ABSTRACT
Background
Autoimmune diseases (such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes, etc) are characterized by the production of autoantibodies against one's own cell components, resulting in the dysfunction of normal organs. At present, therapies for autoimmune diseases involve a variety of nonspecific antiinflammatory and immunosuppressive agents with significant side effects. Current studies have suggested that the germinal center (GC) may be the pathogenic hot spot for the production of autoantibodies in autoimmune disease. Events occurring in the GC—such as the selection of high-affinity B cells, proliferation of B cells, and differentiation of B cells into plasma cells—all depend on T cells. Follicular helper T (Tfh) cells are a recently identified T-cell subset, named for their location in GCs. Tfh cells are characterized by their signature transcription factor (B-cell lymphoma 6), surface molecules (CD40 ligand, chemokine [C-X-C] receptor 5, inducible T-cell costimulator, programmed cell death protein-1, etc), and cytokines (interleukin [IL]-21, IL-6, IL-10, etc). Through these signals, Tfh cells help B cells form GCs and drive B cells to differentiate into memory B cells and plasma cells that produce antibodies. However, uncontrolled generation of Tfh cells in the GCs or peripherals could lead to autoimmunity. Recent studies from our group and others have shown that Tfh cells are expanded in the peripheral blood of patients and in the lymphoid tissues of mice with lupus or rheumatoid arthritis and play an important role in promoting pathogenic autoantibody production.
Methods
In this review, we summarize the latest immunologic findings regarding the characteristics and development of Tfh cells, their relation to other CD4+ T-cell subsets, and the function of Tfh cells in normal immune response and autoimmune diseases.
Conclusion
A clear understanding of the mechanisms of Tfh cell–mediated immunity and pathology may lead to the development of novel therapeutic targets in autoimmune diseases.
Keywords: Antibody formation, autoimmune diseases, germinal center
INTRODUCTION
Follicular helper T (Tfh) cells, a special CD4+ T-cell subset localized in the B-cell follicle, were first reported in tonsils1 where immune cells are constantly exposed to foreign antigens, resulting in the expansion of immune cells and the formation of germinal centers (GCs). The GC is a discrete lymphoid anatomic structure in secondary lymphoid organs (tonsils, lymph nodes, spleen, etc) where clonal expansion, somatic hypermutation, affinity maturation, and the development of B-cell memory and long-lived plasma cells occur, thus playing a key role in the protective immunity against pathogens.2-4
Recently Tfh cells have attracted close attention for their role in providing critical help to B cells and contributing to autoimmunity.5-8 Although Tfh cells and other CD4+ T-cell subsets share some phenotypic and functional properties, Tfh cells bear their specific identity via signature surface markers, cytokines, and transcription factors. Through these specific molecules and cytokines, Tfh cells play an important role in the selection of B-cell clones with high affinity toward foreign antigens in favor of developing a robust humoral immune response, while preventing the selection of B cell clones with weak affinity or affinity toward self-antigens to maintain self-tolerance.
Autoimmune diseases are currently thought to develop in genetically susceptible individuals from environmental exposure that triggers errant immune responses, causing the loss of tolerance to ubiquitous self-antigens and the generation of autoreactive B cells.9 Then these autoreactive B cells obtain excess help from the uncontrolled generation of Tfh cells, leading to increased production of pathogenic autoantibodies, inflammation and tissue injury, the onset of clinical symptoms, continued immune amplification, and eventually irreversible tissue damage. It was believed that Tfh cells may shape the outcome of B cell differentiation and be involved in the pathogenesis of autoimmune diseases. Dysregulation of Tfh cells is associated with the development of several autoimmune diseases, such as systemic lupus erythematosus (SLE),10,11 Sjögren syndrome,10,12 juvenile dermatomyositis,13 and rheumatoid arthritis.14,15
In this review, we summarize the latest immunologic findings regarding the characteristics and development of Tfh cells, their relation to the other CD4+ T cell subsets, and the function of Tfh cells in normal immune response and autoimmune diseases.
