ABSTRACT
Background
Chemotherapeutic resistance and local recurrence or distant organ metastasis are the major causes of cancer mortality. Conventional cancer treatments do not consistently prevent cancer recurrence.
Methods
We illustrate the key roles that cancer stem cells and the tumor microenvironment—particularly the lymph node stromal microenvironment—play in tumor drug resistance, metastasis, and recurrence in 2 representative cancers: colorectal cancer and follicular lymphoma.
Conclusion
We believe that combination treatment with chemotherapeutic agents in conjunction with targeted therapies, such as stromal/cancer stem cell signaling–targeted therapy, may effectively minimize cancer recurrence.
Keywords: Cancer metastasis, cancer stem cells, drug resistance, stromal cells, tumor microenvironment
INTRODUCTION
The current treatments of most solid organ tumors are surgically based with the addition of chemotherapy and radiation depending on tumor stage and histological grade.1 The standard treatment for colon cancer is resectional therapy with the addition of postoperative chemotherapy for lymph node (LN)-positive cancers and those with poor pathologic features such as lymphovascular invasion. Unfortunately, despite appropriate surgery and standard chemotherapeutic treatments, up to 50% of these cancers will recur, making chemotherapeutic resistance and local recurrence or distant organ metastasis the major causes of cancer mortality.2,3 Conventional treatments—surgery with chemotherapy and radiation—fail to effectively prevent extranodal recurrence, even in cases of successful eradication of all visible tumors.4,5 When such recurrences occur, the cancer cells often demonstrate a chemoresistant phenotype. This chemoresistance can be associated with genetic alterations within the cancer cells, but recent studies propose that recurrence is associated with the presence of cancer stem cells (CSCs, also called tumor-initiating cells)6,7 and the interaction with the LN microenvironment.8,9 Current cancer therapies inadequately treat this rare but highly significant population of CSCs.2,10 These therapies do not address the tumor-nurturing role that the microenvironment, specifically the LN microenvironment, plays in cancer recurrence. Thus, an alternative therapeutic approach should be considered.
CANCER STEM CELLS
Recent evidence indicates the functional heterogeneity of cancer cells is a result of cell differentiation,11 and a specific cell population of CSCs exists in various cancers that may be identified by cell surface markers such as CD133, CD44, aldehyde dehydrogenase 1 (ALDH1) enzyme expression, or side population (SP). However, specific CSC markers are cancer dependent.12,13 CSCs are similar to normal stem cells in that they have the ability to self-renew while producing differentiated daughter cells.14 Conventional chemotherapies and radiotherapies target proliferating cells and require active cycling to induce apoptosis. The quiescent nature of CSCs allows for resistance to conventional chemoradiation treatments.7,15,16 Thus, in addition to conventional cancer treatments, targeting the CSC population may be essential to prevent recurrence or metastasis.
Colorectal CSCs
In the United States, colorectal cancer is the third most common malignancy and the second most common cause of cancer-related mortality, with an estimated incidence of 143,000 cases and 51,000 deaths per year.5 Colorectal CSCs (Co-CSCs) express a variety of surface markers, including CD133,10,17,18 ALDH1,19 the epithelial specific antigen (CD326), CD44, and CD166.20 CD133, a transmembrane glycoprotein molecule with a molecular weight of 120 kDa on chromosome 4p15.32, is one of the more promising cell surface markers. CD133+ cancer cells were shown to have the ability to self-renew, retain tumorigenicity, and regenerate a tumor after treatment.17,20 Li et al21 showed that in stage IIIB colon cancer, recurrence correlated with the percentage of CD133+ cells present in the original tumor. While CD133+ cells have been shown to meet some of the characteristics of CSCs, their specificity as a true Co-CSC marker has been questioned.22 CD133+ cells might represent a heterogeneous population of cells, and CD133 might lack a functional role in tumor initiation.23 Do CD133+ cells need other interactions to function as tumor-initiating cells? We recently used colorectal cancer cell lines HT-29 and HCA-7 and human colorectal cancer specimens to show that a small proportion of Co-CSCs expressing both CD133 and C-X-C chemokine receptor type 4 (CXCR4)—a membrane-bound receptor for stromal cell-derived factor-1 or chemokine (C-X-C motif) ligand 12 (CXCL12)—demonstrated increased tumor-initiating ability in immunodeficient mice in the presence of a human LN stromal cell line—HK cells24,25—and HK cell-conditioned media. In addition, these double-positive Co-CSCs were enriched in a chemotherapy-resistant cell population.26 Thus, CD133 and CXCR4 in combination may be better markers for drug-resistant Co-CSC.
