Abstract
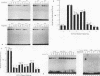
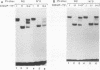
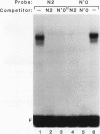
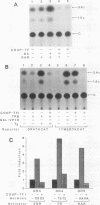
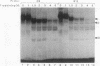
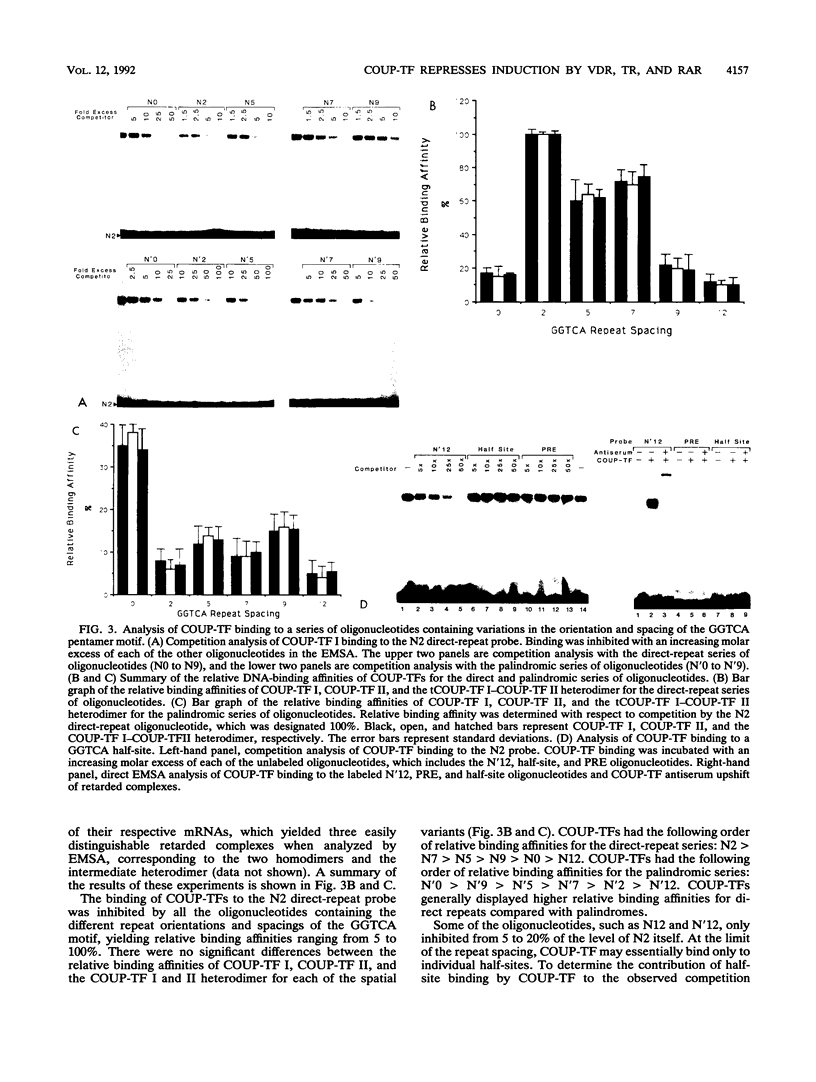
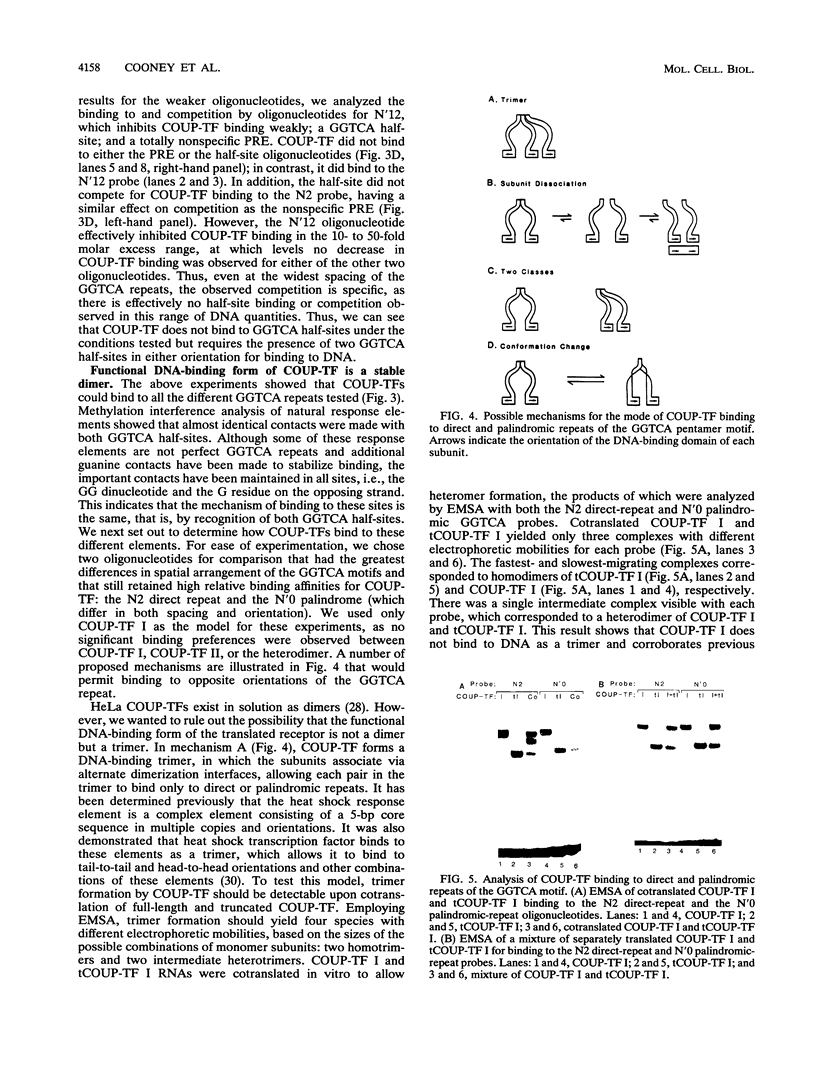
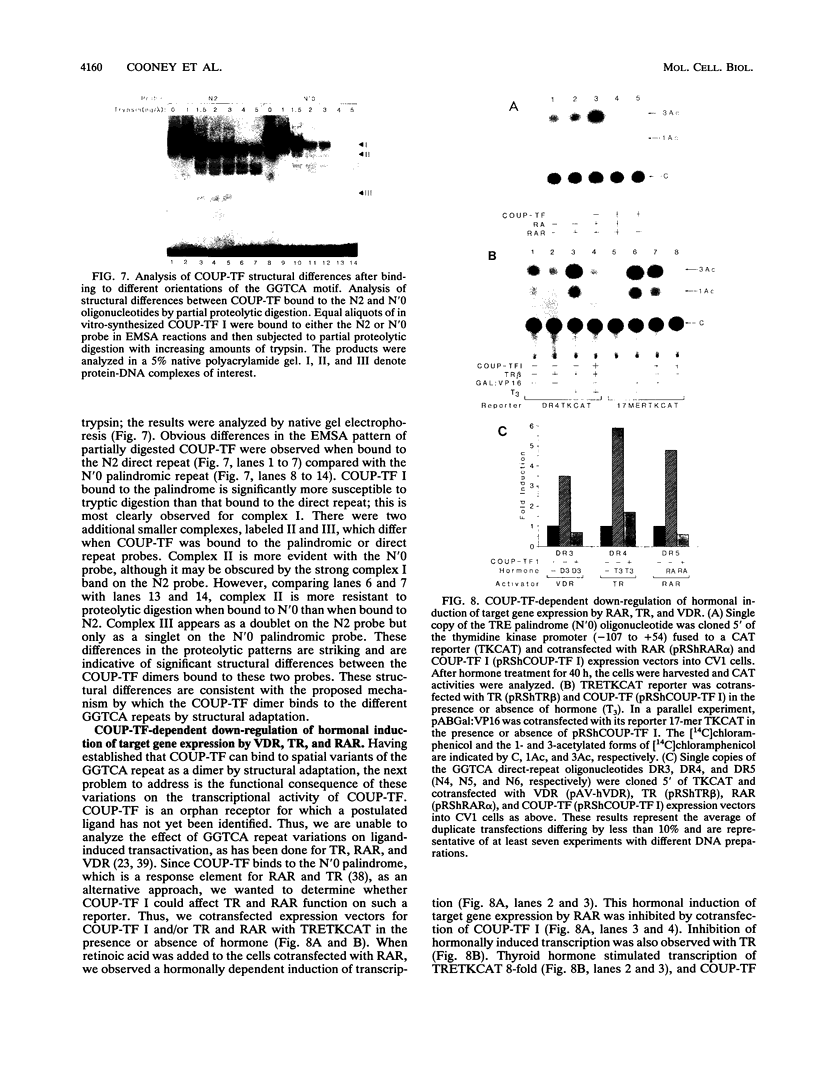
Alignment of natural chicken ovalbumin upstream promoter transcription factor (COUP-TF) response elements shows that, in addition to the predominant direct repeat of the GGTCA motif with a 2-bp spacing, there are other functional COUP elements with variations in the GGTCA orientation and spacing. We systematically analyzed the binding of in vitro-synthesized COUP-TFs and showed that COUP-TF is capable of binding to oligonucleotides containing both direct repeats and palindromes and with different spacings of the GGTCA repeats. Subsequently, we analyzed four possible mechanisms proposed to explain how COUP-TF could bind to these spatial variations of the GGTCA repeat. We demonstrated that the functional DNA-binding form of COUP-TF is a dimer which requires two GGTCA half-sites to bind DNA. We demonstrated that the COUP-TF dimer undergoes a remarkable structural adaptation to accommodate binding to these spatial variants of the GGTCA repeats. A functional consequence of the promiscuous DNA binding of COUP-TF is its ability to down-regulate hormonal induction of target gene expression by other members of the steroid-thyroid hormone receptor superfamily such as the vitamin D3, thyroid hormone, and retinoic acid receptors. Our data indicate that COUP-TF may have an important role in hormonal regulation of gene expression by these receptors.