CHARACTERISTICS OF Tfh CELLS
Tfh cells have been identified as a distinct T helper cell subset based on their characteristic surface phenotype and cytokine profile, as well as their signature transcription factor.16,17 Several surface molecules expressed by Tfh cells (discussed below) are necessary for both the development and maintenance of Tfh cells and are critical to the interaction between Tfh cells and B cells that exerts the B cell response against pathogens.
Chemokine Receptor 5
Chemokine receptor 5 (CXCR5) is involved in Tfh cell homing to the B cell follicles. During GC formation, Tfh cells with strong expression of CXCR5 are attracted to the gradient expression of CXCR5 cognate (C-X-C motif) chemokine ligand 13 (CXCL13) in GCs, allowing Tfh cells to migrate and form stable contacts with antigen-primed B cells in the B cell follicles.18 The homing and colocalization of Tfh cells with B cells set up a center stage for T-B cell interaction, as T cell receptor (TCR) major histocompatibility complex class II (MHC-II) engagement is pivotal to the restriction of cognate B cell help. 19
Inducible T-Cell Costimulator
Inducible T-cell costimulator (ICOS, or CD278) is a costimulatory molecule that belongs to the CD28 superfamily. It interacts with its ligand (ICOSL) expressed on antigen-presenting cells or B cells.20 ICOS plays an important role in the regulation of Tfh cell development, T-cell–dependent antibody response, and GC reactions.21 Mice deficient in ICOS exhibit reduced number of Tfh cells,22 impaired GC formation, and profound defects in B cell maturation and immunoglobulin (Ig) isotype switching.23 Recent evidence showed that ICOS supported Tfh cell formation and maintenance via the production of interleukin (IL)-21 and c-Maf expression.24
Programmed Cell Death Protein-1
Programmed cell death protein-1 (PD-1, or CD279) is generally considered a potent inhibitory receptor expressed by T cells, and associated with T-cell tolerance and CD8 cytotoxic T-cell exhaustion during chronic virus infection and cancer, thus playing a negative role in immune response.25,26 Compared to other T-cell subsets, Tfh cells express the highest levels of PD-1.27 Through the interaction between PD-1 on Tfh cells and its ligand (PD-1L) on GC-B cells, Tfh cells deliver survival signals to GC-B cells.28 The regulation of PD-1 may affect GC magnitude and output, such as the high affinity of long-lived plasma cells.29 Taken together, during Tfh cell activation PD-1 delivers a survival signal to GC-B cells, whereas ICOS functions as a costimulating molecule, thus determining the outcome of the Tfh-cell–mediated B cell response.
CD40 Ligand
Tfh cells also interact with B cells through the expression of surface molecules such as the CD40 ligand (CD40L), a member of the tumor necrosis factor family. CD40L is well known for its role in Ig isotype switching and B-cell survival via its interaction with CD40 on B cells.30 Patients with CD40L deficiencies or mutations have a severe lack of memory B cells and an absence of GCs.31 Of note, CD40L is widely expressed by CD4+ T helper cells and is not limited to Tfh cells.
Other Surface Molecules on Tfh Cells
CD57,32 B and T lymphocyte attenuator (BTLA),33 the members of the signaling lymphocytic activation molecule-associated (SLAM) protein family34 (SAP), CD84, and natural killer T-B antigen also contribute to GC formation and immune homoeostasis.