Follicular Lymphoma CSCs
Follicular lymphoma is the second most common form of non-Hodgkin lymphoma (NHL) in the Western Hemisphere, representing nearly 25% of all NHL cases.27 Follicular lymphoma arises from B cells, with a clinical course that is frequently indolent and responsive to chemotherapy. However, multiple relapses following treatment are common. More than half of the patients who experience a recurrence become refractory to treatment and do not survive more than 5 years.28
A quiescent population of drug-resistant CSCs has been identified in the SP fraction in various malignancies, including Hodgkin lymphoma.29-33 The SP fraction expressed the drug-resistant gene ATP-binding cassette sub-family G member 2 (ABCG2) and demonstrated higher tumorigenic capacity than the non-SP fraction.29,34,35 Recently, we used a CSC enrichment technique to isolate the SP fraction from both the follicular lymphoma cell line and patient specimens. Compared with parental cells, a significantly higher percentage of cells in the SP cell fraction formed colonies, initiated tumors, and were resistant to chemotherapy and irradiation treatments, confirming that SP cells obtained from follicular lymphoma were highly enriched with CSCs. Most important, we found that follicular lymphoma stem cells (FL-SCs) interact with follicular dendritic cells (FDCs)/HK cells in a CXCL12/CXCR4-dependent manner to maintain tumorigenicity.36
Other Cancers
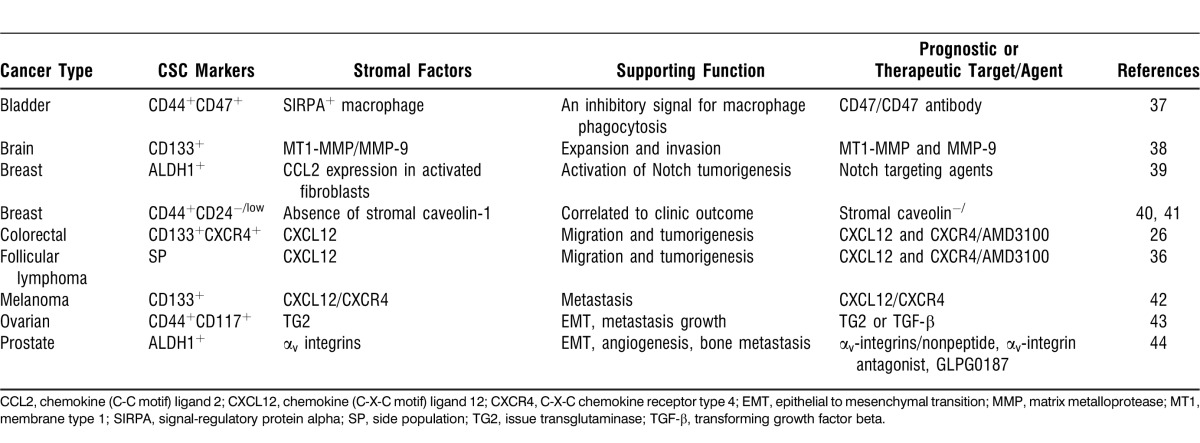
CSCs and their interaction with tumor stromal cells were also discovered in other cancers, such as bladder, brain, breast, ovarian, and prostate cancers (Table).12 CSCs exist in various cancers with cell surface markers dependent on cancer type. Some are supported by interactions with the LN stroma for survival, proliferation, tumorigenesis, drug resistance possible metastasis, and recurrence.
Table.
Cancer Stem Cell (CSC) and Tumor Microenvironment Studies
CANCER STROMAL MICROENVIRONMENT
Studies show that not all cancer cells are tumorigenic.45 Some cancer cells require coinjection with stromal cells to form tumors in immunodeficient mice,26,46 indicating that CSCs are supported by microenvironmental factors produced by the surrounding stroma.47 For example, Gilbertson and Gutmann48 found that in brain tumors, the interaction between brain CSCs and signals from the local microenvironment is significant for region-specific tumorigenesis. In mouse renal carcinoma cell studies, Smith et al49 found that agarose macrobeads selectively support CSCs in a 3-dimensional culture that mimics the in vivo tumor microenvironment. The tumor microenvironment has been recognized as a major factor influencing the growth of cancer and impacting the outcome of therapy. While the niche cells are not malignant per se, their role in supporting cancer growth is vital for tumor survival. Thus, niche cells have become an attractive target for chemotherapeutic agents.50 Clearly, environment-mediated drug resistance is induced by signaling events from the tumor microenvironment and is likely to be reversible because removal of the microenvironment restores the tumor's drug sensitivity.8,51
LN Stromal Microenvironment: FDCs
Evidence shows that CSCs that develop in lymphoid follicles as in follicular lymphoma or CSCs that metastasize to the LN are stimulated by the LN microenvironment. The major problems with follicular lymphoma treatment are relapse and transformation. The transformation of follicular lymphoma to therapy-resistant aggressive large B-cell lymphoma52 is associated with the induction of stromal gene signatures, including CXCL12.53 The potential mechanisms of relapse may involve the interaction of tumorigenic follicular lymphoma cells with the stromal cell counterpart present in the LN.54 One of the putative stromal cell types that may interact with follicular lymphoma cells is FDCs. FDCs are present in the germinal center of lymphoid follicles, the site of follicular lymphoma origin.55 FDCs have been found to initiate and maintain a protumorigenic microenvironment56 by producing appropriate cytokines57 and chemokines that promote lymphoma cell proliferation.36,46,58
FDCs are the most abundant stromal cell type in the LN microenvironment. While their tumor-promoting effects are well known, their origin was recently suggested to arise from ubiquitous perivascular precursors expressing platelet-derived growth factor receptor beta.59 LN metastasis is one of the strongest negative prognostic factors for a number of cancers. For example, the majority of patients with colorectal cancer present with regional LN involvement (stage III disease), suggesting that the LN microenvironment plays a significant role in promoting extranodal recurrence and further metastasis.4 For colorectal cancer, FDCs are unique LN stromal cells that display both autocrine and paracrine properties, analogous to cancer-associated stromal fibroblasts that have been shown to nurture colorectal cancer cells through the production of various cytokines and growth factors.60 Colorectal cancer cells interact with tumor-fostering stromal cells and the extracellular matrix in a protective fashion, decreasing chemotherapy-induced apoptosis.61
To evaluate the role of LN stromal cells in tumor growth, we established in vivo tumor models using CSCs and an FDC cell line—HK cells. Although derived from tonsilar cells, the HK cell line functionally resembles primary FDCs in expressing smooth muscle antigen, von Willebrand factor, and vimentin, but not CD31, and in supporting germinal center B cells and lymphoma growth.46,57 In our in vivo model, the addition of HK cells increased tumor formation, especially in lower dosages of CSCs, suggesting that FDCs/HK cells play a key role in cancer cell survival, tumor initiation, and in vivo growth in both follicular lymphoma and colorectal cancer models.26,36
CXCL12/CXCR4 Signaling
Various chemokines play important roles in stromal cell/CSC niche interaction. CXCL12 is a chemokine that regulates many essential biological processes, including revascularization, cellular adhesion, and tumorigenesis.62 CXCL12 is also one of many soluble microenvironmental factors produced by FDCs in a paracrine fashion.63 It has a negative effect in multiple cancers.64 For example, CXCL12 and CXCR4 are involved in tumor metastasis and extranodal recurrence in colorectal cancer,65-67 and the metastatic activity of CD133+CXCR4+ CSCs is increased in pancreatic cancer.68 Downregulation of CXCR4 significantly decreased cell migration and invasion only in pancreatic CSCs cocultured with pancreatic stromal cells.69
HK cells are known to produce CXCL12. We also found that CXCR4 is active on SP cells in follicular lymphoma and Co-CSCs. In transwell migration assays, we found that FL-SCs specifically migrated toward HK cells and CXCL12 in a dose-dependent manner. Their migration was inhibited by the presence of AMD3100, a specific small molecule inhibitor of CXCL12/CXCR4 signaling.
