ABSTRACT
Objectives
We review the state-of-the-art neurobiology of nerve injury and regeneration, especially as it relates to return of useful function in patients who have sustained injuries to large nerve trunks such as the brachial plexus.
Methods
This review focuses on research conducted in our laboratory at Ochsner and at other laboratories related to the neurobiology of nerve injury with emphasis on how some of the key findings from animal research help us understand the pathophysiology of poor functional recovery after nerve injury.
Conclusions
Published research on the neurobiology of nerve injury and regeneration strongly suggests that chronic Schwann cell denervation, chronic neuronal axotomy, and misdirection of regenerating axons into wrong endoneurial tubes are primarily responsible for poor functional recovery. The effect of muscle denervation atrophy is secondary. Experimental therapeutic strategies (which we are currently investigating in our laboratory at Ochsner) to combat these 3 neurobiologic phenomena have the potential to improve the return of function in patients who have sustained nerve injuries.
Keywords: Axotomy, cytokines, denervation, nerve growth factors, nerve regeneration, neurobiology, peripheral nerve injuries, recovery of function, Schwann cells
INTRODUCTION
Nerve injuries trigger complex cell-molecular interactions that are essential for axonal regeneration and, subsequently, for meaningful functional recovery for patients. These cellular and molecular responses of the nonneuronal cells of the peripheral nervous system (PNS), namely Schwann cells (SCs), create a growth-permissive environment for injured axons contrary to the central nervous system (CNS) response to injuries. However, there is a dichotomy between the capacity of injured PNS neurons to regenerate their axons after injury and suboptimal return of function after nerve injuries.
Significant advancements have been made in the technique of microsurgery of injured nerves that sometimes results in improved outcome for patients.1-3 Unfortunately, functional recovery is frequently still poor after peripheral nerve injuries, especially when nerve trunks close to the spinal cord and far from the target organs are injured. These injuries include most avulsion-type injuries, nerve lacerations, and nerve contusions. After these types of nerve injuries, injured neurons are required to regenerate their axons over long distances at the very slow rate of 1 mm/d. At this sluggish rate of regeneration, reestablishment of a functional motor unit or sense organ reinnervation may take months or even years (Figure 1),1-4 a condition referred to as chronic axotomy.5-7 Likewise, SCs in the distal nerve stumps and the target organs remain denervated for long periods, conditions known as chronic SC denervation2,8-11 and chronic muscle denervation,11 respectively.
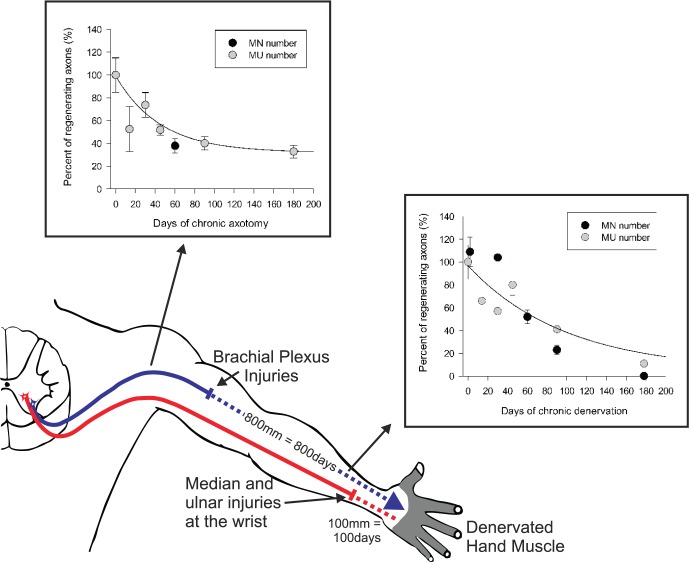
Figure 1.
Schematic illustration of proximal nerve injuries to the brachial plexus that require prolonged regeneration times for the regenerating axons to reinnervate the muscles in the hand. During this time, injured neurons are chronically axotomized and Schwann cells of the distal stump of injured nerves are chronically denervated. The graphs in the inserts demonstrate the progressive decline in the capacity of injured motoneurons that regenerate axons (MN number) and reinnervate muscles (motor unit [MU] number) as a result of chronic Schwann cell denervation and chronic neuronal axotomy.