Cytokines
Tfh cells also exhibit a unique expression profile of cytokines (such as IL-21, IL-4, and IL-10) and other soluble factors (CXCL13, etc).35-37 IL-21 is a signature cytokine for Tfh cells. Reports from our research and that of others showed that IL-21 plays a critical role in promoting B-cell somatic hypermutation, Ig isotype switching, and plasma cell generation.38-40 Through the IL-21 receptor, IL-21 also induces Tfh-cell differentiation in an autocrine fashion.41,42 Furthermore, IL-21 synergizes with IL-6 and B-cell activating factor to regulate Tfh-cell and B-cell differentiation.43
Signature Transcription Factor B-Cell Lymphoma 6
B-cell lymphoma 6 (BCL-6), a protooncogene, encodes a poxvirus-zinc finger transcription factor that acts as a sequence-specific repressor of transcription through recruitment of a silencing mediator for retinoid and thyroid hormone receptors (SMRTs) and histone deacetylase-containing complex.44 Previous studies showed that BCL-6 expression was largely confined in GC-B cells, and its function was closely associated with the fate of GC-B cells.45 Recently BCL-6 has emerged as a master transcription factor for Tfh cells by affecting the differentiation of Tfh cells and the expression of Tfh cell signature molecules,46 as well as controlling long-term CD4+ T cell memory.47 BCL-6–deficient mice lacked GCs, failed to develop Tfh cells, and consequently displayed defective T-cell–dependent antibody responses,48,49 whereas other CD4+ T cell subsets were relatively unaffected, indicating that BCL-6 is critical for programming Tfh cell differentiation and GC-B cell development. Because BCL-6 connects the differentiation of both Tfh cells and GC-B cells, it acts as a master transcription factor for T-cell–dependent antibody responses in GC.
Tfh CELL DEVELOPMENT AND FUNCTION
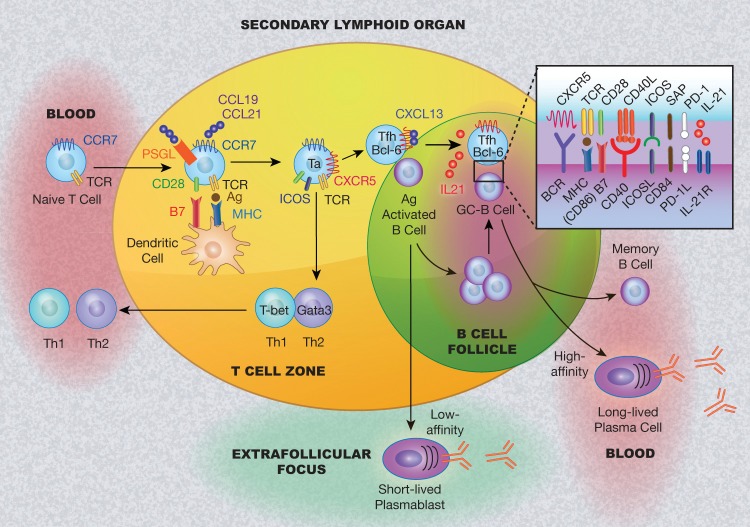
Tfh cells are generated and developed in the secondary lymphoid organs where naïve T cells and plasmoid dendritic cells locate at the T-cell zone in the paracortex and B cells stay at the B-cell follicles in the outer cortex (Figure 1).
Figure 1.
Schematic model of follicular helper T cell (Tfh) development and collaboration with B cells in secondary lymphoid organs. Naïve T cells in the peripheral blood migrate to the T-cell zone in the secondary lymphoid organs (tonsil, spleen, etc) following the gradient of (C-C motif) chemokine ligand (CCL) 19 and CCL21, by engaging to their counterpart (chemokine receptor type 7 [CCR7] and P-selectin glycoprotein ligand-1 [PSGL-1]). T cells become activated (Ta) by recognizing the antigen (Ag) provided by dendritic cells. Dendritic cells provide costimulatory signals to promote T-cell differentiation, resulting in the generation of T-cell subsets. With downregulation of CCR7 and PSGL-1 and upregulation of the expression of chemokine (C-X-C) receptor type 5 (CXCR5) and B-cell lymphoma 6 (BCL-6), Tfh cells are generated and migrate to the T-B border to interact with the antigen-activated B cells. The outcome for this interaction is the development of extrafollicular foci where B cells differentiate into short-lived plasmablasts and/or form germinal centers (GCs). In the GCs, Tfh cells promote B-cell maturation with clone expansion, somatic hypermutation, and class switching through pairs of surface molecules and cytokines (such as interleukin [IL]-21, etc). The interaction of Tfh and B cells leads to the generation of memory B cells and long-lived antibody-producing plasma cells. BCR, B cell receptor; CD40L, CD40 ligand; CXCL13, C-X-C motif chemokine 13; GATA3, GATA binding protein 3; ICOS, inducible T cell costimulator; ICOSL, ICOS ligand; IL-21R, IL-21 receptor; MHC, major histocompatibility complex; PD-1, programmed cell death protein-1; PD-1L, PD-1 ligand; SAP, signaling lymphocytic activation molecule-associated protein; TCR, T cell receptor; Th1, type 1 helper T cells; Th2, type 2 helper T cells.