Similarly, the CXCL12/CXCR4 axis is also associated with in vitro colorectal cancer migration, lymphatic and distant dissemination, disease recurrence, and decreased survival rate.65,67,70 In our experiments, drug-resistant colorectal cancer cells showed increased expression of CD133 and CXCR4. Our observation is in agreement with Dessein et al71; they recently demonstrated that CXCR4 induction acts as a major mechanism underlying invasion in drug-resistant HT-29 cells. This phenomenon is not limited to colorectal cancers; CD133+CXCR4+ migrating CSCs play a crucial role in tumor initiation, growth, and metastasis in human pancreatic, prostate, and breast cancer.68,72
Cell-Cell Contact
The tumor stromal microenvironment could also promote cancer chemoresistance by direct cell-cell contact. For example, Xu et al73 proposed that transforming growth factor beta 1 (TGF-β1) produced by bone marrow stromal cells promotes the survival and chemoresistance of leukemia cells via direct cell-to-cell interactions. They showed that the blockade of TGF-β1 signaling by LY2109761 effectively inhibited prosurvival signaling and could enhance the efficacy of chemotherapy against myelomonocytic leukemic cells in the bone marrow microenvironment. Rafii et al74 demonstrated the capacity of Hospicells—an original type of stromal cells—to confer chemoresistance to ovarian and breast cancer cells by direct cell-cell contact and the exchange of membrane patches and multidrug-resistant proteins.75
In follicular lymphoma, we found that SP cells enriched in cell populations that are adherent to HK cells express ABCG2, a multidrug resistance transporter, and are in close contact with HK cells in the initial stage of tumor formation in immunodeficient mice. The HK cell dependence of FL-SCs in tumorigenesis is in both a cell-cell contact fashion and in a CXCL12/CXCR4-dependent manner because AMD3100 effectively inhibits HK cell-promoted in vivo tumor growth of the FL-SCs.36
Cytokines and Other Factors
In addition to stromal cell factors, CSCs interact with and are regulated by other cells in the tumor microenvironment via inflammatory cytokine networks, such as interleukin (IL)-1, IL-6, and IL-8.76 Recombinant human IL-1 alpha enhanced the chemotherapy-induced growth inhibition in HCT116 colon cancer cells.77 In the particular case of the plasma cell cancer multiple myeloma, the adhesion between multiple myeloma cells and bone marrow fibroblasts led to the increased secretion of IL-6.78 This pleiotropic cytokine has demonstrated the capacity to induce the resistance of multiple myeloma cells to apoptotic stimuli and chemotherapeutic drugs via the Janus kinase/signal transducers and activators of transcription (STAT) pathway and the expression of the antiapoptotic protein Bcl-xL.79 IL-6–mediated STAT3 activation plays a specific role in maintaining an inflammatory positive feedback loop in breast CSCs.80,81 In Co-CSCs, researchers found that STAT3 was constitutively activated and that these cells were sensitive to STAT3 or IL-6 inhibition for tumorigenesis.82 In addition, blockage CXCR1, an IL-8 receptor, targeted breast cancer CSCs using specific antibodies or small molecule inhibitors.83
Autocrine production of IL-4 in colon cancer cells was reported to contribute to chemoresistance84 by protecting tumorigenic CSCs from antitumor therapies through upregulation of antiapoptotic genes.85,86 In contrast, overexpression of IL-12, a potent immunomodulatory cytokine, reduced the expression of IL-4 and STAT6 in Co-CSCs, tumor-sphere formation, and tumor initiation.87 Cytokines are also used as agents for differentiation therapy. In renal cancer, it has been proposed that IL-15 directs the epithelial differentiation of CSCs to differentiated nontumorigenic cells that are sensitive to chemotherapy.88
In addition to cytokines, other factors may also be involved in supporting CSCs. For example, in bladder cancer, when cancer cells undergo the epithelial to mesenchymal transition (EMT), their invasiveness, drug resistance, angiogenesis, and metastatic ability are increased, giving rise to a more aggressive tumor type. Additionally, the tumor-supportive microenvironment (tumor-associated stromal cells and the extracellular matrix) plays a key role in tumorigenesis, tumor progression, and metastasis formation.89
To disseminate and metastasize, the cancer cells activate the EMT pathway, thereby switching toward a migrating CSC (MCSC) phenotype. This switch might be induced by the tumor microenvironment that secretes EMT-inducing growth factors and the interaction with the extracellular matrix. MCSCs can subsequently enter the blood circulation, disseminate, extravasate, and eventually colonize in the target organs to form (macro)-metastases. This process could also explain metastases at distant sites due to the different microenvironment not secreting EMT-inducing signals.89,90
SIGNIFICANCE
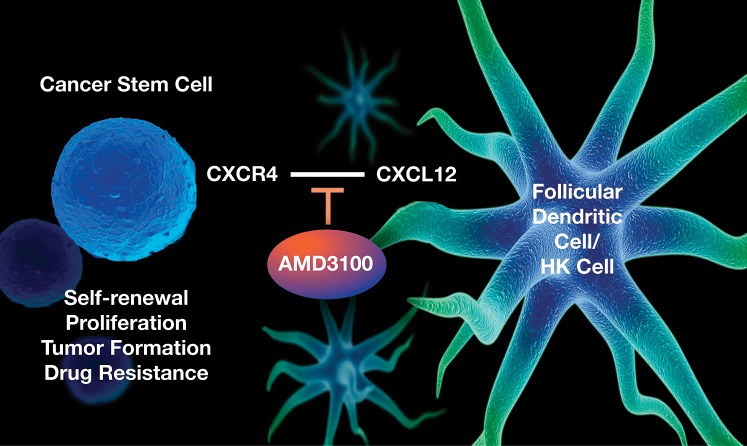
Chemotherapeutic resistance and local recurrence or distant metastasis are the major causes of cancer mortality. The observed interaction between CSCs and stromal cells provides critical insights into the mechanism of cancer drug resistance and recurrence. The CSC models suggest that niche cell signaling plays an important role in CSC-mediated tumorigenesis and evasion of chemotherapy. In both follicular lymphoma and colorectal cancer studies,26,36 we demonstrated in vitro and in vivo that drug-resistant CSCs interact with stromal FDCs via CXCL12/CXCR4 signaling to maintain tumorigenicity, suggesting that CXCL12 is one of the candidate cytokines that FDCs might secrete to modulate the tumorigenicity of these diseases (Figure). In our models, CSCs in both follicular lymphoma and colorectal cancer were enriched by chemotherapy in the presence of stromal cells. This finding suggests that targeting CSCs alone may not suffice to minimize recurrence and that future treatments must address LN microenvironmental support as well.