The failure of functional return in the hand, for example, is generally attributed to irreversible atrophy of the denervated muscles and sense organs and fatty tissue replacement.1 However, our experiments over the past ∼15 years provide strong evidence that the progressive failure of chronically axotomized neurons to regenerate their axons and the progressive failure of chronically denervated SCs to support the regenerating axons play the predominant role in the clinical failures of functional recovery after microsurgical repair.2,5,7,8,10-18 Poor functional recovery is also exacerbated by the delay incurred in microsurgical repair of injured nerves while patients are being observed for spontaneous functional recovery, typically for 3-6 months after blunt nerve injuries. Regeneration of axons into wrong endoneurial tubes and end organs—termed misdirection of regenerating axons—is another factor that contributes to poor functional recovery.
REGENERATIVE RESPONSE AFTER NERVE INJURY AND ITS ROLE IN FUNCTIONAL OUTCOME
Initial Phase of Axonal Regeneration
After nerve injury, the proximal and distal stumps of the injured nerve undergo structural and molecular changes in preparation for the process of axonal regeneration. The proximal stump undergoes die back degeneration up to the first node of Ranvier and then each injured axon elaborates multiple daughter axons.19,20 Many of the daughter axons are pruned; those that remain begin the process of elongation through the distal nerve stumps and constitute regenerating units.21 This initial stage of axonal regeneration is sustained by both the availability of locally produced cytoskeletal materials and the neuronally produced and anterogradely transported cytoskeletal proteins such as actin and tubulin (Figure 2A).6,20,22,23 Axon regeneration proceeds at a rate of 1-3 mm/d, the rate corresponding with the slow rate of transport of the cytoskeletal materials. Further elongation and regeneration through the distal nerve stump are dependent on the growth-supportive milieu provided by the SCs of the distal nerve stumps. Lack of Schwann cell–laden endoneurial channels (bands of Büngner) results in misdirected regeneration and formation of neuromas.3,24-26
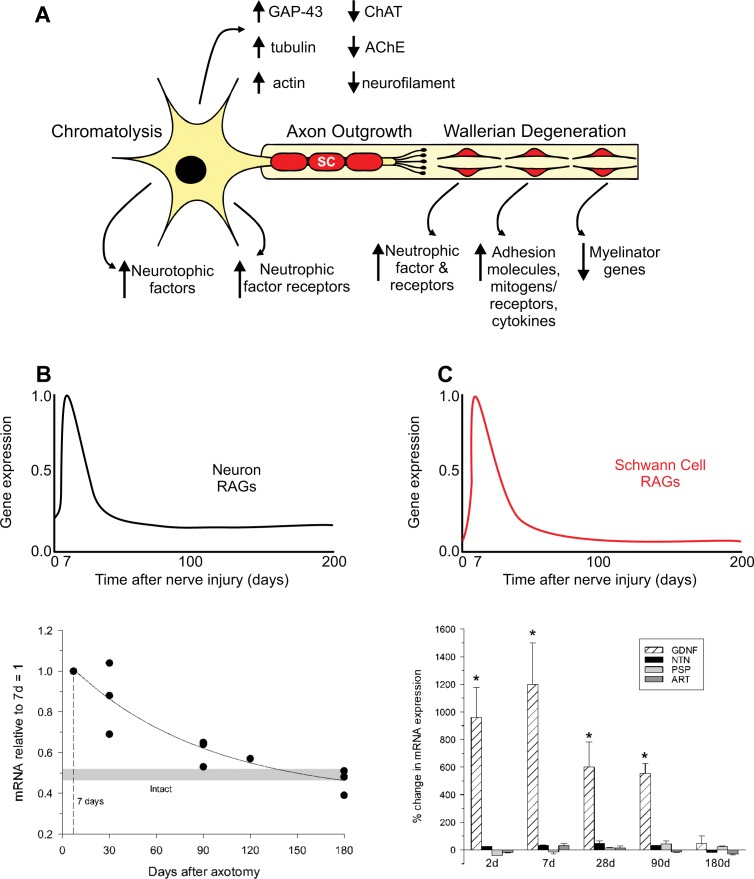
Figure 2.
After nerve injury, regeneration-associated genes (RAGs) are upregulated transiently in the neurons while genes associated with normal synaptic transmission are downregulated (A and B). Schwann cells in the denervated nerve stumps undergo proliferation during Wallerian degeneration and express many RAGs while myelin-associated genes are downregulated (A). The gene profiles support the outgrowth of axons, but the expression is very short lived such that over time (while regenerating axons grow at a slow rate of 1 mm/d) the expression of RAGs is downregulated and the capacity of injured neurons to regenerate their axons and Schwann cells to support regeneration is diminished (C). Examples of progressive decline in mRNA levels are plotted for tubulin in neurons and GDNF in Schwann cells in the graphs.
