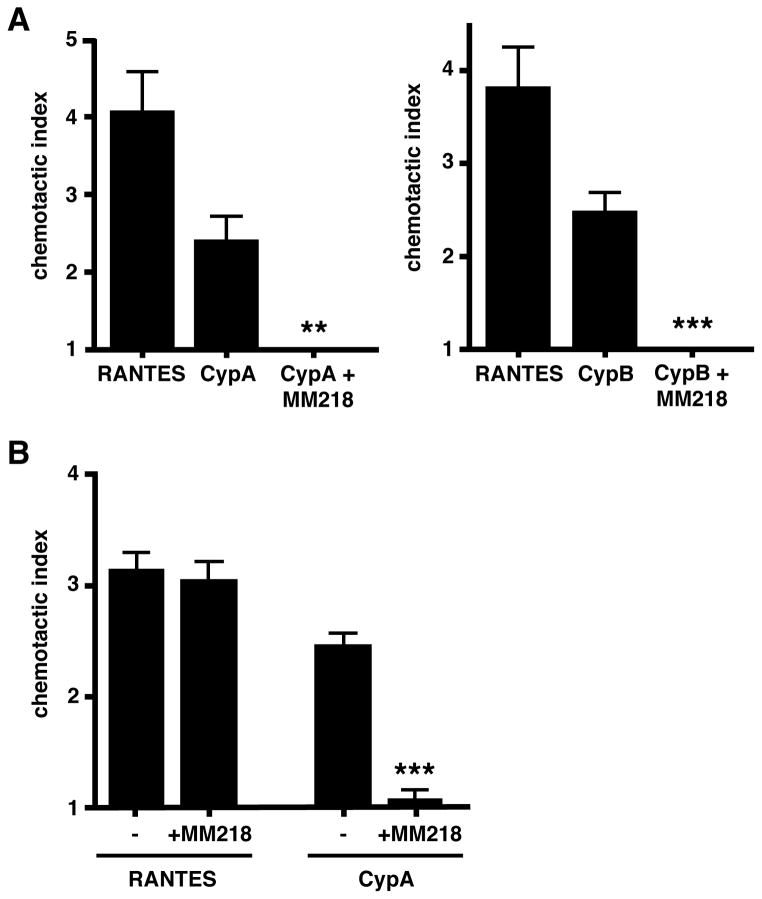
The above findings suggest that extracellular cyclophilins likely contribute to allergic lung inflammation by promoting the recruitment of pro-inflammatory leukocytes into the respiratory tract and that blocking the function of these cyclophilins using cell-impermeable CsA impacts this recruitment. In previous studies we showed that both CypA and CypB have the capacity to induce potent migration of activated CD4+ T cells in vitro (21). However, we (21) and others (23) have found that the same cyclophilins are unable to induce eosinophils either to migrate or to initiate signaling events. These findings have led us to previously suggest that, in vivo, extracellular cyclophilins likely mediate their chemotactic effect directly on effector/memory T cells and indirectly on eosinophils via T cell-derived cytokines (21). To provide supporting evidence that cell-impermeable CsA has the capacity to directly inhibit the migration of CD4+ T cells, in vitro chemotaxis assays were conducted in which activated CD4+ T cells were stimulated either with CypA or CypB in the presence or absence of MM218. Figure 7A shows that MM218 was indeed able to completely inhibit the migration of activated CD4+ T cells in response to both CypA and CypB. This effect was shown to be cyclophilin-specific, as the presence of MM218 did not alter the response of the same cells to a T cell attracting chemokine, RANTES (Fig. 7B).
FIGURE 7. MM218 inhibits in vitro CD4+ T cell migration in response to extracellular cyclophilins.