Figure.
Lymph node stromal cells support cancer stem cells via paracrine C-X-C chemokine receptor type 4 (CXCR4) and chemokine (C-X-C motif) ligand 12 (CXCL12) signaling. The HK cell line is a human follicular dendritic cell or lymph node stromal cell line. AMD3100 is a specific small molecule inhibitor of CXCL12/CXCR4 signaling.
Our findings are in agreement with a recent report that Co-CSC marker Wnt signaling activity is regulated by the microenvironmental myofibroblast-secreted factors,47 implicating the microenvironment as a dominant factor in Co-CSCs. CXCL12 expression in B-cell lymphoma was associated with poor prognosis.53 FDCs may promote CSC tumor growth in 2 ways: (1) by supporting CSC survival and activation for tumor initiation by providing soluble factors such as CXCL12 and (2) by enhancing the host response to increase tumor angiogenesis.
Thus, the CSCs and the environment that protects them could be therapeutic targets that eradicate recurrence. Studies that identify and characterize CSCs and their interactions with the tumor microenvironment are important steps in the development of biologically based, curative treatments for cancer. Understanding the essential signals for tumor chemoresistance and survival produced by tumor stromal cells may help develop prognostic biomarkers and novel therapeutic strategies.91 For example, a predictive gene signature was identified in bladder cancer studies that could serve as an indicator of tumor progression.37 In addition, CD47 plays a significant role in inhibiting phagocytosis, making it a prime drug target so that CD47 inhibition would enhance macrophage phagocytosis of tumor cells (Table).
Prognostic Markers
Tumor invasion and regional LN metastasis are important factors for determining cancer prognosis. For example, the 5-year survival rate for stage I colorectal cancer is 90%, but the rate decreases to 75% and 50% for stage II and III patients, respectively.4 Prognostic markers are urgently needed for cancers like colorectal cancer, especially for stage II patients. Currently, 2 prognostic biomarkers of colorectal cancer recurrence are used in the clinical setting: the Oncotype DX colon cancer assay (Genomic Health, Inc., Redwood City, CA) in the United States and ColoPrint (Agendia, Amsterdam, The Netherlands) in Europe.92,93 Their use has been limited because they are only prognostic biomarkers and not predictive biomarkers.
The development of biomarkers that are both prognostic and predictive can greatly impact the treatment of LN-positive colorectal cancer. Recently, several studies have focused on such prognostic markers. Saiki et al,94 using reverse transcription polymerase chain reaction analysis, revealed that the expression of stem cell–related genes, including LIN28 and SOX2, correlated with LN metastasis. Although the colorectal cancer tumor tissues examined had been collected by laser microdissection, these results may indicate that the Co-CSC population is increased at metastatic sites. When putative CSC markers were tested in colorectal cancer tumor buds, ABCG5 and EpCAM were significantly associated with a poorer prognosis.95 However, most published studies have been conducted with bulk tumor samples without differentiating the response between Co-CSCs and non-CSCs. Because Co-CSCs are such a small population, precise analysis of prognostic markers within Co-CSCs may yield more accurate prognostic significance.
In follicular lymphoma, CXCL12 and CXCR4 expression may also serve as prognostic markers for risk of disease transformation, because FL-SCs express higher levels of CXCR4 and inhibition of CXCL12/CXCR4 interaction abolished FLK-1 cell—a follicular lymphoma cell line—tumor formation in nonobese diabetic/severe combined immunodeficiency mice. Recent reports showed that migrating populations enrich CSCs in neuroblastoma SP cells,29 and migrating CD133+CXCR4+ CSCs are essential for pancreatic adenocarcinoma metastasis.68
The prognostic biomarkers may be used to identify patients who are at greatest risk of recurrence and in need of the most aggressive and novel therapies. For example, Witkiewicz et al40 identified the loss of stromal caveolin-1 as a surrogate biomarker associated with an increase in cell cycle progression, the secretion of growth factors, and angiogenic potential in the tumor microenvironment.41
Novel Therapeutic Targets
Current treatments for cancers are not curative for the majority of patients and were designed and deemed successful based on the response of the bulk population of tumor cells.14 Effects on a rare CSC population or on cells in the supportive microenvironment are largely unknown but presumed to be inadequate given the high rates of cancer recurrence. A deeper understanding of the essential signals produced by tumor stromal cells to promote CSC survival could suggest new therapeutic targets. For example, targeting the stem cell niche interaction may be an attractive approach for targeting CSCs.
