ABSTRACT
Background
Tumor necrosis factor-α (TNF-α) is a potent proinflammatory cytokine involved in a variety of disease pathologies, including ischemia/reperfusion (I/R) injuries in transplantation. The interaction of TNF-α with its cognate receptor TNF receptor I (TNFRI) results in the activation of signal transduction pathways that regulate either cell survival or cell death. Hepatocytes express TNFRI and respond to TNF-α released by resident Kupffer cells as well as leukocytes that migrate to the liver during I/R injury. Upon binding TNF-α, the hepatocyte proliferates or undergoes apoptosis or necroptosis. The decision by the cell to commit to one path or the other is not understood. The damaged tissue exhibits cell death and hemorrhaging from the influx of immune mediators. TNF-α inhibitors ameliorate the injury in animal models, suggesting that lowering (but not eliminating) TNF-α levels shifts the balance of TNF-α toward its beneficial functions.
Methods
We review TNF-α signal transduction pathways and the role of TNF-α in liver I/R injury.
Conclusions
Because TNF-α plays an important role in hepatocyte proliferation, complete inhibition of TNF-α is not desirable in treating liver I/R injury. The strategy for developing pharmacological therapies may be the identification of specific intermediates in the TNF-α/TNFR1 signal transduction pathway and directed targeting of proapoptotic and pronecroptotic events.
Keywords: Inflammation, ischemia, liver, reperfusion, tumor necrosis factor-alpha
INTRODUCTION
Orthotopic liver transplantation is the only treatment option for end-stage liver disease. National 1-year and 5-year patient survival rates have risen to 89% and 80%, respectively.1 While treatment plans often focus on the recipient, 2 donor events directly affect the recipient's outcome. First, the condition of the donor liver has an impact on the success of the transplant. As of October 2012, 16,832 recipients awaited livers, but only 3,917 deceased donor livers were available and transplanted.2 Thus, with recipients outnumbering donors 4 to 1, surgeons are increasingly turning toward the use of live donors, split livers, and marginal livers. Marginal livers include livers from older donors, donors with significant fatty livers, and donors with anticipated long cold ischemia times. Second, the degree of I/R injury caused by cold preservation of the excised liver and warm reperfusion upon implantation has an equal and profound effect on the outcome. While the donor liver condition and I/R injury involve multiple physiological events, the inflammatory response is a key mediator in both liver damage and liver regeneration. This review examines the mechanism of action by the cytokine TNF-α in regulating both hepatocyte death and survival pathways. Pharmacological interventions to inhibit TNF-α in vivo, therefore, must take into account the beneficial functions of TNF-α.
The table lists the molecules discussed and assists the reader with the numerous abbreviations used in this review.
TNF-α: STRUCTURE AND FUNCTION
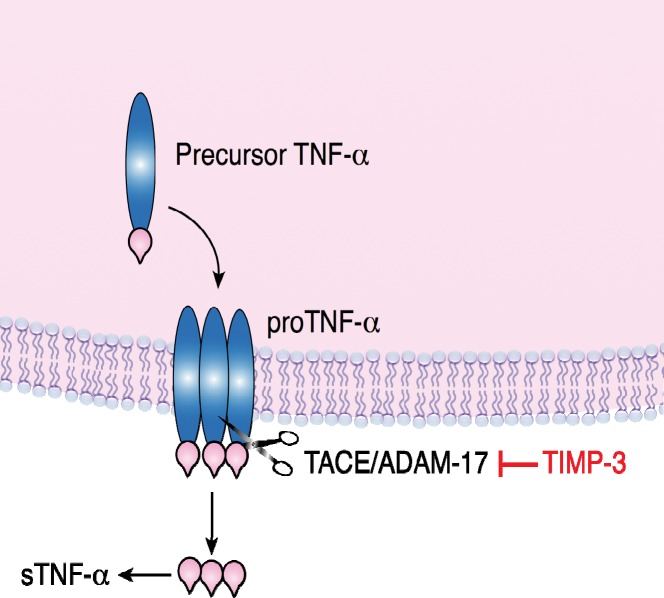
The immune system responds to liver injury and/or stress by activating resident Kupffer cells and recruiting an influx of leukocytes to release proinflammatory cytokines, chemokines, and other factors. Numerous immune proteins are involved; however, TNF-α is implicated as the primary mediator of inflammation during I/R injury in several tissues, including the lung, heart, liver, eye, kidney, and brain. TNF-α is a potent proinflammatory cytokine that targets various cell types through receptor-mediated signal transduction pathways. TNF-α is encoded by a 1,686-ribonucleotide mRNA that is translated into a 26 kDa, nonglycosylated, membrane-bound, precursor protein, mTNF-α.3-6 The mTNF-α monomer is assembled at the cell surface as a homotrimer known as proTNF-α (Figure 1). Active, soluble TNF-α is generated by the enzyme activity of TACE/ADAM17 between Ala-76 and Val-77 of mTNF-α.7,8 TACE/ADAM17 cleavage of mTNF-α releases the 51 kDa trimeric TNF-α (or sTNF-α), which contains three 17 kDa monomers. TACE/ADAM17 is a member of the zinc-dependent MMP family that degrades ECM.9-11 MMPs have been implicated in the pathology of several diseases, including arthritis and cancer. Approximately 26 MMPs have been identified to date.12
Figure 1.
TNF-α is modified posttranslationally. TNF-α is expressed as a monomeric precursor protein of 233 amino acids, 26 kDa. The membrane-bound form (proTNF-α; mTNF-α) is a trimer. Cleavage between Ala-76 and Val-77 of proTNF-α by TACE/ADAM17 releases the active, soluble 51 kDa form that contains three 17 kDa monomers. TIMP-3 inhibits TACE/ADAM17, preventing the release of sTNF-α.