Physiologically attracted to the T-cell zone chemokine gradient of C-C motif chemokine ligand 19 (CCL19) and CCL21, peripheral naïve CD4+ T cells expressing the counterparts (CCR7 and P-selectin glycoprotein ligand-1 [PSGL-1]) of these chemokines migrate to the T-cell zone from the circulation.50 Upon encountering the antigens provided by dendritic cells (DCs), naïve CD4+ T cells gain expression of CXCR5 and transcription factor BCL-6 while losing expression of CCR7 and PSGL-1, are attracted to the chemokines (such as CXCL13) expressed by B cells, migrate to the B-cell follicle border, and become Tfh cells.51 Meanwhile, the B cells encounter antigens presented by DCs or macrophages in the follicle and are activated through their B-cell receptor, which leads to the upregulation of CCR7 and migration to the B-cell follicle border.52,53 Thus, at the B-cell follicle border, Tfh cells and B cells migrate toward each other and finally engage in a stable cognate interaction, ultimately leading to a series of molecular events that shapes the humoral immune response, including the development of extrafollicular foci and GCs. First, the T-B cell interaction—via the TCR/MHC peptide and ICOS and its ligand (ICOSL)—promotes B-cell survival and then consequently leads B-cell migration and formation of the extrafollicular foci where B cells differentiate into short-lived antibody-producing plasmablasts.54 Alternatively, with maintenance of CXCR5 expression for homing to the follicle55 and greatest TCR signal strength on T cells,56 both Tfh and B cells are selected and migrate into the follicle and lead to B-cell clonal expansion and GC formation. In GCs, Tfh cells then interact with GC-B cells though various pairs of molecules, such as ICOS-ICOSL, PD-1–PD-1L, CD40-CD40L, and IL-21–IL-21R, resulting in the generation of memory B cells and long-lived antibody-producing plasma cells (Figure 1).57,58
The location and mobilization of these immune cells are a dynamic process that was revealed by multiphoton intraviral imaging.4 The studies showed that the interaction between Tfh and B cells in the GC is less frequent and far shorter compared to that in the T-B border, suggesting that cytokines are more efficient in T-B interaction in GCs and that cell-cell contact is critical in the T-B border. Indeed, our studies on GC-B cells showed that the cytokines secreted by GC-Tfh cells determine the fate of GC-B cell differentiation.38,59 IL-4 directs GC-B cells to differentiate into memory B cells, whereas IL-10 and IL-21 steer them into plasma cells.
The preferential localization of Tfh cells in the GC suggests a unique, intimate relationship between Tfh and B cells. Events occurring in the GC, such as selection of high-affinity B cells, proliferation of B cells, and differentiation of B cells into plasma cells, all depend on the provision of help by Tfh cells, indicating that Tfh cells are a key participant in controlling B-cell peripheral tolerance and antibody production.