Antibody-mediated inhibition of CXCL12/CXCR4 signaling completely abrogated the CXCL12-mediated cell migration of lymphocytic leukemias and lymphomas, as well as the migration of lymphoma cells toward LN stromal cells.96-98 Additionally, AMD3100 inhibited infiltration of lymphoma cells into liver and lung tissues by inhibiting CXCL12/CXCR4 signaling, supporting the theory that AMD3100 disrupts the CSC niche and makes CSCs more susceptible to chemotherapy.99 Therefore, our findings26,36 that inhibition of CXCL12/CXCR4 signaling reduced the migration of lymphoma cells and Co-CSCs toward stromal cells and inhibited tumor formation are consistent with previous reports and highlight the idea that one mechanism of action of AMD3100 in these diseases may be the elimination of CSCs. AMD3100 has been used clinically in the setting of mobilization of normal hematopoietic stem cells prior to autologous stem cell transplantation. The safety and efficacy of AMD3100 in combination with chemotherapy in a variety of hematologic malignancies, including lymphomas, are being investigated in clinical trials. We have hypothesized that AMD3100 disrupts the CSC niche and makes CSCs more susceptible to chemotherapy.36
Most solid-tumor in vivo studies generate subcutaneous human tumor xenografts using immunodeficient mice.12 The mouse microenvironment is artificial for human tumors. Thus, drug regimens that are curative in mouse subcutaneous xenograft models often do not have a significant effect on human disease.100 To overcome this hurdle, we have established humanized tumor microenvironment models for follicular lymphoma and colorectal cancer.26,36 In these models, coinoculation of human stromal cells with cancer cells re-creates a humanized microenvironment similar to that of an LN. Our data show that the humanized microenvironment is essential for CSCs to form a tumor in immunodeficient mice, selectively supports CSCs' survival from chemotherapeutic drugs, and enhances drug-resistant CSC tumor formation through CXCL12/CXCR4 signaling. The CSC and stromal interaction in vivo model could serve as a humanized microenvironment model to identify and analyze key interactions and signaling molecules in CSCs and evaluate response to targeted agents.
In addition to colorectal cancers and follicular lymphoma, as summarized in the Table, stromal/CSC interaction in various cancers requires the involvement of many molecules and agents. Annabi et al38 suggested that membrane type 1 matrix metalloprotease (MMP) and MMP-9—two MMPs that contribute to the blood-brain barrier opening and to the radioresistance phenotype in brain tumor cells—may be promising new targets in the annihilation of CSCs as an anticancer therapy. In the breast cancer model, Tsuyada et al39 identified chemokine ligand 2, STAT3, and Notch1 as potential therapeutic targets in deterring CSC-stimulating cancer-host crosstalk, providing a method for defeating CSC-mediated disease progression and treatment resistance. This study furthered our understanding of how the tumor microenvironment influences CSCs as the cancer and host niche coevolve.39 Kim et al42 suggested that the combined targeting of the CXCL12/CXCR4 axis and the implementation of dacarbazine treatment could serve as a therapy to block chemoresistant CD133+ melanoma CSC metastasis toward a lymphatic metastatic niche. In addition, the potential use of TGF-β1 as a therapy in the intervention of ovarian cancer may be helpful because of its involvement in cancer invasion and tumor progression through the regulation of tissue transglutaminase.43 Thus, closer scrutiny of CSCs and their supporting microenvironment, especially LN microenvironmental factors, will lead to a better understanding of mechanisms of cancer recurrence and metastasis and, ultimately, to novel targeted therapies.
Cancer growth and metastasis are dynamic processes. Many stromal environmentally dependent tumors become independent in recurrence. We have also observed some FDC-dependent B-cell lymphoma cells adapt detour growth pathways and bypass the FDC requirement for their growth and tumor formation.101 Our humanized tumor microenvironment models for follicular lymphoma and colorectal cancer are focused on CSC/stromal interaction in early stages of cancer progress. These models may bring future potential therapeutic targets to light that will prevent and reduce recurrence.
CONCLUSION
Not all cancer cells constituting a tumor are the same; a small population of CSCs exists.102 Cancer poses a problem not only with its cancer cells, but also in its involvement with a microenvironment that specifically supports CSCs. Thus, the CSC population is responsible for recurrence and metastasis with help from its stromal environment.64,68 Therefore, combination treatment with chemotherapy drugs in conjunction with other therapies, such as stromal/CSC signaling—targeted therapy may effectively minimize cancer recurrence.103
ACKNOWLEDGMENT
We are very grateful to Ms Barbara Siede for the artistry of the illustration.
Footnotes
Funding: This work was partially supported by NIH/NCI grant R01 CA089057 (LL) and Ladies Leukemia League, Metairie, LA (LL).