Many activated immune cells express TNF-α, including neutrophils, B lymphocytes, CD4+ T lymphocytes, NK cells, NKT cells, and cells of the monocyte lineage. Resident macrophages—astroglia, microglia, Langerhans cells, Kupffer cells, and alveolar macrophages—are primary producers of TNF-α.13 Binding TNF-α to one of its receptors, either TNFR1 or TNFR2, activates signal transduction pathways.14-18 TNFR1 is expressed constitutively on the surface of all cell types as a trimer of 55 kDa subunits, and TNFR2 is expressed in activated immune cells as a trimer of 75 kDa subunits. Although TNF-α binds either receptor, TNF-α mediates its effects primarily through its interactions with TNFR1.19
TNF-α ACTIVATES PROGRAMMED DEATH PATHWAYS
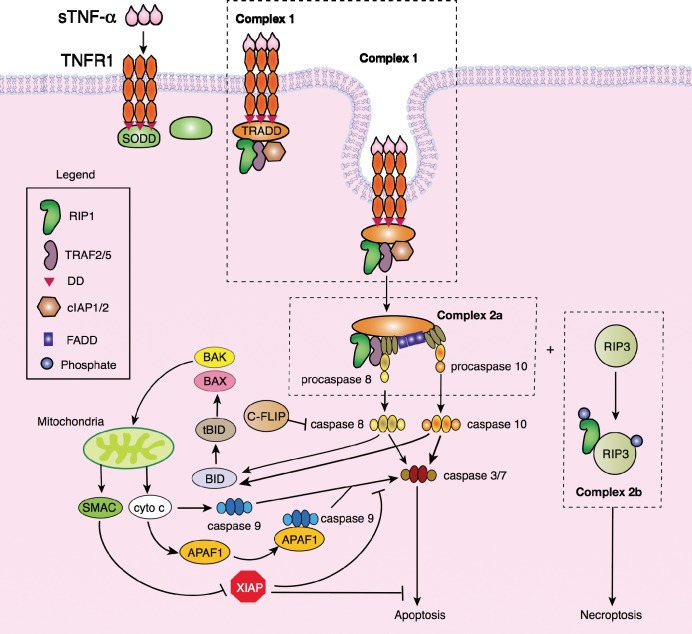
TNFR1 is a membrane-bound protein that contains a DD in its cytoplasmic tail that is associated with the 60 kDa SODD protein. Soluble TNF-α binds TNFR1, resulting in the trimerization of TNFR1 and the release of SODD (Figure 2).20,21 TRADD binds the trimeric DD of TNFR1, which recruits RIP1, TRAF2/5, and cIAP1/2 to form Complex 1.22 Endocytosis of Complex 1 leads to the degradation of cIAP1/2 and the formation of proapoptotic Complex 2a or the dissociation of Complex 1 and the formation of pronecroptotic Complex 2b. Thus, Complex 2a leads to apoptosis (programmed cell death), and Complex 2b results in necroptosis (programmed necrosis) of the hepatocyte. The transition between Complex 1 and Complex 2a/2b has yet to be elucidated. Complex 2a consists of TRADD, RIP1, TRAF2/5, FADD, and procaspase-8 and -10. As zymogens, procaspases are inactive forms of the caspases (cysteine-aspartic proteases or cysteine-dependent aspartate-directed proteases) that consist of 2 groups: upstream initiator (apical) caspases and downstream effector (executioner) caspases.23 Initiator caspase-2/8/9/10 cleaves and activates the effector caspase-3/6/7. From Complex 2, procaspase-8 and -10 are converted to caspase-8 and -10, which initiate apoptosis through caspase-3, -6, and -7 and the mitochondria death pathway. Caspase-8 and -10 cleave BID into the 15 kDa tBID.24-26 tBID activates BAX and BAK to reassemble into heterodimeric pore units in the mitochondrial membrane, resulting in the release of cytochrome c and SMAC.26-29 Cytochrome c activates caspase-9 either directly or through APAF-1 in an ATP-dependent manner, resulting in the binding of APAF-1 to caspase-9, a complex known as the apoptosome.30-33 Whether APAF-1 is required for cytochrome c–dependent activation of caspase-9 is unclear. SMAC binds and blocks XIAP from binding to caspases.34,35 Caspase-9 activates executioner caspase-3 and -7, leading to apoptosis.36,37
Figure 2.
Signal transduction by TNF-α leads to apoptosis or necroptosis. Invagination of Complex 1 results in the formation of Complex 2a (apoptosome) or 2b (necroptosome). Soluble TNF-α binds to its cognate receptor, TNFR1, which is bound to SODD via the TNFR1 DD. Binding of TNF-α to TNFR1 releases SODD, enabling the binding of TRADD, followed by the assembly of RIP1, TRAF2/5, and cIAP1/2. Endocytosis of Complex 1 leads to the degradation of cIAP1/2 and the formation of Complex 2a, which consists of TRADD, TRAF2/5, RIP1, FADD, and the zymogens procaspase-8 and -10. Procaspase-8 and -10 are cleaved, and caspase-8 and -10 cleave BID into tBID, activating the mitochondrial death pathway. Caspase-8 and -10 also activate caspase-3, -6, and -7, leading to apoptosis.