DIFFERENTIATION AND REGULATION OF Tfh CELLS
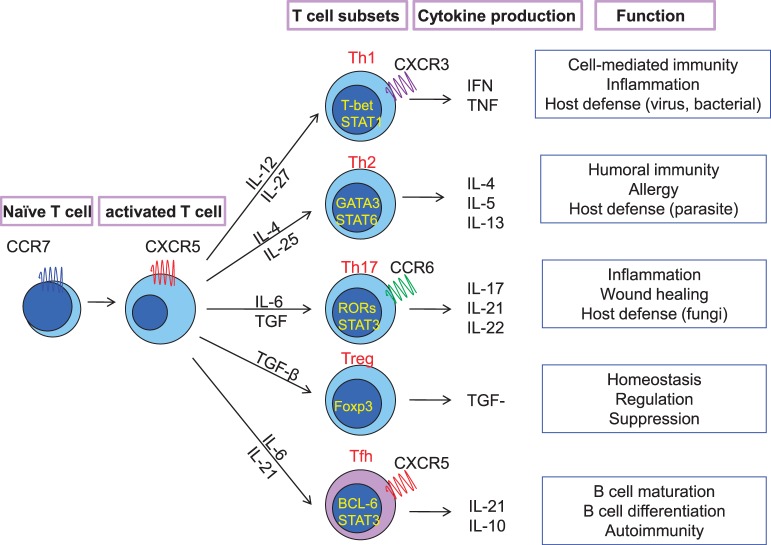
The differentiation of the CD4+ T cells depends on microenvironmental factors, including signals from B cells and the cytokines' milieu that influence their transcription factor expression and determine the type of T-cell subpopulations (T helper [Th]1, Th2, Th17, T regulatory, and Tfh cells) (Figure 2). Each T-cell subset produces specific cytokines and exerts its unique functions in host defense.16,60 In contrast to the other T-helper subsets—such as Th1, Th2, and Th17 cells that are controlled by T-bet, GATA3, and RORγt, respectively—the differentiation of Tfh cells depends on transcription factor BCL-6.
Figure 2.
T helper cell lineage development and function. The cytokines produced by dendritic cells regulate the T helper cell lineage (Th1, Th2, Th17, T regulatory [Treg], and follicular helper T cells [Tfh]). Each of them exhibits a unique phenotype, cytokine, and transcriptional profile and exerts different functions in immune response. BCL-6, B cell lymphoma 6 protein; CCR, C-C chemokine recetor; CXCR, CXC chemokine receptor; Foxp3, forkhead box P3; IFN, interferon; IL, interleukin; RORs, retinoid-acid receptor related orphan receptors; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor-beta; TNF, tumor necrosis factor.
BCL-6 is an important arbiter of the Tfh-cell lineage fate. It acts as a transcription repressor by binding to the promoter region of T-bet, GATA3, and RORγt, thus suppressing Th1, Th2, and Th17 differentiation,61,62 and it dominantly directs T cells toward Tfh cells. BCL-6 also affects the signature molecule expression on Tfh cells.46 Blimp-1 is another transcription factor that acts as an antagonist of BCL-6. The balance between BCL-6 and Blimp-1 in T cells is critical for the fate of Tfh-cell differentiation.63 By repressing Blimp-1 expression, BCL-6 directs CD4+ T cells toward non-Tfh-cell (Th1, Th2, Th17) differentiation. Of note, BCL-6 is also expressed on GC-B cells and controls GC-B cell differentiation with a set of regulating genes separate from those in Tfh cells.
Tfh CELLS IN THE PATHOGENESIS OF AUTOIMMUNITY
Autoimmune disease is characterized by the production of autoantibodies directing against the patient's own cells, contributing to tissue damage. Research studies have suggested that GCs may be the pathogenic hot spot for the production of autoantibodies in autoimmune diseases. Many lupus-prone murine models show that spontaneous generation of GCs positively correlates with the production of autoantibodies.64,65 Within GCs, somatically mutated self-reactive B cells are normally eliminated at GC reaction via soluble antigen-induced apoptosis without T-cell help. In autoimmune disease, however, with dysfunctional B-cell selection and cognate help from Tfh cells, these self-reactive B cells could be able to survive, expand, differentiate, ultimately produce antibodies, and cause tissue or organ damage.