Role of Schwann Cells in Axonal Regeneration
The distal nerve stumps of severed nerves undergo a degenerative process named after Augustus Waller, Wallerian degeneration.27 We now understand that Wallerian degeneration is an essential preparatory stage of the process of axonal regeneration via which molecules that could be inhibitory to regeneration are eliminated. A recent review breaks the events into three morphologically discernible stages: acute axonal degeneration, a latency period, and an abrupt granular degenerative stage.28 Within an hour of injury, die back in both the proximal and distal stumps is mediated by channel-mediated calcium influx and activation of calpains that cleave neurofilament and microtubular-associated components such as spectrin and tubulin. In the proximal stump, this die back is to the first node of Ranvier. The next stage of approximately 24 hours, during which time axon potentials continue to be conducted,29 is followed by a longer period of disassembly of cytoskeletal proteins by calcium-activated calpains and the ubiquitin-proteasome system.30 SCs play a major role in this process by way of phagocytosis of the axonal and myelin debris. They also secrete chemoattractive factors such as interleukin-1 and monocyte chemoattractant protein-1 that recruit macrophages into the denervated distal nerve stumps which contribute significantly to the phagocytosis of axon and myelin debris.23,31,32 The axon debris releases mitogens that promote mitotic SC division and initiates a network of cytokines and transcription factors that stimulates myelin breakdown and macrophage invasion and targets the myelin for phagocytosis by the SCs and macrophages.6,33
Conversion From an Action-Potential- Conduction Mode to a Regeneration-Supportive Mode: Role of Regeneration-Associated Genes
Immediately after a nerve is injured, loss of axonal contact triggers the SCs to proliferate and switch their phenotype from a myelinating to a nonmyelinating growth-supportive phenotype.6,23,34 The messenger RNA (mRNA) expression of myelin-associated proteins such as P0 and myelin-associated glycoprotein are downregulated. Neurotrophins (eg, nerve growth factor, brain-derived neurotrophic factor, and glial-derived neurotrophic factor), their receptors (eg, p75, GFRA-1, GFRA-2), and adhesion molecules (eg, neural-cell adhesion molecule) are upregulated in preparation for the process of axonal regeneration (Figure 2).6,26,35,36 These upregulated genes are collectively called regeneration-associated genes (RAGs). The change in the gene expression and the myelin and axonal degeneration and clearance are key features of the process of Wallerian degeneration.6,23,34
Likewise, neurons whose nerves have been injured downregulate mRNAs of proteins required for neurotransmission and upregulate those for rebuilding their peripheral processes.6,12,37 Hence, actin, tubulin, and GAP-43 are upregulated immediately after injury (Figure 2A and 2B).6,37 However, the upregulation of RAGs is not sustained in either the injured neurons or the SCs. By 6 months in experimental animals, most of the upregulated mRNAs are downregulated, thereby losing the growth-supportive environment for regenerating axons (Figure 2A and 2B).6,37 The implication of the time-limited upregulation of RAGs is demonstrated in the progressive decline in the capacities of injured neurons to regenerate their axons and of SCs to support regenerating axons as the duration of nerve repair is prolonged (Figure 3).
Figure 3.