(A) Activated CD4+ T cells were generated in vitro and set up in Boyden chambers using recombinant CypA and CypB ± MM218. RANTES was used as a positive control for T cell chemotaxis. Data show mean ± SE chemotactic indices for each group (n=6 wells per group). Student’s t-test established ** P<0.01 and *** P<0.001 levels of statistical differences between + MM218 and no MM218 for CypA and CypB stimulation, respectively. (B) The same assay was used with activated CD4+ T cells set up either with RANTES or CypA ± MM218. Data show mean ± SE chemotactic indices for each group (n=6 wells per group). Student’s t-test established a *** P<0.001 statistical difference between + MM218 and no MM218 for CypA stimulation only. These data are representative of 3 independent experiments.
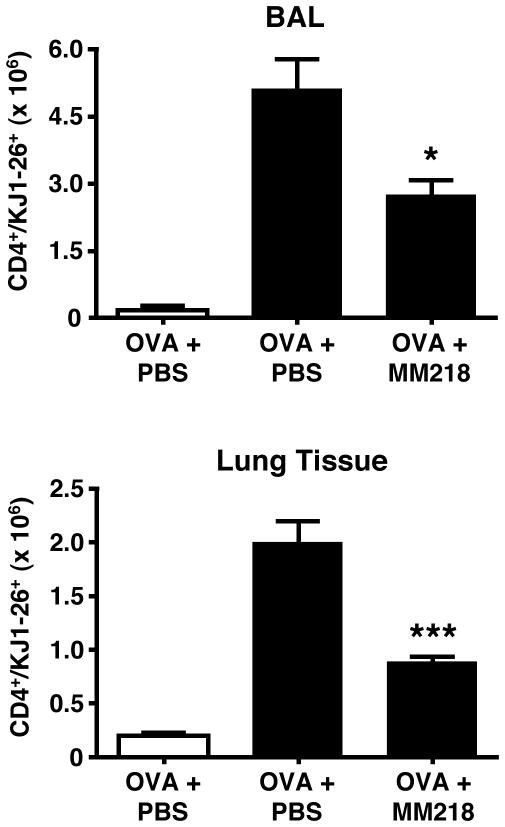
Further supporting evidence that cell-impermeable CsA can impact directly on the recruitment of activated CD4+ T cells was provided by in vivo studies using a well-established T cell transfer model of allergic lung inflammation in which OVA-specific activated TH2 CD4+ T cells are generated in vitro from DO11.10 TCR Tg mice and then transferred into naive WT recipients, followed by intranasal OVA challenge to induce lung inflammatory responses (22). This approach enables populations of antigen-specific T cells to be tracked as they migrate into lung tissues and airways and to observe any changes in migration during drug treatment. Figure 8 shows a robust recruitment of OVA-specific (KJ1-26+) CD4+ T cells in response to OVA challenge in recipient mice that had received OVA-primed (activated) DO11.10 TCR Tg CD4+ T cells, but not unprimed (naïve) DO11.10 CD4+ T cells. Mice treated with cell-impermeable CsA showed a significant reduction in the number of OVA-specific (KJ1-26+) CD4+ T cells recruited both into lung tissues and airways. Taken together, the results from these studies provide compelling evidence that cell-impermeable CsA has the capacity to directly inhibit the recruitment of activated CD4+ T cells in response to extracellular cyclophilins both in vitro and in vivo.
FIGURE 8. MM218 intervention inhibits the in vivo migration of OVA-specific CD4+ T cells to lung tissues and airways of allergic mice.
Activated OVA-specific TH2 CD4+ T cells were transferred into BALB/c mice. A separate group of mice received naïve OVA-specific CD4+ T cells. All recipient mice were challenged over four days in the airways with OVA. Groups of mice (n=6) were treated i.p. with MM218 or PBS during OVA challenge. Data show significant reductions in the number of OVA-specific (KJ1-26+) effector/memory CD4+ T cells in the airways and lung tissues of mice given activated CD4+ T cells. No response was induced in mice given naïve CD4+ T cells (white bars). Student’s t-test test established * = P <0.05 and *** P<0.001 levels of statistical difference in the airways and lung tissue, respectively when comparing OVA + MM218 to OVA + PBS groups given activated T cells. These data are representative of 2 independent experiments.