The contribution of Tfh cells in autoimmune disease has been mainly studied in murine models (Table) that show disease-prone mice to have an aberrantly expanded Tfh population.66,67 The most striking evidence of Tfh involvement in autoimmunity is from the sanroque mouse model.67 Sanroque mice have a single recessive mutation in the roquin gene that encodes a RING-type ubiquitin ligase protein that disrupts a repressor of ICOS expression. These mice demonstrated a spontaneous GC formation and SLE-like pathologies, such as anti-dsDNA antibodies, immune-complex mediated glomerulonephritis, and excessive Tfh cells with increased expression of ICOS and IL-21. Furthermore, deficiency of SAP molecules or deletion of an allele of BCL-6 in the sanroque mouse model resulted in significant reduction of Tfh cell numbers and IL-21 production, abrogated GC formation, and ameliorated SLE-like pathological features.68,69 Conversely, adoptive transfer of Tfh cells from the sanroque mouse into wild-type recipients induced spontaneous GC formation and autoantibody production,70 providing direct evidence of the role of Tfh cells in the pathogenesis of autoimmune diseases. Thus, the data from autoimmune mice strongly suggest that excessive Tfh cells are responsible for autoimmunity and the dysregulation of the GC response.
Table.
Follicular Helper T (Tfh) Cells and Autoimmune Pathology in Mouse Models and Human Disease

In addition to the number of Tfh cells, the quality of help from Tfh cells also controls the extent of the immune response. Evidence has shown that excessive production of the Tfh signature cytokine IL-21 leads to autoimmunity.71 Similar to the sanroque mouse model, BXSB mice—in which lupus-like autoimmunity is promoted by excessive signaling induced by self-RNA autoantigens as a result of duplication of the gene encoding toll-like receptor 7 (TLR-7)—also demonstrated spontaneous GC formation with significant Tfh cell expansion and IL-21 production.72 Blocking IL-21 signaling in BXSB mice and lupus-prone MRLlpr mice led to diminished pathogenic autoantibody production and other lupus-like features,73 indicating that IL-21 is required for autoreactive B cell differentiation and that blocking IL-21 could be a potential therapy to treat SLE.
Studies from other lupus-prone mice give further insight into how Tfh cells help support autoantibody production.70 Unlike the spontaneous GC formation observed in sanroque mice, plasmablasts in MRLlpr mice form extrafollicular foci lacking GCs and their Tfh cells are located in extrafollicular sites. Tfh cells in both extrafollicular and GC sites involving the lupus pathogenesis were observed in lupus-prone NZB/WF1 mice.71 Their Tfh cells also expand beyond GCs to extrafollicular sites, exaggerating the lupus progress.
While the role of Tfh cells in autoimmunity has been established in mice, the association of Tfh cells with human autoimmune diseases remains largely unexplored. The fact that many pathogenic high-affinity anti-DNA antibodies detected in patients with autoimmune diseases exhibit heavy chain class switching and somatic hypermutation72 suggests the involvement of GC-Tfh or Tfh-committed extrafollicular cells in the pathogenesis of autoimmune diseases. Recently, we and others discovered the expansion of circulating Tfh cells in patients with juvenile dermatomyositis,13 rheumatoid arthritis,14,15 SLE,10,11 and Sjögren syndrome.10 Furthermore, our recent study showed that the accumulation of circulating Tfh cells strongly correlated with high levels of plasmablasts, anti-dsDNA, and antinuclear antibodies, as well as the severity of disease activity in patients with SLE.11 These observations suggest that the expanded circulating Tfh cells may act as the key mediators of autoantibody production and the pathogenesis of lupus. Our data also show that the circulating Tfh cells in patients with lupus and the synovial fluid in patients with rheumatoid arthritis shared similar phenotypic and functional properties with GC-Tfh cells in the follicles, indicating circulating Tfh cells derived from GC-Tfh cells and shuffling between peripheral blood and lymphoid tissues.