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012 Jul-Aug;62(4):220–241. doi: 10.3322/caac.21149. Epub 2012 Jun 14. Erratum in: CA Cancer J Clin. 2012 Sep-Oct;62(5):348. [DOI] [PubMed] [Google Scholar]
- 2.Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012 Mar;30(3):363–371. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EC, Hessman C, Levin TG, Monroe MM, Wong MH. The role of colorectal cancer stem cells in metastatic disease and therapeutic response. Cancers (Basel) 2011 Jan 1;3(1):319–339. doi: 10.3390/cancers3010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. SEER Stat Fact Sheets: Colon and Rectum. 2012 http://seer.cancer.gov/statfacts/html/colorect.html. Accessed September 18, 2012. [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. Epub 2012 Jan 4. [DOI] [PubMed] [Google Scholar]
- 6.Gerger A, Zhang W, Yang D, et al. Common cancer stem cell gene variants predict colon cancer recurrence. Clin Cancer Res. 2011 Nov 1;17(21):6934–6943. doi: 10.1158/1078-0432.CCR-11-1180. Epub 2011 Sep 14. [DOI] [PubMed] [Google Scholar]
- 7.Merlos-Suárez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011 May 6;8(5):511–524. doi: 10.1016/j.stem.2011.02.020. Epub 2011 Mar 17. [DOI] [PubMed] [Google Scholar]
- 8.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009 Sep;9(9):665–674. doi: 10.1038/nrc2714. Epub 2009 Aug 20. [DOI] [PubMed] [Google Scholar]
- 9.Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011 Aug 1;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. Epub 2011 Jun 13. [DOI] [PubMed] [Google Scholar]
- 10.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010 Jun;138(6):2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 12.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008 Oct;8(10):755–768. doi: 10.1038/nrc2499. Epub 2008 Sep 11. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011 Mar;17(3):313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001 Nov 1;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Moore N, Quiescent Lyle S. slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. 2011 doi: 10.1155/2011/396076. 2011; pii 396076. Epub 2010 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overdevest JB, Thomas S, Kristiansen G, et al. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011 Jun 1;71(11):3802–3811. doi: 10.1158/0008-5472.CAN-11-0519. Epub 2011 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007 Jan 4;445(7123):106–110. doi: 10.1038/nature05372. Epub 2006 Nov 19. [DOI] [PubMed] [Google Scholar]
- 18.Haraguchi N, Ohkuma M, Sakashita H, et al. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008 Oct;15(10):2927–2933. doi: 10.1245/s10434-008-0074-0. Epub 2008 Jul 29. [DOI] [PubMed] [Google Scholar]
- 19.Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009 Oct 15;69(20):8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. Epub 2009 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10158–10163. doi: 10.1073/pnas.0703478104. Epub 2007 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CY, Li BX, Liang Y, et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med. 2009 Jul 7;7:56. doi: 10.1186/1479-5876-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008 Jun;118(6):2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horst D, Scheel SK, Liebmann S, et al. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009 Dec;219(4):427–434. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994 Oct 1;153(7):2951–2961. [PubMed] [Google Scholar]
- 25.Kim HS, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995 Aug 1;155(3):1101–1109. [PubMed] [Google Scholar]
- 26.Margolin DA, Silinsky J, Grimes C, et al. Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1α/CXCR4 paracrine signaling. Neoplasia. 2011 Sep;13(9):874–886. doi: 10.1593/neo.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998 Aug;16(8):2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 28.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006 Nov 15;108(10):3295–3301. doi: 10.1182/blood-2006-05-021113. Epub 2006 Jul 27. [DOI] [PubMed] [Google Scholar]
- 29.Das B, Tsuchida R, Malkin D, et al. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008 Jul;26(7):1818–1830. doi: 10.1634/stemcells.2007-0724. Epub 2008 May 8. [DOI] [PubMed] [Google Scholar]
- 30.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007 May 15;67(10):4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 31.Jones RJ, Gocke CD, Kasamon YL, et al. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009 Jun 4;113(23):5920–5926. doi: 10.1182/blood-2008-11-189688. Epub 2009 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008 Sep 8;268(1):1–9. doi: 10.1016/j.canlet.2008.03.048. Epub 2008 May 19. [DOI] [PubMed] [Google Scholar]
- 33.Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009 May 18;277(2):227–234. doi: 10.1016/j.canlet.2008.12.015. Epub 2009 Jan 30. [DOI] [PubMed] [Google Scholar]
- 34.Jakubikova J, Adamia S, Kost-Alimova M, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011 Apr 28;117(17):4409–4419. doi: 10.1182/blood-2010-02-267344. Epub 2011 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger JA, Kaplan CD, Luo Y, et al. Characterization of stem cell-like cancer cells in immune-competent mice. Blood. 2006 Dec 1;108(12):3906–3912. doi: 10.1182/blood-2006-05-024687. Epub 2006 Aug 15. [DOI] [PubMed] [Google Scholar]
- 36.Lee CG, Das B, Lin TL, et al. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol. 2012 Jul;158(1):79–90. doi: 10.1111/j.1365-2141.2012.09123.x. Epub 2012 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14016–14021. doi: 10.1073/pnas.0906549106. Epub 2009 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annabi B, Rojas-Sutterlin S, Laflamme C, et al. Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res. 2008 Jun;6(6):907–916. doi: 10.1158/1541-7786.MCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 39.Tsuyada A, Chow A, Wu J, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012 Jun 1;72(11):2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. Epub 2012 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witkiewicz AK, Casimiro MC, Dasgupta A, et al. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009 Jun 1;8(11):1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 41.Witkiewicz AK, Dasgupta A, Sotgia F, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009 Jun;174(6):2023–2034. doi: 10.2353/ajpath.2009.080873. Epub 2009 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M, Koh YJ, Kim KE, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010 Dec 15;70(24):10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. Epub 2010 Nov 5. [DOI] [PubMed] [Google Scholar]