Studies in the past 3 years have shown that necrosis can occur in a genetically encoded, regulated manner similar to apoptosis, known as necroptosis or programmed necrosis.38 Unlike apoptosis, necroptosis does not require caspases but, rather, the kinases RIP1 and RIP3. The formation of Complex 2b, or the necrosome, during TNF-α signaling has only recently been described.39 RIP1 is found in Complexes 2a and 2b, and an antiapoptotic domain within RIP1 may control whether RIP1 participates in apoptosis or necroptosis.40 The association of RIP3 with RIP1 leads to phosphorylation of both kinases; however, the identity of the activating kinases is not known. He et al39 suggest that RIP3 undergoes autophosphorylation, while Cho et al41 suggest that an as-yet-to-be-determined kinase phosphorylates RIP3. It is not clear which kinase phosphorylates RIP1. Necroptosis has been identified as a mechanism of cell death in renal, cardiac, and retinal I/R injuries.42-45 We have shown that rat livers undergoing I/R injury are characterized by massive necrosis that may be caused by TNF-α–mediated necroptosis.46-48 Characterization of the processes involved to activate necroptosis during liver I/R injury has not yet been undertaken.
TNF-α ACTIVATES CELL SURVIVAL AND PROLIFERATION
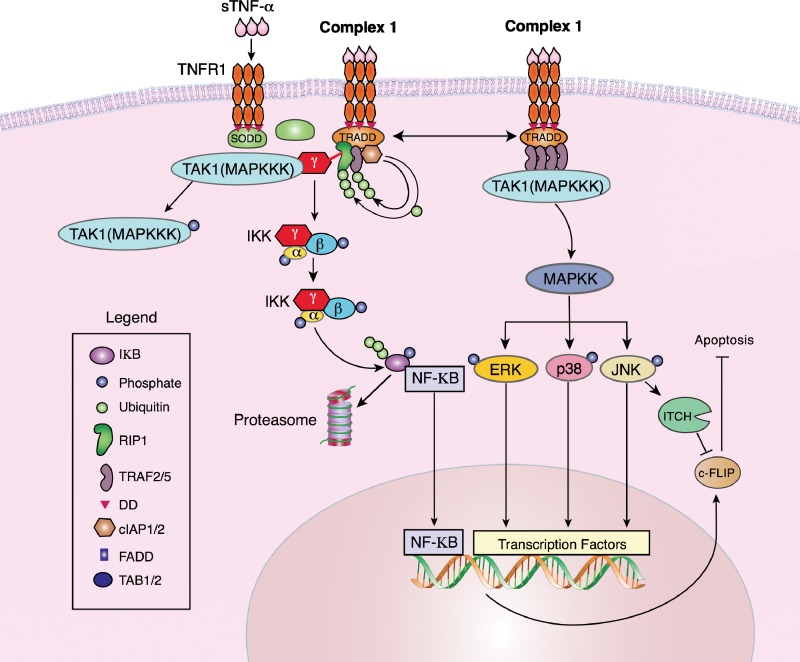
Engagement of sTNF-α with TNFR1 activates cell survival and proliferation pathways if Complex 1 is retained on the cell membrane. Complex 1 leads to either of 2 signal transduction pathways: the canonical (classical) NF-κB pathway or the MAPK pathway (Figure 3). The polyubiquitination of RIP1 and TRAF2/5 by cIAP1/2 results in the recruitment of NEMO to the complex.49-52 NEMO is associated with TAK1, a member of the MAPKKK family. TAK1 activates IKK, which phosphorylates IκB.53 IκB becomes polyubiquitinated, releasing NF-κB, and IκB is targeted to the proteasome for degradation. NF-κB translocates to the nucleus and activates transcription of genes that regulate cell survival and proliferation. The canonical NF-κB pathway has been well studied, and Hayden and Ghosh54 have provided a recent overview of the progress made in NF-κB research.
Figure 3.
Signal transduction by TNF-α leads to cell survival and proliferation. Retention of Complex 1 on the cell surface commits the cell to proliferate via 2 signal transduction pathways: NF-κB or MAPK. Polyubiquitination of RIP1 releases NEMO or IKK and recruits both TAK1 (MAPKKK) and IKK. Active TAK1 phosphorylates the IKK complex (IKK-α, -β, and -γ), which phosphorylates IκB. Phospho-IκB is ubiquitinated, released from the transcription factor NF-κB, and targeted for degradation by the proteasome. NF-κB translocates to the nucleus to activate transcription. Binding of TNF-α to TNFR1 may also form another version of Complex 1 that consists of oligomers of TRAF2/5 bound to TNFR1. TRAF2/5 recruits TAK1 to the complex. TAK1, a MAPKKK, phosphorylates MAPKK, which in turn phosphorylates the terminal MAPKs: p38 MAPK, JNK, and ERK1/2. Phospho-JNK and phospho-ERK1/2 translocate to the nucleus to activate transcription.
Alternatively, Complex 1 leads to the activation of the MAPK signal transduction pathway through TRADD and TRAF2/5. TRAF2/5 oligomerizes, resulting in the binding of TAK1 (a MAPKKK) to TRAF2/5.55,56 Activated MAPKKK follows the classical MAPK phosphorylation cascade by activating a MAPKK that, in turn, phosphorylates the 3 terminal MAPKs: p38 MAPK, JNK, and ERK1/2. Phosphorylated MAPKs translocate into the nucleus to activate transcription factors. The MAPK pathway has been the focus of intense efforts in designing and applying pharmacological inhibitors in vivo and in vitro with some inhibitors advancing to clinical trials for a variety of pathologies, including inflammatory diseases and cancer.57 As with NF-κB, MAPK signaling pathways are an active area of research, and excellent reviews have been published that provide the recent progress in MAPK studies.58
Although genes that upregulate cell division are transcribed through NF-κB and MAPK signal transduction, antiapoptotic genes are also expressed. NF-κB induces the expression of c-FLIP.59 Three isoforms of FLIP have been identified: c-FLIPL, c-FLIPS, and c-FLIPR. All three regulate caspase-8 activation and DR-induced apoptosis. However, c-FLIP consists of FLIPS and FLIPL in the literature.60-64 Recent data indicate that c-FLIP has pro- and antiapoptotic functions and is regulated by its intracellular stoichiometry.65 Low, moderate, or no levels of c-FLIP mediate apoptosis, while high levels of c-FLIP may stimulate proliferation.62,66,67 Interestingly, JNK phosphorylates the E3 ubiquitin ligase ITCH, which ubiquitinates c-FLIP to induce c-FLIP degradation, leading to apoptosis.68 Thus, JNK antagonizes NF-κB during TNF-α–mediated Complex 1 signal transduction.