Cell-impermeable CsA is as effective at reducing leukocyte recruitment when administered locally to the airways
There is currently much interest to develop drug therapies and small molecule antagonists that have specific targets and can be administered locally to asthma patients (24). Having demonstrated the efficacy of our cell-impermeable CsA analog to reduce allergic lung inflammation when delivered systemically, we next tested whether it would also be effective administered directly to the airways. For these studies, OVA primed/challenged mice were given two different doses of MM218 (10 μg or 2 μg) by i.n. delivery. As shown in Figure 9, intranasal administration of MM218 was able to induce a very marked and significant reduction in the number of effector/memory CD4+ T cells and eosinophils detected in the airways of allergic mice. Similar reductions of both leukocyte subsets were observed in lung tissues (data not shown). It is particularly striking that a dose as low as 2 μg was found to be effective because this dose is 100-fold lower than the optimal dose for systemic administration. These findings provide promising evidence that cell-impermeable CsA analogs, such as MM218, may have potential for therapeutic use.
FIGURE 9. MM218 is effective when administered directly to the airways.
Mice were primed/challenged with OVA, with groups of mice (n=6) given difference doses of MM218 by i.n. delivery during challenge. Data show a significant reduction in effector/memory CD4+ T cells and eosinophils in MM218 treated mice. Student’s t-test established *** P<0.001 and ** P<0.01 levels of statistical difference comparing OVA + MM218 to OVA + PBS groups. These data are representative of 2 independent experiments.
Discussion
Cyclophilins, traditionally known as intracellular PPIases, have been shown to be potent chemoattractants for leukocytes. Furthermore, elevated levels of extracellular cyclophilins have been observed in several inflammatory diseases, implicating a role for these proteins in promoting inflammation. In the current study, we utilized a mouse model of acute allergic lung inflammation characterized by the influx of leukocytes, notably activated TH2 CD4+ T cells and eosinophils, into lung tissues and airways to investigate the contribution of extracellular cyclophilins to various parameters of lung inflammation. We made use of two different non-immunosuppressive analogs of CsA to block cyclophilin activity.
Our findings demonstrate that elevated levels of extracellular CypA and CypB are present throughout the challenge phase of the inflammatory response. Interestingly, the release of CypA and CypB appeared to be differentially regulated since their initial secretion as well as peak production followed different kinetics profiles. For example, CypA levels showed a marked increase after only a single OVA challenge, whereas little increase was observed in CypB levels until after four challenges. Such findings suggest that the two cyclophilins may regulate different aspects of leukocyte recruitment, with CypA contributing more to the initial wave of recruitment and CypB being more important for sustaining leukocyte influx. In the absence of reagents with the capacity to inhibit the function of individual members of the cyclophilin family we were unable to provide direct evidence for this possibility. Although a new small molecule compound designed to specifically target CypA, and not CypB, was recently described (25), this compound is not currently compatible for in vivo use due to its poor solubility in aqueous diluents. However, studies are currently underway to design more soluble versions of such compounds (G. Fischer, unpublished observations). In addition, we have also initiated studies to develop an immunohistochemical approach that might enable us to formally investigate any differential kinetics of expression between distinct cyclophilins, as well as their cell source. Thus, in the current studies our approach was to inhibit the function of all cyclophilins present throughout the leukocyte recruitment phase.