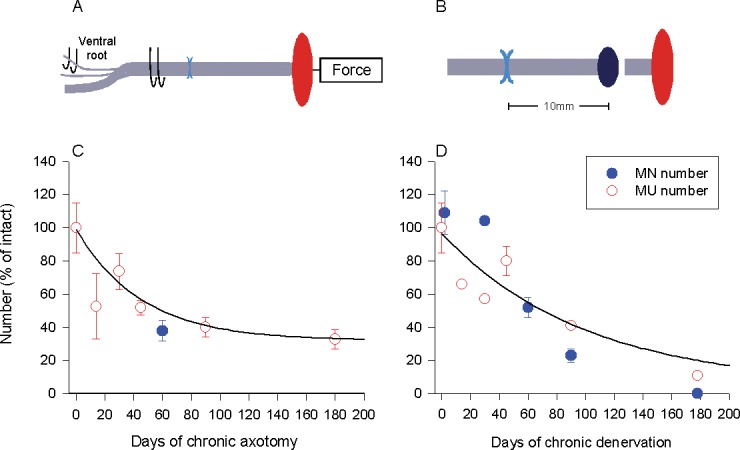
Regenerative capacity declines with time due to prolonged axotomy and Schwann cell denervation. In rats, we experimentally prolonged either (A) the duration of time during which motoneurons were prevented from regenerating their axons (chronic axotomy; by delaying the suture of the proximal nerve stump to a freshly denervated distal nerve stump) or (B) the denervation of Schwann cells in the distal nerve stumps (chronic denervation; by delaying the suture of a freshly cut proximal nerve stump to a nerve stump that was chronically denervated prior to nerve repair). The capacity of the neurons for regeneration after chronic axotomy of motoneurons or chronic denervation of Schwann cells and target muscles was determined either by (a) calculating the number of reinnervated muscle units (the nerve and the muscle fibers that the one motoneuron supplies) using force measurements in response to stimulation of single axons and the muscle nerve or (b) counting backlabelling motoneurons that had regenerated their axons successfully by application of a retrograde dye to the regenerating axons in the nerve stump distal to the site of nerve repair. The evaluations of regenerative success obtained by the methods of motoneuron counts and counts of motor units were in good agreement demonstrating the progressive decline in regenerative capacity as a function of (C) chronic axotomy of the motoneurons and (D) chronic denervation of the Schwann cells.
EXPERIMENTAL PARADIGMS AND ASSESSMENT OF AXONAL REGENERATION AFTER NERVE INJURY AND MICROSURGICAL REPAIR
Experimental studies that were conducted during World War II by Gutmann and Young indicated that the rate of outgrowth and axon numbers were not affected by delayed nerve repair.38,39 These findings, with evidence of extreme atrophy of the denervated muscles, led to the erroneous conclusion that poor functional recovery after nerve injury was due to irreversible denervation atrophy of muscle and its inability to accept innervation, especially after long periods of time.38,39 This conclusion became quite popular and unfortunately is often repeated even in recent publications. Recent evidence from our laboratory and others disapproved the erroneous conclusion mainly because (1) the authors did not directly estimate the numbers of injured neurons that regenerated into the distal nerve stump and those that reinnervated denervated muscles and (2) counting nerve fibers in the distal nerve stump overestimates the numbers of regenerated nerve fibers because many daughter axons arise from single regenerating nerve fibers in the proximal nerve stump and may be erroneously counted as representative of the individual nerve fibers in the proximal stump.
In several experiments, we studied the process of axonal regeneration after immediate and delayed repairs of nerve injury. Further, we used quantitative methods of counting the motoneurons that regenerated their axons into distal nerve stumps and of counting the number of reinnervated motor units in the target muscles to assess the capacities of motoneurons to regenerate their axons and to reinnervate muscle. Using a cross-suture technique in rats, which allows for independent study of the effects of delayed reinnervation of the distal nerve stump (termed chronic or prolonged denervation) and delayed neuronal regeneration to their targets (termed chronic or prolonged axotomy), we evaluated the numbers of regenerated motoneurons using the back-labeling technique whereby retrogradely transported fluorescent dyes are applied to the cut ends of reinnervated distal nerve stumps which label the motoneurons that regenerated their axons. This way, we were able to enumerate the number of motoneurons that regenerated their axons in the ventral horn of the spinal cord after our terminal experiments. We estimated the number of motor units by applying supramaximal stimulation to individual ventral roots and measuring total muscle force. Then we teased out ventral root fibers and stimulated them individually to determine motor unit force. The number of reinnervated motor units was then calculated by a division of muscle force by motor unit force. Figure 3 illustrates our surgical paradigm and methods of assessment of axonal regeneration and muscle reinnervation after nerve injury and repair.