TNF-α AND THE IMMUNE RESPONSE IN HEPATIC I/R INJURY
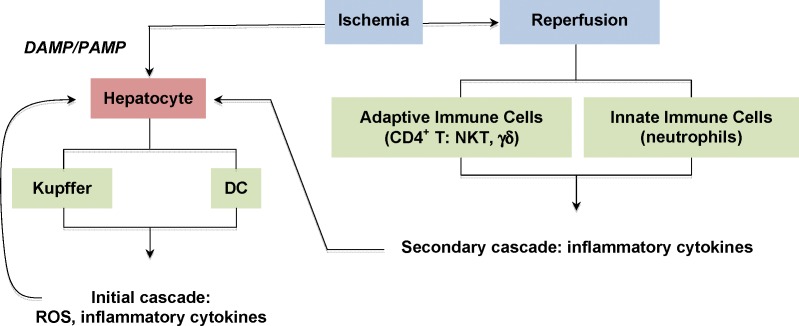
Hepatic I/R injury occurs in numerous clinical settings, including but not limited to liver hemorrhage and shock, surgical resection, and transplantation. Although the pathophysiology of I/R injury involves multiple pathways, inflammatory cells and soluble factors are key mediators. Two general immune mechanisms have been identified during liver transplantation. The lack of ATP production because of glycogen consumption and oxygen depletion triggers the surface expression of DAMP/PAMP during ischemia (Figure 4). Kupffer cells and DCs express TLRs that bind the endogenous DAMPs/PAMPs expressed by the ischemic cells in the liver.69-72 The Kupffer cells and DCs become activated and respond with a classic inflammatory reaction cascade, producing ROS and proinflammatory cytokines such as TNF-α.73-77
Figure 4.
I/R injury to hepatocytes is mediated by immune cells. The initial immune cascade is caused by the expression of DAMP/PAMP on the surfaces of cells, including hepatocytes. The resident macrophages, Kupffer cells, and dendritic cells express TLRs that bind DAMPs/PAMPs and activate the immune cells, releasing ROS, cytokines, and chemokines. Reperfusion activates adaptive immune cells (CD4+ T cells, γδ T cells, and NKT cells) and innate immune cells (primarily neutrophils) that are recruited to the site of tissue injury. The cells release a second cascade of mediators. TNF-α is produced in both the initial and secondary cascades. Hepatocytes express TNFRI in response to the massive influx of TNF-α.
A second immune-mediated response occurs during the reperfusion phase. The initial inflammatory response during ischemia leads to the recruitment of leukocytes, particularly neutrophils and CD4+ T cells. These cells activate and secrete a secondary wave of cytokines and chemokines, amplifying the immune reaction at the site of I/R injury.78-83 T cell–deficient mice have reduced I/R injury, and systemic treatment with immunosuppressive drugs attenuates I/R injury in various organs, suggesting that decreasing T cell function is beneficial to organ survival.84-86 The activated T cells constitutively express their surface stimulatory molecules CD28 and CD154, which are recognized by B7 and CD40, respectively, on antigen-presenting cells during I/R injury.80,87-90 The costimulation of the CD4+ T cell's CD28 and CD154 leads to the phosphorylation of CD28 by Lck and the activation of the PI signaling pathway. The phosphorylation events initiating from these kinases, as well as signal transduction events from the antigen-TCR complex and cytokine/cytokine receptor complexes, result in gene transcription, including TNF-α, which leads to additional T cell proliferation and cytokine and chemokine production, further damaging the tissue.
Platelets also express CD40 that binds to the T cell's CD154 receptor and mediates tissue damage following I/R injury.91-93 The infiltration of NKT cells into renal and hepatic I/R injured tissue recruits neutrophils, and activated NKTs produce various cytokines, including IFN-γ, IL-2, IL-13, and TNF-α.94-97 NKT cells are found in high quantities in the liver, and the production of TNF-α by NKT cells is yet another level of redundancy by the immune system in response to I/R injury. CD8+ T cells have been implicated in renal and intestinal I/R injury. Mice that underwent renal I/R exhibited increased IL-1β, IL-6, TNF-α, IFN-γ, MIP-2, and RANTES expression.98,99 CD8-deficient mice showed lower cytokine expression levels, but kidney histology was unchanged after I/R induction, suggesting a chronic effect of CD8+ T cell infiltration.99 Another type of inflammatory cells, mast cells, has not been shown to be involved in I/R injury.100 Thus, multiple immune system events generate proinflammatory cytokines that initiate and amplify the responses that lead to tissue injury.