Our results show that treatment of allergic mice with drugs that target the function of cyclophilins, using either a cell-permeable analog of CsA (NIM811) or a cell-impermeable analog of CsA (MM218), significantly reduces the number of effector/memory CD4+ T cells as well as eosinophils in inflamed lung tissues and airways. While the current studies were focused on investigating changes in populations of pro-inflammatory effector cell subsets, it should be noted that MM218 treatment also reduced the influx of monocytic cells into airways (Supp. Fig. 1). This finding is not surprising due to the fact that murine monocytes express CD147, the cell surface receptor for extracellular cyclophilins (9), and they migrate potently in response to cyclophilins (26). Interestingly, the reduction in monocyte numbers was not as marked as that seen in eosinophils and T cells (64% for monocytes versus >80% for the other two subsets). This likely reflects the fact that most resident lung leukocytes are monocytic (macrophages and dendritic cells), thus fewer are proportionally recruited relative to other subsets of leukocytes. These findings further support the idea that MM218 inhibits active leukocyte recruitment and has little impact on resident leukocytes.
As part of our studies we also demonstrated that cell-impermeable MM218 CsA has the capacity to inhibit the recruitment of activated CD4+ T cells both in vitro (using cyclophilin-specific chemotaxis assays) and in vivo (using an adoptive cell transfer approach). As previously discussed (21), we believe that extracellular cyclophilins likely mediate a chemotactic effect directly on CD4+ T cells and indirectly on eosinophils, via alterations in TH2 cytokine production. The net result is that inhibiting the function of extracellular cyclophilins impacts on the recruitment of both leukocyte subsets. A capacity to reduce the influx of effector/memory CD4+ T cells into the pulmonary microenvironment is of particular importance in the context of immune-mediated lung inflammation since this population regulates many parameters of immune lung pathology (27). Indeed, several different studies have shown that inhibition of the cytokines IL-5 and IL-13 alone can lead to reduction in airway hyperreactivity, mucus hypersecretion, IgE generation, as well as eosinophil influx (28–31).
Of particular interest is the finding that treatment with the cell-impermeable MM218 analog was markedly more effective at reducing leukocyte influx (75–88% reduction) compared to the cell-permeable NIM811 analog (29–50% reduction). Furthermore, the augmented effectiveness of MM218 was achieved using smaller amounts of compound per injection (0.67 mM versus 1.66 mM of MM218 and NIM811, respectively). We think the likely explanation is that the cell-impermeable nature of MM218 prevents it from being trapped at intracellular sinks that are known to sequester cell-permeable cyclosporines, such as CsA and NIM811 (32, 33). This enables the entire amount of drug to be available for the inhibition of extracellular cyclophilins. Indeed, studies using fluorescent-tagged CsA have demonstrated that cell-permeable analogs accumulate at very high concentrations within the cell cytosol (20) which would reduce their availability to interact with extracellular ligands. We were particularly pleased to observe that a localized delivery of MM218 was just as effective at reducing leukocyte recruitment into asthmatic lungs as systemic delivery. Moreover, this effect could be mediated with 100-fold lower doses of the drug. These findings provide strong supporting evidence that the primary target of our cell-impermeable CsA analog is extracellular cyclophilins generated in the respiratory tract, which has significant positive implications from a therapeutic perspective.
Collectively, our results demonstrate the potent capacity of a cell-impermeable analog of CsA to reduce several parameters of acute allergic lung inflammation and provide initial evidence that targeting extracellular cyclophilins could have therapeutic potential for reducing tissue inflammation. The findings that a CsA derivative was so highly effective at reducing allergic lung inflammation might be questioned in the light of human data reporting only modest improvements following CsA treatment (34). However, other studies have demonstrated CsA to have a potent inhibitory effect on allergic lung inflammation both in animal models (35–37) and in humans (38–40), particularly in cases of severe refractory asthma (41). Regardless of these contradictory findings, it is clear that the side effects associated with the high doses and need for long-term usage reduce the appeal of CsA as a drug of choice (34). We propose that the use of cell-impermeable derivatives of CsA, as described in the current studies, offer a safer and more efficacious approach than unmodified CsA due to their remaining in extracellular spaces, rather than being sequestered within cytoplasmic compartments as previously demonstrated (20, 32, 33). By remaining extracellular, cell-impermeable CsA derivatives have no impact on the function of intracellular proteins and will require lower doses for optimal inhibition of extracellular cyclophilin function. An additional advantage of the new cell-impermeable derivatives is their high solubility in aqueous solutions, making them more manageable for local delivery, for example via inhalation. Interestingly, a study in which CsA was complexed to cylodextrin to improve its solubility demonstrated an equivalent reduction in murine allergen-induced eosinophilia at 9-fold lower doses than non-complexed CsA (36), suggesting CsA functions more efficiently in water-soluble forms. In conclusion, we propose that using drugs and other compounds that remain outside of cells, such as the current cell-impermeable CsA, will enable us to more specifically target extracellular molecules and reduce side effects due to uptake by non-target cells.