Chronic Schwann Cell Denervation
The provision of the growth-supportive environment by SCs is intimately related to the loss and timely reestablishment of axonal contact with the cells.10 SCs whose reinnervation by regenerating axons is delayed are said to be chronically denervated and the deleterious effect of chronic denervation on SCs worsens as the duration of chronic denervation is prolonged.2,10,18 In our experiments, to test the effect of chronic denervation, the common peroneal (CP) branch of the sciatic nerve is cut and its reinnervation is delayed for periods of 1-6 months prior to cross-suturing the proximal stump of a freshly cut tibial (TIB) nerve into the chronically denervated distal CP nerve. This way, the effect of chronic CP denervation on the regeneration of freshly injured TIB motoneurons can be directly estimated. Using this paradigm, chronic denervation reduced the number of motoneurons that were back labeled with fluororuby dye that was applied to the distal nerve stump 10 mm from the cross-suture site to less than 10% of the number that regenerated after immediate suture of nerve stumps (Figure 3). There was excellent correspondence between this proportion of motoneurons that regenerated their axons into the chronically denervated nerve stump and the proportion of freshly axotomized motoneurons that regenerated and reinnervated the denervated muscle after 4 to 6 months (Figure 3).10 The reduced number of motoneurons that did regenerate their axons through the chronically denervated SCs not only reinnervated the denervated muscle fibers but also reinnervated up to 3 times the number of muscle fibers that they normally do. Since this enlargement of the reinnervated motor units constitutes the maximum sprouting capacity of the motoneurons,2,10 it follows that it is not the inability of the chronically denervated muscle fibers to accept reinnervation that limits functional recovery after chronic nerve injuries.2,10,23 The sustained capacity of denervated muscle to accept reinnervation was also demonstrated by the parallel recovery of both muscle weight and isometric force. Nonetheless, isolation of the effects of SC denervation from muscle denervation showed that chronically denervated muscle fibers did not fully recover their former size, arguing that limited numbers of satellite cells are incorporated into each atrophic muscle fiber to recover muscle fiber cross-sectional area.11 Hence, progressive deterioration of the growth-supportive capacity of SCs in the distal nerve stumps plays a primary role in poor functional recovery after nerve injury and the role of muscle atrophy is secondary. It was striking that the long-term chronically denervated SCs maintained their capacity to remyelinate the fewer axons that regenerated,2 particularly in view of the progressive regression of the capacity of the denervated SCs to sustain their growth-permissive phenotype and the progressive decline in numbers of growth-supportive SCs in the chronically denervated distal nerve stumps.36,40
A clinical correlate of our experimental cross-suture paradigm is the injuries to large nerve stumps in which SCs of the distal nerve stumps are left chronically denervated due to the slow rate of regenerating axons. The longer it takes for regenerating axons to reinnervate SCs in the distal nerve stumps, the more prolonged the period of chronic denervation is during which the SCs do not have contact with axons and the higher the likelihood that their capacity to support regenerating axons is impaired.2,6,10 We believe that this is partly why reinnervation and subsequent functional recovery in muscle targets located close to regenerating motoneurons are better than in more distally placed muscle targets.
Chronic Neuronal Axotomy
Injured neurons whose axons may or may not be regenerating but have not made target connections are said to be subjected to chronic axotomy.5,12,14 For example, after brachial plexus injury, the injured neurons have to regenerate over a long distance before they can reinnervate some of the denervated muscles and are thereby being subjected to chronic axotomy. To the test the effect of chronic axotomy, we also used the cross-suture technique by using the 2 branches of rat sciatic nerve. The TIB branch of the sciatic nerve is cut and its regeneration is delayed for periods of 1-6 months prior to cross-suturing the proximal stump of the chronically axotomized proximal stump of the TIB nerve into a freshly cut distal CP nerve. This way, the effect of chronic TIB axotomy on the reinnervation of a freshly injured distal CP nerve can be directly estimated. Using this paradigm and the outcome measures described above, we found that the number of motoneurons that regenerated their axons fell progressively as a function of prolonged time of chronic axotomy to ∼37% of those that regenerated without the effect of chronic axotomy.5,7 This reduced capacity to regenerate after chronic axotomy is significant, especially when combined with the deleterious effect of chronic denervation, both of which occur concurrently particularly after injuries to large nerve trunks such as the brachial plexus. The progressive decline in regenerative capacity of the chronically axotomized motoneurons was paralleled by a decline in the upregulated RAGs.41 The dramatic negative effect of reduced regenerative capacity of the chronically axotomized motoneurons did not affect the ability of the neurons to reinnervate up to 3 times as many muscle fibers as normal, the fewer motoneurons enlarging their motor units and the number of muscle fibers supplied by each reinnervating motoneuron.5 Thus, chronic axotomy impairs the regenerative capacity of motoneurons but does not impair the capacity of the smaller number of regenerated motor axons to make functional connections with denervated muscle fibers.