PHARMACOLOGICAL TARGETS OF TNF-α-MEDIATED SIGNAL TRANSDUCTION IN HEPATIC I/R INJURY
The dual roles of TNF-α present a conundrum when using inhibitors against TNF-α. Complete knockout of the TNF-α, TNFR1, or TIMP-3 gene in mice results in the inability of the liver to regenerate after tissue damage.101-103 In the case of TIMP-3 knockout in mice, the deregulation of TACE/ADAM17 leads to sustained production of soluble TNF-α, which leads to increased inflammation and increased cell death.102 Thus, TIMP-3 is critical to maintaining the homeostasis of the liver by regulating TNF-α release. Monoclonal antibodies (etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol) against TNF-α have been approved for inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, psoriasis, ankylosing spondylitis, and inflammatory bowel disease.104,105 Applications of TNF-α monoclonal antibodies in liver I/R studies appear to attenuate tissue injury.75,106,107 We used recombinant TIMP-3 in a rat I/R model and showed that liver damage is dramatically decreased in TIMP-3–treated animals prior to I/R induction as compared to untreated animals.46-48 Hernandez et al108 recently generated shRNAs to silence the TNF-α gene in a mouse liver I/R model. Although the data showed a correlation between decreased liver injury and shRNA pretreatment, the ALT levels in shRNA-treated mice were higher than in the control group, suggesting that tissue damage was still occurring. We suggest that this result may be caused by the lack of TNF-α for cell survival signal transduction that is required for liver regeneration.
Some researchers have targeted TNF-α's proliferative effects by using specific inhibitors of downstream signal transduction proteins. The rationale of these efforts is to diminish or inhibit immune cell proliferation. A review of the literature indicates that MAPK inhibitors, especially those targeting p38 MAPK, provide insights into the contrasting roles of MAPK in I/R injury. MAPK induces gene expression that leads to cell proliferation, and data in I/R injury studies indicate that upregulation of immune cell proliferation is a direct result. Studies assessing the effect of MAPK induction with small molecules in I/R injury are well documented in the retina, heart, kidney, lung, brain, and liver and show mixed results. In myocardial I/R injury, p38 MAPK aggravates lethal injury but can also protect the heart under certain circumstances, although this theory remains controversial.109-117
In liver I/R injury, p38 MAPK inhibitors appear to attenuate tissue damage in animals.118-120 However, these studies have not determined the mechanism of action by the inhibitor, whether immune cells and/or hepatocytes are targeted. Nilotinib, a second-generation receptor tyrosine kinase inhibitor, protects against liver I/R injury in the mouse by reducing p38 MAPK in liver nonparenchymal cells and reducing JNK activation in hepatocytes.121 Nilotinib did not inhibit p38 MAPK in bulk liver and may be selective for nonparenchymal cells that are involved in TLR signaling.70 Interestingly, nilotinib did not inhibit its known receptor tyrosine kinases and may be exerting its effects through another pathway.
CONCLUSIONS
TNF-α's opposing functions—survival versus death—present a challenge in understanding the mechanism of I/R injury and designing treatments to prevent tissue damage, particularly during liver transplantation. The regenerative capability of the liver must be retained, and TNF-α plays an important role in hepatocyte proliferation. Thus, complete inhibition of TNF-α is not desirable in treating liver I/R injury. Identification of specific intermediates in the TNF-α/TNFR1 signal transduction pathway and directed targeting of proapoptotic and pronecroptotic events may be the strategy for developing pharmacological therapies in liver I/R injury.
ACKNOWLEDGMENT
The authors are grateful to Ms Barbara Siede of Medical Illustrations, Ochsner Clinic Foundation, for generating the figure diagrams and for her infinite patience in editing the diagrams.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
Table.
Definitions of Abbreviations Used in the Text

REFERENCES
- 1.Scientific Registry of Transplant Recipients. Liver, 2010 SRTR & OPTN Annual Data Report. 2012 http://srtr.transplant.hrsa.gov/annual_reports/2010/flash/03_liver/index.html. Accessed November 1. [Google Scholar]
- 2.HRSA Health Resources and Services Administration. 2012 http://optn.transplant.hrsa.gov/data/. Accessed September 13, [Google Scholar]
- 3.Pennica D, Nedwin GE, Hayflick JS, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312(5996):724–729. doi: 10.1038/312724a0. Dec 20-1985 Jan 2. [DOI] [PubMed] [Google Scholar]
- 4.Wang AM, Creasey AA, Ladner MB, et al. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- 5.Shirai T, Yamaguchi H, Ito H, Todd CW, Wallace RB. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. Nature. 1985;313(6005):803–806. doi: 10.1038/313803a0. Feb 28-Mar 6. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. NCBI Reference Sequence. 2012 http://www.ncbi.nlm.nih.gov/nuccore/NM_000594.3. Accessed November 1. [Google Scholar]
- 7.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997 Feb 20;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 8.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997 Feb 20;385(6618):733–736. doi: 10.1038/385733a0. Erratum in: Nature. 1997 Apr 17;386(6626):738. [DOI] [PubMed] [Google Scholar]
- 9.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002 Oct 1;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 10.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 11.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008 Oct;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. Epub 2008 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal A, Chhabra A, Malhotra U, Kohli S, Rani V. Comparative analysis of human matrix metalloproteinases: Emerging therapeutic targets in diseases. Bioinformation. 2011 Mar 2;6(1):23–30. doi: 10.6026/97320630006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohmann HP, Remy R, Brockhaus M, van Loon AP. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha) J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- 15.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993 Sep 5;268(25):18542–18548. [PubMed] [Google Scholar]