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Purification and characterization of chicken ovalbumin gene upstream promoter transcription factor from homologous oviduct cells. Mol Cell Biol. 1987 Dec;7(12):4151–4158. doi: 10.1128/mcb.7.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw M. S., Tsai S. Y., Leng X. H., Dobson A. D., Conneely O. M., O'Malley B. W., Tsai M. J. Studies on the mechanism of functional cooperativity between progesterone and estrogen receptors. J Biol Chem. 1991 Sep 5;266(25):16684–16690. [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991 Jun;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Drouin J., Nemer M., Charron J., Gagner J. P., Jeannotte L., Sun Y. L., Therrien M., Tremblay Y. Tissue-specific activity of the pro-opiomelanocortin (POMC) gene and repression by glucocorticoids. Genome. 1989;31(2):510–519. doi: 10.1139/g89-099. [DOI] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Nemer M. Glucocorticoid repression of pro-opiomelanocortin gene transcription. J Steroid Biochem. 1989;34(1-6):63–69. doi: 10.1016/0022-4731(89)90066-6. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Haddad I. A., Ordovas J. M., Fitzpatrick T., Karathanasis S. K. Linkage, evolution, and expression of the rat apolipoprotein A-I, C-III, and A-IV genes. J Biol Chem. 1986 Oct 5;261(28):13268–13277. [PubMed] [Google Scholar]
- Hwung Y. P., Crowe D. T., Wang L. H., Tsai S. Y., Tsai M. J. The COUP transcription factor binds to an upstream promoter element of the rat insulin II gene. Mol Cell Biol. 1988 May;8(5):2070–2077. doi: 10.1128/mcb.8.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwung Y. P., Wang L. H., Tsai S. Y., Tsai M. J. Differential binding of the chicken ovalbumin upstream promoter (COUP) transcription factor to two different promoters. J Biol Chem. 1988 Sep 15;263(26):13470–13474. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Berrodin T. J., Harding H. P. Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol. 1991 Oct;11(10):5005–5015. doi: 10.1128/mcb.11.10.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Ozono K., Sone T., McDonnell D. P., Pike J. W. Vitamin D receptor interaction with specific DNA requires a nuclear protein and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. H., Teng C. T. Characterization of estrogen-responsive mouse lactoferrin promoter. J Biol Chem. 1991 Nov 15;266(32):21880–21885. [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Martinez E., Givel F., Wahli W. The estrogen-responsive element as an inducible enhancer: DNA sequence requirements and conversion to a glucocorticoid-responsive element. EMBO J. 1987 Dec 1;6(12):3719–3727. doi: 10.1002/j.1460-2075.1987.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N., Kadowaki Y., Fukushige S., Shimizu S., Semba K., Yamanashi Y., Matsubara K., Toyoshima K., Yamamoto T. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988 Dec 9;16(23):11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Orchard K., Perkins N., Chapman C., Harris J., Emery V., Goodwin G., Latchman D., Collins M. A novel T-cell protein which recognizes a palindromic sequence in the negative regulatory element of the human immunodeficiency virus long terminal repeat. J Virol. 1990 Jul;64(7):3234–3239. doi: 10.1128/jvi.64.7.3234-3239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorcic M., Wang H., Elbrecht A., Tsai S. Y., Tsai M. J., O'Malley B. W. Control of transcription initiation in vitro requires binding of a transcription factor to the distal promoter of the ovalbumin gene. Mol Cell Biol. 1986 Aug;6(8):2784–2791. doi: 10.1128/mcb.6.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K., Leff T., Breslow J. L. Human apolipoprotein CIII gene expression is regulated by positive and negative cis-acting elements and tissue-specific protein factors. J Biol Chem. 1988 May 15;263(14):6857–6864. [PubMed] [Google Scholar]
- Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol. 1986 Dec;6(12):4259–4267. doi: 10.1128/mcb.6.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Evans R. M. Trans-activation by thyroid hormone receptors: functional parallels with steroid hormone receptors. Proc Natl Acad Sci U S A. 1989 May;86(10):3494–3498. doi: 10.1073/pnas.86.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Sagami I., Wang H., Tsai M. J., O'Malley B. W. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell. 1987 Aug 28;50(5):701–709. doi: 10.1016/0092-8674(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Sagami I., Tsai M. J., O'Malley B. W. Purification and characterization of chicken ovalbumin upstream promoter transcription factor from HeLa cells. J Biol Chem. 1987 Nov 25;262(33):16080–16086. [PubMed] [Google Scholar]
- Widom R. L., Ladias J. A., Kouidou S., Karathanasis S. K. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991 Feb;11(2):677–687. doi: 10.1128/mcb.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J., Muller E., Ab G. Oestrogen facilitates the binding of ubiquitous and liver-enriched nuclear proteins to the apoVLDL II promoter in vivo. Nucleic Acids Res. 1991 Jan 11;19(1):33–41. doi: 10.1093/nar/19.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J., Philipsen J. N., Ab G. Tissue-specific and steroid-dependent interaction of transcription factors with the oestrogen-inducible apoVLDL II promoter in vivo. EMBO J. 1988 Sep;7(9):2757–2763. doi: 10.1002/j.1460-2075.1988.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]