Staggered Axon Regeneration and Misdirection of Regenerating Axons
One of the key prerequisites for successful functional recovery is that regenerating axons regenerate into the correct endoneurial tubes that direct them back to their original target organs. However, we found that regenerating axons encounter a significant delay at the injury site and traverse the injury site into the distal nerve stumps in a staggered fashion (staggered axonal regeneration). Indeed, the crossing of regenerating axons occurs slowly prior to the entry of the regenerating axons into the distal nerve stumps. It is only once the axons enter the distal stumps that they regenerate at the slow rate of transport of 1-3 mm/d.
Functional outcomes after axonal injuries are best after nerve crush injuries in which the endoneurium remains anatomically intact all the way to the target, and axonal sprouts of the regenerating unit are contained within the original endoneurium so the regenerating axons are led back to their original targets.3 With time, reinnervated muscle fibers display the normal mosaic distribution of muscle fiber types, motor unit and muscle isometric forces recover fully, the original number of functional motor units is restored, and the numbers and diameters of the myelinated nerve fibers return to normal.42
Nerve injuries that disrupt the endoneurial tubes (such as axonotmetic or neurotmetic injuries) whereby regenerating axons have to find their original endoneurial tubes introduces potential for errors during the regenerative process. Axonal sprouts emanating from the proximal nerve stump may enter several different endoneurial tubes with target destinations that they did not formerly supply as in the case of motor axons that regenerate within pathways leading to the skin rather than muscle.43 Experiments that applied retrograde dyes to regenerated axons of transected sciatic and facial nerves in rats directly demonstrated the misdirection of regenerating motor axons from several different motoneuron pools to muscles that the motoneurons did not formerly supply.44 In cases where the nerves supplied muscles with antagonistic actions, axonal misdirection was associated with inappropriate movements, quite consistent with findings that flexor and extensor motoneurons sustain their normal but inappropriate pattern of firing when they are directed to inappropriately reinnervate extensor and flexor muscles, respectively.45 We believe that this misdirection of regenerating injured axons plays an important role in reducing functional recovery after nerve injuries.
There are some exceptions to the misdirection of regenerating axons even after a transaction injury. These include several peripheral nerve trunks whose branches innervate skeletal muscles and sense organs in the skin and the joints. Examples include the femoral nerve with two branches, the muscle branch containing motor and sensory nerves to the quadriceps muscle, and the other pure sensory saphenous nerve branch to the skin.43 Although reinnervation of motor and sensory nerve branches is initially random, the motoneurons that progressively regenerate their axons across the suture site send their axons into the appropriate motor branch.43,46 This preferential motor reinnervation emerged in parallel with the progressive or staggered regeneration of motor axons into the distal nerve stumps.46 An unusual acidic glycan associated with myelin profiles of motor but not sensory mouse axons that is recognized by a monoclonal antibody L2/HNK-1 may be one mechanism of the preferential reinnervation of appropriate pathways.47,48 The differential neurotrophic factor profile in SCs derived from sensory and motor axons with the BDNF/NT4/5 profile is also likely to play an important role in preferential reinnervation of motor pathways.49
CONCLUSIONS
A tremendous amount of progress has been made in our understanding of the microanatomy, pathophysiology, and microsurgical management of injured nerves. These advancements have improved the quality of care provided to patients inflicted with nerve injuries and often result in better functional recovery. However, patients who fail to recover good function despite excellent microsurgical care pose a challenge to the nerve surgeon. The upregulation of RAGs is short lived, and there seems to be a time window of opportunity during which SCs provide a growth-supportive environment and the injured neurons can regenerate their axons. The time-limited upregulation of RAGs and the slow rate of axonal regeneration result in progressive loss of neurotrophic support for the injured neurons and their regenerating axons, hence chronic SC denervation and chronic neuronal axotomy.5,6,23 Therefore, in our laboratory at Ochsner, we focus on 2 possible approaches to combat the gap between the timing of upregulation