- 16.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995 Dec 1;83(5):793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 17.Schmid EF, Binder K, Grell M, Scheurich P, Pfizenmaier K. Both tumor necrosis factor receptors, TNFR60 and TNFR80, are involved in signaling endothelial tissue factor expression by juxtacrine tumor necrosis factor alpha. Blood. 1995 Sep 1;86(5):1836–1841. [PubMed] [Google Scholar]
- 18.Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W. Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol. 1995 Mar 15;154(6):2904–2913. [PubMed] [Google Scholar]
- 19.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998 Jan 15;160(2):943–952. [PubMed] [Google Scholar]
- 20.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995 May 19;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999 Jan 22;283(5401):543–546. doi: 10.1126/science.283.5401.543. Erratum in: Science. 1999 Mar 19;283(5409):1852. [DOI] [PubMed] [Google Scholar]
- 22.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003 Jul 25;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 23.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004 Jan 23;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998 Aug 21;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 25.Gross A, Yin XM, Wang K, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999 Jan 8;274(2):1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 26.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000 Aug 15;14(16):2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Kolluri SK, Gu J, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000 Aug 18;289(5482):1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 28.Korsmeyer SJ, Wei MC, Saito M, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000 Dec;7(12):1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 29.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001 Apr 27;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997 Nov 14;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000 Oct 6;275(40):31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 32.Baliga B, Kumar S. Apaf-1/cytochrome c apoptosome: an essential initiator of caspase activation or just a sideshow? Cell Death Differ. 2003 Jan;10(1):16–18. doi: 10.1038/sj.cdd.4401166. [DOI] [PubMed] [Google Scholar]
- 33.Ekert PG, Read SH, Silke J, et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004 Jun 21;165(6):835–842. doi: 10.1083/jcb.200312031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deveraux QL, Roy N, Stennicke HR, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998 Apr 15;17(8):2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004 Aug;24(16):7003–7014. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999 Jan 25;144(2):281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001 Mar 9;276(10):7320–7326. doi: 10.1074/jbc.M008363200. Epub 2000 Oct 31. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Galluzzi L, Vandenabeele P, et al. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009 Jan;16(1):3–11. doi: 10.1038/cdd.2008.150. Epub 2008 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009 Jun 12;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Duprez L, Bertrand MJ, Vanden Berghe T, et al. Intermediate domain of receptor-interacting protein kinase 1 (RIPK1) determines switch between necroptosis and RIPK1 kinase-dependent apoptosis. J Biol Chem. 2012 Apr 27;287(18):14863–14872. doi: 10.1074/jbc.M111.288670. Epub 2012 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009 Jun 12;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum DM, Degterev A, David J, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010 May 15;88(7):1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012 Sep;27(9):3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 44.Linkermann A, Bräsen JH, Himmerkus N, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012 Apr;81(8):751–761. doi: 10.1038/ki.2011.450. Epub 2012 Jan 11. [DOI] [PubMed] [Google Scholar]
- 45.Oerlemans MI, Koudstaal S, Chamuleau SA, et al. Targeting cell death in the reperfused heart: Pharmacological approaches for cardioprotection. Int J Cardiol. 2012 Mar 27; doi: 10.1016/j.ijcard.2012.03.055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Tang ZY, Loss G, Carmody I, Cohen AJ. TIMP-3 ameliorates hepatic ischemia/reperfusion injury through inhibition of tumor necrosis factor-alpha-converting enzyme activity in rats. Transplantation. 2006 Dec 15;82(11):1518–1523. doi: 10.1097/01.tp.0000243381.41777.c7. [DOI] [PubMed] [Google Scholar]
- 47.Zetzmann CP, Swamy OR, Loss GE, Jr, Bohorquez H, Cohen AJ. Improving donor livers by inhibiting TNF-α production. Ochsner J. 2010 Winter;10(4):250–255. [PMC free article] [PubMed] [Google Scholar]
- 48.Shuh M, Swamy OR, Zetzmann CP, et al. Tissue inhibitor of metalloproteinase-3 ameliorates total sublethal hepatic ischemia/reperfusion injury in a rat model. J Transplant Technol Res. 2012:S3–003. [Google Scholar]
- 49.Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000 Mar;12(3):301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- 50.Devin A, Cook A, Lin Y, et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000 Apr;12(4):419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 51.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006 Apr 21;22(2):245–257. doi: 10.1016/j.molcel.2006.03.026. Epub 2006 Apr 6. [DOI] [PubMed] [Google Scholar]