- 43.Cao L, Shao M, Schilder J, et al. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012 May 17;31(20):2521–2534. doi: 10.1038/onc.2011.429. Epub 2011 Oct 3. [DOI] [PubMed] [Google Scholar]
- 44.van der Horst G, van den Hoogen C, Buijs JT, et al. Targeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011 Jun;13(6):516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007 Apr 15;67(8):3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Yoon SO, Fu DD, Zhang X, Choi YS. Novel follicular dendritic cell molecule, 8D6, collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line, L3055. Blood. 2004 Aug 1;104(3):815–821. doi: 10.1182/blood-2004-01-0292. Epub 2004 Apr 15. [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen L, De Sousa E, Melo F, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010 May;12(5):468–476. doi: 10.1038/ncb2048. Epub 2010 Apr 25. [DOI] [PubMed] [Google Scholar]
- 48.Gilbertson RJ, Gutmann DH. Tumorigenesis in the brain: location, location, location. Cancer Res. 2007 Jun 15;67(12):5579–5582. doi: 10.1158/0008-5472.CAN-07-0760. [DOI] [PubMed] [Google Scholar]
- 49.Smith BH, Gazda LS, Conn BL, et al. Three-dimensional culture of mouse renal carcinoma cells in agarose macrobeads selects for a subpopulation of cells with cancer stem cell or cancer progenitor properties. Cancer Res. 2011 Feb 1;71(3):716–724. doi: 10.1158/0008-5472.CAN-10-2254. Epub 2011 Jan 24. [DOI] [PubMed] [Google Scholar]
- 50.Basak GW, Srivastava AS, Malhotra R, Carrier E. Multiple myeloma bone marrow niche. Curr Pharm Biotechnol. 2009 Apr;10(3):345–346. doi: 10.2174/138920109787847493. [DOI] [PubMed] [Google Scholar]
- 51.Roodhart JM, Daenen LG, Stigter EC, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. 2011 Sep 13;20(3):370–383. doi: 10.1016/j.ccr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 52.de Jong D, de Boer JP. Predicting transformation in follicular lymphoma. Leuk Lymphoma. 2009 Sep;50(9):1406–1411. doi: 10.1080/10428190903093815. [DOI] [PubMed] [Google Scholar]
- 53.Lenz G, Wright G, Dave SS, et al. Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008 Nov 27;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000 May;64(5):323–332. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 55.Klein U, Goossens T, Fischer M, et al. Somatic hypermutation in normal and transformed human B cells. Immunol Rev. 1998 Apr;162:261–280. doi: 10.1111/j.1600-065x.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 56.Torlakovic E, Torlakovic G, Brunning RD. Follicular pattern of bone marrow involvement by follicular lymphoma. Am J Clin Pathol. 2002 Nov;118(5):780–786. doi: 10.1309/EG2M-YHB9-WEFW-7H1R. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Choi YS. Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells. Semin Immunol. 2002 Aug;14(4):259–266. doi: 10.1016/s1044-5323(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Zhang X, Kovacic S, et al. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000 Mar 20;191(6):1077–1084. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012 Jul 6;150(1):194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagawa H, Liyanarachchi S, Davuluri RV, et al. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene. 2004 Sep 23;23(44):7366–7377. doi: 10.1038/sj.onc.1208013. [DOI] [PubMed] [Google Scholar]
- 61.Kouniavsky G, Khaikin M, Zvibel I, et al. Stromal extracellular matrix reduces chemotherapy-induced apoptosis in colon cancer cell lines. Clin Exp Metastasis. 2002;19(1):55–60. doi: 10.1023/a:1013880326925. [DOI] [PubMed] [Google Scholar]
- 62.Madlambayan GJ, Butler JM, Hosaka K, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009 Nov 5;114(19):4310–4319. doi: 10.1182/blood-2009-03-211342. Epub 2009 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Husson H, Carideo EG, Cardoso AA, et al. MCP-1 modulates chemotaxis by follicular lymphoma cells. Br J Haematol. 2001 Dec;115(3):554–562. doi: 10.1046/j.1365-2141.2001.03145.x. [DOI] [PubMed] [Google Scholar]
- 64.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005 Aug;23(7):879–894. doi: 10.1634/stemcells.2004-0342. Epub 2005 May 11. [DOI] [PubMed] [Google Scholar]
- 65.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005 Apr 20;23(12):2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 66.Zeelenberg IS. Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003 Jul 1;63(13):3833–3839. [PubMed] [Google Scholar]
- 67.Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005 Mar 1;11(5):1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 68.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007 Sep 13;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Moriyama T, Ohuchida K, Mizumoto K, et al. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010 Jul 15;116(14):3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 70.Matsusue R, Kubo H, Hisamori S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009 Sep;16(9):2645–2653. doi: 10.1245/s10434-009-0599-x. Epub 2009 Jul 9. [DOI] [PubMed] [Google Scholar]
- 71.Dessein AF, Stechly L, Jonckheere N, et al. Autocrine induction of invasive and metastatic phenotypes by the MIF-CXCR4 axis in drug-resistant human colon cancer cells. Cancer Res. 2010 Jun 1;70(11):4644–4654. doi: 10.1158/0008-5472.CAN-09-3828. Epub 2010 May 11. [DOI] [PubMed] [Google Scholar]
- 72.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004 Aug;18(11):1240–1242. doi: 10.1096/fj.03-0935fje. Epub 2004 Jun 4. [DOI] [PubMed] [Google Scholar]
- 73.Xu Y, Tabe Y, Jin L, et al. TGF-beta receptor kinase inhibitor LY2109761 reverses the anti-apoptotic effects of TGF-beta1 in myelo-monocytic leukaemic cells co-cultured with stromal cells. Br J Haematol. 2008 Jun;142(2):192–201. doi: 10.1111/j.1365-2141.2008.07130.x. Epub 2008 May 19. [DOI] [PubMed] [Google Scholar]