Supplementary Material
Acknowledgments
We thank Dr. Fabrice Allain and Dr. Christophe Vanpouille for providing us with the necessary reagents to generate recombinant CypB.
This research was supported by the National Institutes of Health (R01-AI67254 to S.L.C.) and by the Deutsche Forschungsgemeinschaft (SFB 610 to G.F.).
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- CsA
cyclosporine A
- Cyp
cyclophilin
- PPIase
peptidyl-prolyl cis-trans isomerase
References
- 1.Velazquez J, Teran L. Chemokines and their receptors in the allergic airway inflammatory process. Clinic Rev Allerg Immunol. 2010 doi: 10.1007/s12016-010-8202-6. Epub ahead of print March 30. [DOI] [PubMed] [Google Scholar]
- 2.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Heitman J. The cyclophilins. Genome Biology. 2005;6:226.221–226.226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiene C, Fischer G. Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol. 2000;10:40–45. doi: 10.1016/s0959-440x(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 5.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol. 2002;23:323–325. doi: 10.1016/s1471-4906(02)02237-8. [DOI] [PubMed] [Google Scholar]
- 6.Sherry B, Yartlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 8.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci USA. 2002;99:2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M. Active-site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 10.Arora K, Gwinn W, Bower M, Watson A, Okwumabua I, MacDonald H, Bukrinsky M, Constant S. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997;185:975–980. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki J, Jin ZG, Meoli D, Matoba T, Berk B. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 13.Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol. 1997;17:380–386. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 14.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–317. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handschumacher RE, Harding M, Rice J, Drugge R, Speicher D. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 16.Kallen J, Spitzfaden C, Zurini M, Wider G, Widmer H, Wuthrich K, Walkinshaw M. Sructure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 17.Ke H, Zhao Y, Luo F, Weissman I, Freidman J. Crystal structure of murine cyclophilin C complexed with immunosuppressive drug cyclosporin A. PNAS. 1993;90:11850–11854. doi: 10.1073/pnas.90.24.11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikol V, Kallen J, Walkinshaw M. X-ray structure of a cyclophilin B/cyclopsorine complex: comparison with cyclophilin A and delineation of its calcineurin-binding domain. PNAS. 1994;91:5183–5186. doi: 10.1073/pnas.91.11.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM811, a nonimmunosuppressive cyclosporine A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malesevic M, Kuhling J, Erdmann F, Balsley M, Bukrinsky M, Constant S, Fischer G. A cyclosporin derivative discriminates between extracellular and intracellular cyclophilins. Angew Chem Int Ed. 2010;49:213–215. doi: 10.1002/anie.200904529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwinn W, Damsker J, Falahati R, Okwumabua I, Kelly-Welch A, Keegan A, Vanpouille C, Lee J, Dent LA, Leitenberg D, Bukrinsky M, Constant S. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cormier S, Taranova A, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur S, O’Neilll K, Colbert D, Lombari T, Constant S, McGarry M, Lee J, Lee N. Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holgate S, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 25.Daum S, Schumann M, Mathea S, Aumuller T, Balsley M, Constant S, de Lacriox BF, Kruska F, Braun M, Schiene-Fischer C. Isoform-specific inhibition of cyclophilins. Biochemistry. 