- 52.Varfolomeev E, Goncharov T, Fedorova AV, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008 Sep 5;283(36):24295–24299. doi: 10.1074/jbc.C800128200. Epub 2008 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Deng L, Hong M, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001 Jul 19;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 54.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012 Feb 1;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natoli G, Costanzo A, Ianni A, et al. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997 Jan 10;275(5297):200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 56.Baud V, Liu ZG, Bennett B, et al. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999 May 15;13(10):1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002 Jan;23(1):40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- 58.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011 Mar;75(1):50–83. doi: 10.1128/MMBR.00031-10. Erratum in: Microbiol Mol Biol Rev. 2012 Jun;76(2):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001 Aug;21(16):5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death—inducing signaling complex. Cell. 1996 Jun 14;85(6):817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 61.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999 Jan 15;274(3):1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 62.Chang DW, Xing Z, Pan Y, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002 Jul 15;21(14):3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprick MR, Rieser E, Stahl H, et al. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 2002 Sep 2;21(17):4520–4530. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and −10 by FLIP(L) Biochem J. 2004 Sep 1;382(Pt 2):651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fricker N, Beaudouin J, Richter P, et al. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010 Aug 9;190(3):377–389. doi: 10.1083/jcb.201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Micheau O, Thome M, Schneider P, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002 Nov 22;277(47):45162–45171. doi: 10.1074/jbc.M206882200. Epub 2002 Sep 4. [DOI] [PubMed] [Google Scholar]
- 67.Peter ME. The flip side of FLIP. Biochem J. 2004 Sep 1;382(Pt 2):e1–e3. doi: 10.1042/BJ20041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang L, Kamata H, Solinas G, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006 Feb 10;124(3):601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Zhai Y, Shen XD, O'Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004 Dec 15;173(12):7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 70.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005 Dec 1;175(11):7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 71.Shen XD, Ke B, Zhai Y, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007 Oct;13(10):1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 72.Ellett JD, Evans ZP, Atkinson C, et al. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009 Sep;15(9):1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hisama N, Yamaguchi Y, Miyanari N, et al. Ischemia-reperfusion injury: the role of Kupffer cells in the production of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family. Transplant Proc. 1995 Apr;27(2):1604–1606. [PubMed] [Google Scholar]
- 74.Hisama N, Yamaguchi Y, Ishiko T, et al. Kupffer cell production of cytokine-induced neutrophil chemoattractant following ischemia/reperfusion injury in rats. Hepatology. 1996 Nov;24(5):1193–1198. doi: 10.1053/jhep.1996.v24.pm0008903397. [DOI] [PubMed] [Google Scholar]
- 75.Wanner GA, Müller PE, Ertel W, et al. Differential effect of anti-TNF-alpha antibody on proinflammatory cytokine release by Kupffer cells following liver ischemia and reperfusion. Shock. 1999 Jun;11(6):391–395. [PubMed] [Google Scholar]
- 76.Nakamitsu A, Hiyama E, Imamura Y, Matsuura Y, Yokoyama T. Kupffer cell function in ischemic and nonischemic livers after hepatic partial ischemia/reperfusion. Surg Today. 2001;31(2):140–148. doi: 10.1007/s005950170198. [DOI] [PubMed] [Google Scholar]
- 77.Mosher B, Dean R, Harkema J, et al. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res. 2001 Aug;99(2):201–210. doi: 10.1006/jsre.2001.6217. [DOI] [PubMed] [Google Scholar]
- 78.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997 Jul 15;100(2):279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colletti LM, Cortis A, Lukacs N, et al. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998 Sep;10(3):182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Ke B, Shen XD, Gao F, et al. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005 May 15;79(9):1078–1083. doi: 10.1097/01.tp.0000161248.43481.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khandoga A, Kessler JS, Hanschen M, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006 Jun;79(6):1295–1305. doi: 10.1189/jlb.0805468. Epub 2006 Mar 21. [DOI] [PubMed] [Google Scholar]
- 82.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008 Sep 15;86(5):710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 83.Kuboki S, Sakai N, Tschöp J, et al. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009 May;296(5):G1054–G1059. doi: 10.1152/ajpgi.90464.2008. Epub 2009 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaudel CP, Frink M, van Griensven M, et al. FTY720 application following isolated warm liver ischemia improves long-term survival and organ protection in a mouse model. Transplant Proc. 2007 Mar;39(2):493–498. doi: 10.1016/j.transproceed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Kaudel CP, Frink M, Schmiddem U, et al. FTY720 for treatment of ischemia-reperfusion injury following complete renal ischemia; impact on long-term survival and T-lymphocyte tissue infiltration. Transplant Proc. 2007 Mar;39(2):499–502. doi: 10.1016/j.transproceed.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Parra C, Salas P, Dominguez J. Effects of immunosuppressive drugs on rat renal ischemia reperfusion injury. Transplant Proc. 2010 Jan-Feb;42(1):245–247. doi: 10.1016/j.transproceed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 87.Shen XD, Ke B, Zhai Y, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002 Aug 15;74(3):315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 88.Moore TM, Shirah WB, Khimenko PL, et al. Involvement of CD40-CD40L signaling in postischemic lung injury. Am J Physiol Lung Cell Mol Physiol. 2002 Dec;283(6):L1255–L1262. doi: 10.1152/ajplung.00016.2002. Epub 2002 Aug 9. [DOI] [PubMed] [Google Scholar]
- 89.Ishikawa M, Vowinkel T, Stokes KY, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005 Apr 5;111(13):1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. Epub 2005 Mar 28. [DOI] [PubMed] [Google Scholar]
- 90.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006 Feb;43(2):306–315. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- 91.Esch JS, Jurk K, Knoefel WT, et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets. 2010;21(5):348–359. doi: 10.3109/09537101003739897. [DOI] [PubMed] [Google Scholar]