of RAGs and the slow rate of regeneration including experimental strategies that accelerate the rate of axonal regeneration and sustain the neurotrophic environment for regenerating axons for longer periods. We demonstrated the positive effect of brief (1 h) low-frequency electrical stimulation in accelerating axonal regeneration by modulating the expression of RAGs.46,50-52 We also showed the positive effect of transforming growth factor beta in reactivating and sustaining the expression of RAGS by chronically denervated SCs. We are now exploring the role of transplantation of stem cells into the distal nerve stumps and the use of enhanced nerve guidance channels or conduits that will not only eliminate the need for autografts but will also allow for positive modulation of the growth-permissive environment for axons traversing the guidance channels into the distal nerve stumps.53,54
We believe that the ultimate solution to improve functional recovery in the subset of patients who currently do not do well with microsurgical repair is a combination of several neurobiological approaches to overcome the challenge of the limited time window for optimal nerve regeneration.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Sulaiman OAR, Midha R, Gordon T. Pathophysiology of surgical nerve disorders. In: Winn HR, editor. Youmans Neurological Surgery. 6th ed. Philadelphia, PA: Saunders;; 2011. pp. 2368–2379. In. ed. [Google Scholar]
- 2.Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000 Dec;32(3):234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Sunderland S. Nerves and Nerve Injuries. Edinburgh, Scotland: Churchill Livingstone;; 1978. [Google Scholar]
- 4.Sulaiman WA, Kline DG. Nerve surgery: a review and insights about its future. Clin Neurosurg. 2006;53:38–47. [PubMed] [Google Scholar]
- 5.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995 May;15(5 Pt 2):3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997 Feb-Apr;14(1-2):67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 7.Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002 Feb;15(4):613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 8.Sulaiman OA, Midha R, Munro CA, et al. Chronic Schwann cell denervation and the presence of a sensory nerve reduce motor axonal regeneration. Exp Neurol. 2002 Aug;176(2):342–354. doi: 10.1006/exnr.2002.7928. [DOI] [PubMed] [Google Scholar]
- 9.Sulaiman OA, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic Schwann cell denervation. Exp Neurol. 2002 May;175(1):127–137. doi: 10.1006/exnr.2002.7878. [DOI] [PubMed] [Google Scholar]
- 10.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995 May;15(5 Pt 2):3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011 Apr 6;31(14):5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003 Jun;27(3):277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 13.Sulaiman OA, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002 Mar 1;37(3):206–218. doi: 10.1002/glia.10022. [DOI] [PubMed] [Google Scholar]
- 14.Gordon T, Fu SY. Long-term response to nerve injury. Adv Neurol. 1997;72:185–199. [PubMed] [Google Scholar]
- 15.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003 Oct;183(2):610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 16.Sulaiman OAR, Gordon T. TGF-beta reverses the deleterious effect of long-term Schwann cell denervation on nerve regeneration by inducing erbB3 receptor expression. Glia. 2003 Sep;43(Suppl 2):24. [Google Scholar]
- 17.Midha R, Munro CA, Chan S, Nitising A, Xu QG, Gordon T. Regeneration into protected and chronically denervated peripheral nerve stumps. Neurosurgery. 2005 Dec;57(6):1289–1299. doi: 10.1227/01.neu.0000187480.38170.ec. [DOI] [PubMed] [Google Scholar]
- 18.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009 Oct;65(4 Suppl):A105–A114. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- 19.Cajal SR. Degeneration and Regeneration of the Nervous System. Oxford, UK: Oxford University Press; 1928. Translated by R. M. May. [Google Scholar]
- 20.Ertürk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 2007 Aug 22;27(34):9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the “regenerating unit. Z Zellforsch Mikrosk Anat. 1972;124(1):103–130. [PubMed] [Google Scholar]
- 22.McQuarrie IG. Effect of conditioning lesion on axonal sprout formation at nodes of Ranvier. J Comp Neurol. 1985 Jan 8;231(2):239–249. doi: 10.1002/cne.902310211. [DOI] [PubMed] [Google Scholar]
- 23.Sulaiman OAR, Boyd JG, Gordon T. Axonal regeneration in the peripheral system of mammals. In: Kettenmann H, Ransom BR, editors. Neuroglia. 2nd ed. Oxford, UK: Oxford University Press; 2005. pp. 454–466. In. eds. [Google Scholar]
- 24.Aitken JT, Sharman M, Young JZ. Maturation of peripheral nerve fibres with various peripheral connections. J Anat. 1947;81:1–22. [PubMed] [Google Scholar]