2009;48:6268–6277. doi: 10.1021/bi9007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damsker J, Okwumabua I, Pushkarsky T, Arora K, Bukrinsky MI, Constant SL. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02877.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn L, Elias J, Chupp G. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 28.Leckie M. Anti-interleukin-5 monoclonal antibodies: preclinical and clinical evidence in asthma models. Am J Respir Med. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Herbert C, Webb D, Li L, Foster P. Effects of anticytokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med. 2004;170:1043–1048. doi: 10.1164/rccm.200405-681OC. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Walter DM, McIntire JJ, Berry G, McKenzie ANJ, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 32.Shibata N, Shimakawa H, Minouchi T, Yamaji A. Pharmacokinetics of cyclosporin A after intravenous administration to rats in various disease states. Biol Pharm Bull. 1993;16:1130–1135. doi: 10.1248/bpb.16.1130. [DOI] [PubMed] [Google Scholar]
- 33.Ryffel B, Foxwell B, Gee A, Greiner B, Woerly G, Mihatsch M. Cyclosporine--relationship of side effects to mode of action. Transplantation. 1988;46:90S–96S. doi: 10.1097/00007890-198808001-00017. [DOI] [PubMed] [Google Scholar]
- 34.Evans D, Cullinan P, Geddes D. Cyclosporin as an oral corticosteroid sparing agent in stable asthma. Cochrane Database Syst Rev. 2001;2:CD002993. doi: 10.1002/14651858.CD002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeum H, Lee Y, Kim S, Roh S, Lee J, Seo Y. Fritillaria cirrhosa, Anemarrhena asphodeloides, Lee-Mo-Tang and cyclosporine A inhibit ovalbumin-induced eosinophil accumulation and Th2-mediated bronchial hyperresponsiveness in a murine model of asthma. Basic Clin Pharmacol Toxicol. 2007;100:205–213. doi: 10.1111/j.1742-7843.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukaya H, Limura A, Hoshiko K, Fuyumuro T, Noji S, Nabeshima T. A cyclosporin A/maltosyl-alpha-cyclodextrin complex for inhalation therapy of asthma. Eur Respir J. 2003;22:213–219. doi: 10.1183/09031936.03.00018202. [DOI] [PubMed] [Google Scholar]
- 37.Roh S, Kim S, Lee Y, Seo Y. Effects of radix adenophorae and cyclosporine A on an OVA-induced murine model of asthma by suppressing to T cells activity, eosinophilia, and bronchial hyperresponsiveness. Mediators Inflamm. 2008;2008:ID781425. doi: 10.1155/2008/781425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan L, Kon O, Macfarlane A, Meng Q, Ying S, Barnes N, Kay A. Attenuation of the allergen-induced late asthmatic reaction by cyclosporin A is associated with inhibition of bronchial eosinophils, interleukin-5, granulocyte macrophage colony-stimulating factor, and eotaxin. Am J Respir Crit Care Med. 2000;162:1377–1382. doi: 10.1164/ajrccm.162.4.9911117. [DOI] [PubMed] [Google Scholar]
- 39.Sihra B, Kon O, Durham S, Walker S, Barnes N, Kay A. Effect of cyclosporin A on the allergen-induced late asthmatic reaction. Thorax. 1997;52:447–452. doi: 10.1136/thx.52.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda T, Asakawa J, Motojima S, Makino S. Cyclosporine A reduces T lymphocyte activity and improves airway hyperresponsiveness in corticosteroid-dependent chronic severe asthma. Ann Allergy Asthma Immunol. 1995;75:65–72. [PubMed] [Google Scholar]
- 41.Polosa R, Morjaria J. Immunomodulatory and biologic therapies for severe refractory asthma. Respir Med. 2008;102:1499–1510. doi: 10.1016/j.rmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.