- 74.Rafii A, Mirshahi P, Poupot M, et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 2008;3(12) doi: 10.1371/journal.pone.0003894. :e3894. Epub 2008 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lis R, Touboul C, Mirshahi P, et al. Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer. 2011 Feb 1;128(3):715–725. doi: 10.1002/ijc.25619. [DOI] [PubMed] [Google Scholar]
- 76.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin Cancer Res. 2011 Oct 1;17(19):6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. Epub 2011 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geoffroy FJ, Allegra CJ, Sinha B, Grem JL. Enhanced cytotoxicity with interleukin-1 alpha and 5-fluorouracil in HCT116 colon cancer cells. Oncol Res. 1994;6(12):581–591. [PubMed] [Google Scholar]
- 78.Thomas X, Anglaret B, Magaud JP, Epstein J, Archimbaud E. Interdependence between cytokines and cell adhesion molecules to induce interleukin-6 production by stromal cells in myeloma. Leuk Lymphoma. 1998 Dec;32(1-2):107–119. doi: 10.3109/10428199809059251. [DOI] [PubMed] [Google Scholar]
- 79.Shain KH, Landowski TH, Dalton WS. The tumor microenvironment as a determinant of cancer cell survival: a possible mechanism for de novo drug resistance. Curr Opin Oncol. 2000 Nov;12(6):557–563. doi: 10.1097/00001622-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010 Aug 27;39(4):493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009 Nov 13;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. Epub 2009 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin L, Liu A, Peng Z, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011 Dec 1;71(23):7226–7237. doi: 10.1158/0008-5472.CAN-10-4660. Epub 2011 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010 Feb;120(2):485–497. doi: 10.1172/JCI39397. Epub 2010 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Stefano AB, Iovino F, Lombardo Y, et al. Survivin is regulated by interleukin-4 in colon cancer stem cells. J Cell Physiol. 2010 Nov;225(2):555–561. doi: 10.1002/jcp.22238. [DOI] [PubMed] [Google Scholar]
- 85.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007 Oct 11;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 86.Todaro M, Perez Alea M, Scopelliti A, Medema JP, Stassi G. IL-4-mediated drug resistance in colon cancer stem cells. Cell Cycle. 2008 Feb 1;7(3):309–313. doi: 10.4161/cc.7.3.5389. Epub 2007 Nov 30. [DOI] [PubMed] [Google Scholar]
- 87.Yin XL, Wang N, Wei X, et al. Interleukin-12 inhibits the survival of human colon cancer stem cells in vitro and their tumor initiating capacity in mice. Cancer Lett. 2012 Sep 1;322(1):92–97. doi: 10.1016/j.canlet.2012.02.015. Epub 2012 Feb 22. [DOI] [PubMed] [Google Scholar]
- 88.Azzi S, Bruno S, Giron-Michel J, et al. Differentiation therapy: targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst. 2011 Dec 21;103(24):1884–1898. doi: 10.1093/jnci/djr451. Epub 2011 Oct 31. [DOI] [PubMed] [Google Scholar]
- 89.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 2012 Aug;10(8):995–1009. doi: 10.1158/1541-7786.MCR-12-0274. Epub 2012 Jun 19. [DOI] [PubMed] [Google Scholar]
- 90.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005 Sep;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 91.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009 Oct 15;114(16):3367–3375. doi: 10.1182/blood-2009-06-225326. Epub 2009 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark-Langone KM, Sangli C, Krishnakumar J, Watson D. Translating tumor biology into personalized treatment planning: analytical performance characteristics of the Oncotype DX Colon Cancer Assay. BMC Cancer. 2010 Dec 23;10:691. doi: 10.1186/1471-2407-10-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan IB, Tan P. Genetics: an 18-gene signature (ColoPrint®) for colon cancer prognosis. Nat Rev Clin Oncol. 2011 Mar;8(3):131–133. doi: 10.1038/nrclinonc.2010.229. Epub 2011 Feb 8. [DOI] [PubMed] [Google Scholar]
- 94.Saiki Y, Ishimaru S, Mimori K, et al. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann Surg Oncol. 2009 Sep;16(9):2638–2644. doi: 10.1245/s10434-009-0567-5. Epub 2009 Jun 25. [DOI] [PubMed] [Google Scholar]
- 95.Hostettler L, Zlobec I, Terracciano L, Lugli A. ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J Gastroenterol. 2010 Feb 14;16(6):732–739. doi: 10.3748/wjg.v16.i6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawaguchi A, Orba Y, Kimura T, et al. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood. 2009 Oct 1;114(14):2961–2968. doi: 10.1182/blood-2008-11-189308. Epub 2009 Aug 5. [DOI] [PubMed] [Google Scholar]
- 97.O'Callaghan K, Lee L, Nguyen N, et al. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 2012 Feb 16;119(7):1717–1725. doi: 10.1182/blood-2011-04-347518. Epub 2011 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, Cui GH, Liu F, Wu QL, Chen Y. Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol Sin. 2006 Nov;27(11):1438–1446. doi: 10.1111/j.1745-7254.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 99.Yang ZJ, Wechsler-Reya RJ. Hit 'em where they live: targeting the cancer stem cell niche. Cancer Cell. 2007 Jan;11(1):3–5. doi: 10.1016/j.ccr.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Huynh AS, Abrahams DF, Torres MS, et al. Development of an orthotopic human pancreatic cancer xenograft model using ultrasound guided injection of cells. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020330. :e20330. Epub 2011 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoon SO, Zhang X, Freedman AS, et al. Down-regulation of CD9 expression and its correlation to tumor progression in B lymphomas. Am J Pathol. 2010 Jul;177(1):377–386. doi: 10.2353/ajpath.2010.100048. Epub 2010 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011 Feb;8(2):90–100. doi: 10.1038/nrgastro.2010.211. [DOI] [PubMed] [Google Scholar]
- 103.Tysnes BB. Tumor-initiating and -propagating cells: cells that we would like to identify and control. Neoplasia. 2010 Jul;12(7):506–515. doi: 10.1593/neo.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]