- 25.Toft PB, Fugleholm K, Schmalbruch H. Axonal branching following crush lesions of peripheral nerves of rat. Muscle Nerve. 1988 Aug;11(8):880–889. doi: 10.1002/mus.880110813. [DOI] [PubMed] [Google Scholar]
- 26.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007 Jul;82(4):163–201. doi: 10.1016/j.pneurobio.2007.06.005. Epub 2007 Jun 22. [DOI] [PubMed] [Google Scholar]
- 27.Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos Trans R Soc London. 1850;140:423–429. [Google Scholar]
- 28.Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012 Jan 9;196(1):7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai Q, Wang J, Kim A, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003 Jul 17;39(2):217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 31.Sulaiman OAR, Cellular Gordon T. and molecular interactions after peripheral and central nerve injury. Biomed Rev. 2003;14:51–62. [Google Scholar]
- 32.Höke A, Mi R. In search of novel treatments for peripheral neuropathies and nerve regeneration. Discov Med. 2007 Aug;7(39):109–112. [PubMed] [Google Scholar]
- 33.Brushart TM. Nerve Repair. New York, NY: Oxford University Press;; 2011. [Google Scholar]
- 34.Scherer SS, Salzer JL. Axon-Schwann cell interactions during peripheral nerve degeneration and regeneration. In: Jessen KR, Richardson WD, editors. Glial Cell Development: Basic Principles and Clinical Relevance. Oxford, UK: Bios Scientific;; 1996. pp. 169–196. In. eds. [Google Scholar]
- 35.Höke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002 Jan;173(1):77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 36.You S, Petrov T, Chung PH, Gordon T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia. 1997 Jun;20(2):87–100. doi: 10.1002/(sici)1098-1136(199706)20:2<87::aid-glia1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Bisby MA, Tetzlaff W. Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration. Mol Neurobiol. 1992;6(2-3):107–123. doi: 10.1007/BF02780547. Summer-Fall. [DOI] [PubMed] [Google Scholar]
- 38.Gutmann E. Effect of delay of innervation on recovery of muscle after nerve lesions. J Neurophysiol. 1948 Jul;11(4):279–294. doi: 10.1152/jn.1948.11.4.279. [DOI] [PubMed] [Google Scholar]
- 39.Gutmann E, Young JZ. The re-innervation of muscle after various periods of atrophy. J Anat. 1944 Jan;78(Pt 1-2):15–43. [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Terenghi G, Hall SM. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997 Aug;20(4):333–347. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Tetzlaff W, Leonard C, Krekoski CA, Parhad IM, Bisby MA. Reductions in motoneuronal neurofilament synthesis by successive axotomies: a possible explanation for the conditioning lesion effect on axon regeneration. Exp Neurol. 1996 May;139(1):95–106. doi: 10.1006/exnr.1996.0084. [DOI] [PubMed] [Google Scholar]
- 42.Gordon T, Pattullo MC. Plasticity of muscle fiber and motor unit types. Exerc Sport Sci Rev. 1993;21:331–362. [PubMed] [Google Scholar]
- 43.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993 Jun;13(6):2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brushart TM, Mesulam MM. Transganglionic demonstration of central sensory projections from skin and muscle with HRP-lectin conjugates. Neurosci Lett. 1980 Apr;17(1-2):1–6. doi: 10.1016/0304-3940(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 45.Gordon T, Stein RB, Thomas CK. Organization of motor units following cross-reinnervation of antagonistic muscles in the cat hind limb. J Physiol. 1986 May;374:443–456. doi: 10.1113/jphysiol.1986.sp016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000 Apr 1;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994 Nov;14(11 Pt 2):7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberhardt KA, Irintchev A, Al-Majed AA, et al. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol. 2006 Apr;198(2):500–510. doi: 10.1016/j.expneurol.2005.12.018. Epub 2006 Feb 7. [DOI] [PubMed] [Google Scholar]
- 49.Höke A, Redett R, Hameed H, et al. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006 Sep 20;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000 Dec;12(12):4381–4390. [PubMed] [Google Scholar]
- 51.Brushart TM, Hoffman PN, Royall RM, et al. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002 Aug 1;22(15):6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon T, Sulaiman OA, Ladak A. Chapter 24: Electrical stimulation for improving nerve regeneration: where do we stand? Int Rev Neurobiol. 2009;87:433–444. doi: 10.1016/S0074-7742(09)87024-4. [DOI] [PubMed] [Google Scholar]
- 53.Midha R. Emerging techniques for nerve repair: nerve transfers and nerve guidance tubes. Clin Neurosurg. 2006;53:185–190. [PubMed] [Google Scholar]
- 54.Pfister LA, Papaloïzos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007 Jun;12(2):65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]