- 92.Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Activation of CD40 with platelet derived CD154 promotes reactive oxygen species dependent death of human hepatocytes during hypoxia and reoxygenation. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030867. e30867. Epub 2012 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lapchak PH, Ioannou A, Kannan L, et al. Platelet-associated CD40/CD154 mediates remote tissue damage after mesenteric ischemia/reperfusion injury. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032260. e32260. Epub 2012 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimamura K, Kawamura H, Nagura T, et al. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005 Mar;234(1):31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 95.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006 Nov 27;203(12):2639–2648. doi: 10.1084/jem.20061097. Epub 2006 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li L, Huang L, Sung SS, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007 May 1;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 97.Cao Z, Yuan Y, Jeyabalan G, et al. Preactivation of NKT cells with alpha-GalCer protects against hepatic ischemia-reperfusion injury in mouse by a mechanism involving IL-13 and adenosine A2A receptor. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G249–G258. doi: 10.1152/ajpgi.00041.2009. Epub 2009 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-gamma mediates ischemia-reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am J Physiol Gastrointest Liver Physiol. 2009 Mar;296(3):G659–G663. doi: 10.1152/ajpgi.90495.2008. Epub 2008 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ascon M, Ascon DB, Liu M, et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 2009 Mar;75(5):526–535. doi: 10.1038/ki.2008.602. Epub 2008 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shibamoto T, Tsutsumi M, Kuda Y, et al. Mast cells are not involved in the ischemia-reperfusion injury in perfused rat liver. J Surg Res. 2012 May 1;174(1):114–119. doi: 10.1016/j.jss.2010.11.900. Epub 2010 Dec 18. [DOI] [PubMed] [Google Scholar]
- 101.Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998 Oct;28(4):959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- 102.Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004 Sep;36(9):969–977. doi: 10.1038/ng1413. Epub 2004 Aug 22. [DOI] [PubMed] [Google Scholar]
- 103.Knight B, Yeoh GC. TNF/LTalpha double knockout mice display abnormal inflammatory and regenerative responses to acute and chronic liver injury. Cell Tissue Res. 2005 Jan;319(1):61–70. doi: 10.1007/s00441-004-1003-6. Epub 2004 Nov 3. [DOI] [PubMed] [Google Scholar]
- 104.Murdaca G, Colombo BM, Cagnati P, et al. Update upon efficacy and safety of TNF-α inhibitors. Expert Opin Drug Saf. 2012 Jan;11(1):1–5. doi: 10.1517/14740338.2012.630388. Epub 2011 Oct 20. [DOI] [PubMed] [Google Scholar]
- 105.Kerensky TA, Gottlieb AB, Yaniv S, Au SC. Etanercept: efficacy and safety for approved indications. Expert Opin Drug Saf. 2012 Jan;11(1):121–139. doi: 10.1517/14740338.2012.633509. Epub 2011 Nov 11. [DOI] [PubMed] [Google Scholar]
- 106.Ben-Ari Z, Hochhauser E, Burstein I, et al. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002 Jun 27;73(12):1875–1880. doi: 10.1097/00007890-200206270-00004. [DOI] [PubMed] [Google Scholar]
- 107.Mahmoud MF, El Shazly SM, Barakat W. Inhibition of TNF-α protects against hepatic ischemia-reperfusion injury in rats via NF-κB dependent pathway. Naunyn Schmiedebergs Arch Pharmacol. 2012 May;385(5):465–471. doi: 10.1007/s00210-012-0729-z. Epub 2012 Feb 8. [DOI] [PubMed] [Google Scholar]
- 108.Hernandez-Alejandro R, Zhang X, Croome KP, et al. Reduction of liver ischemia reperfusion injury by silencing of TNF-α gene with shRNA. J Surg Res. 2012 Aug;176(2):614–620. doi: 10.1016/j.jss.2011.10.004. Epub 2011 Nov 1. [DOI] [PubMed] [Google Scholar]
- 109.Cain BS, Meldrum DR, Meng X, et al. p38 MAPK inhibition decreases TNF-alpha production and enhances postischemic human myocardial function. J Surg Res. 1999 May 1;83(1):7–12. doi: 10.1006/jsre.1998.5548. [DOI] [PubMed] [Google Scholar]
- 110.Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000 Dec;95(6):472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- 111.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK alpha/beta reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 112.Gao F, Yue TL, Shi DW, et al. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc Res. 2002 Feb 1;53(2):414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 113.Kaiser RA, Lyons JM, Duffy JY, et al. Inhibition of p38 reduces myocardial infarction injury in the mouse but not pig after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2747–H2751. doi: 10.1152/ajpheart.01280.2004. Epub 2005 Sep 2. [DOI] [PubMed] [Google Scholar]
- 114.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1107–H1114. doi: 10.1152/ajpheart.00455.2007. Epub 2007 May 11. [DOI] [PubMed] [Google Scholar]
- 115.Gao F, Yan WL, Zhang HF, Shi DW, Mo QZ, Ma XL. [Anti-apoptotic effect of insulin in myocardial ischemia-reperfusion and its principal signaling pathway] Zhonghua Nei Ke Za Zhi. 2003 Mar;42(3):153–156. Chinese. [PubMed] [Google Scholar]
- 116.Bell JR, Eaton P, Shattock MJ. Role of p38-mitogen-activated protein kinase in ischaemic preconditioning in rat heart. Clin Exp Pharmacol Physiol. 2008 Feb;35(2):126–134. doi: 10.1111/j.1440-1681.2007.04794.x. Epub 2007 Sep 24. [DOI] [PubMed] [Google Scholar]
- 117.Bassi R, Heads R, Marber MS, Clark JE. Targeting p38-MAPK in the ischaemic heart: kill or cure? Curr Opin Pharmacol. 2008 Apr;8(2):141–146. doi: 10.1016/j.coph.2008.01.002. Epub 2008 Mar 4. [DOI] [PubMed] [Google Scholar]
- 118.Yoshinari D, Takeyoshi I, Kobayashi M, et al. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to University of Wisconsin solution on reperfusion injury in liver transplantation. Transplantation. 2001 Jul 15;72(1):22–27. doi: 10.1097/00007890-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 119.Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002 Mar;131(3):344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Tian FZ, Tang LJ, Shi L, Zhang XQ. [The role of p38 MAPK pathway in ischemia-reperfusion injury of isolated liver] Zhonghua Gan Zang Bing Za Zhi. 2003 Mar;11(3):170–172. Chinese. [PubMed] [Google Scholar]
- 121.Ocuin LM, Zeng S, Cavnar MJ, et al. Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol. 2012 Oct;57(4):766–773. doi: 10.1016/j.jhep.2012.05.012. Epub 2012 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]