CONCLUSION
Progress has been made in understanding the immunobiology of Tfh cells. Tfh cells have emerged as distinct subsets of T-helper cells that provide critical help to B cells and play a critical role in GC generation and maintenance of humoral immune responses. Growing evidence also has implicated Tfh cells in the development of autoimmune disease. Currently, no effective anti-Tfh-cell antibody or inhibitor has been applied to the patients with autoimmune diseases. A deeper understanding of the role and the origins of Tfh cells will help advance translational research and lead to the development of a novel therapeutic protocol for autoimmune disease.
ACKNOWLEDGMENTS
We would like to thank Barbara Siede of Medical Illustrations for her hard work on our figures. We also would like to thank Dr Rodwig and Sherron Hammond of the Ochsner Blood Bank and Dr Guarisco of the Department of Surgery for providing clinical material to support our research.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000 Dec 4;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 3.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997 Apr;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 4.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007 Aug;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009 Mar 20;30(3):324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 7.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011 Nov 23;35(5):671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Linterman MA, Liston A, Vinuesa CG. T-follicular helper cell differentiation and the co-option of this pathway by non-helper cells. Immunol Rev. 2012 May;247(1):143–159. doi: 10.1111/j.1600-065X.2012.01121.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Davidson A. Taming lupus—a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012 Jun 6;18(6):871–882. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010 Jan;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 11.Lindwall E, Gauthier C, Alarakhia A, et al. Circulating T helper cells in patients with systemic lupus erythematosus share the phenotypic properties with lymphoid T follicular helper cells. [abstract] Arthritis Rheum. 2011 Oct;63(10):646. [Google Scholar]
- 12.Li XY, Wu ZB, Ding J, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren's syndrome. Biochem Biophys Res Commun. 2012 Jun 1;422(2):238–244. doi: 10.1016/j.bbrc.2012.04.133. Epub 2012 Apr 30. [DOI] [PubMed] [Google Scholar]
- 13.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011 Jan 28;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. Epub 2011 Jan 6. Erratum in: Immunity. 2011 Jan 28;34(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ing S, Alarakhia A, Lindwall E, et al. Identification of follicular helper T cells as a novel cell population potentially involved in the pathogenesis of rheumatoid arthritis. [abstract] Arthritis Rheum. 2012 Oct;64(10):1195. [Google Scholar]
- 15.Ma J, Zhu C, Ma B, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012 doi: 10.1155/2012/827480. 2012:827480. Epub 2012 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. 2001 Sep;2(9):876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002 Dec;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 18.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000 Dec 4;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001 Jun 18;193(12):1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdam AJ, Greenwald RJ, Levin MA, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001 Jan 4;409(6816):102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 21.Akiba H, Takeda K, Kojima Y, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005 Aug 15;175(4):2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 22.Tafuri A, Shahinian A, Bladt F, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001 Jan 4;409(6816):105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 23.Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001 Jan 4;409(6816):97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 24.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009 Feb;10(2):167–175. doi: 10.1038/ni.1690. Epub 2008 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009 May 15;182(10):5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haymaker C, Wu R, Bernatchez C, Radvanyi L. PD-1 and BTLA and CD8(+) T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology. 2012 Aug 1;1(5):735–738. doi: 10.4161/onci.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, Guo Z, Ju W, et al. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012 Sep;9(5):375–379. doi: 10.1038/cmi.2012.18. Epub 2012 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012 Oct;12(10):2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. Epub 2012 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Good-Jacobson KL, Szumilas CG, Chen L, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010 Jun;11(6):535–542. doi: 10.1038/ni.1877. Epub 2010 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renshaw BR, Fanslow WC, III, Armitage RJ, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994 Nov 1;180(5):1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994 Jun;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim JR, Lim HW, Kang SG, Hillsamer P, Kim CH. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. 2005 Feb 4;6:3. doi: 10.1186/1471-2172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashiwakuma D, Suto A, Hiramatsu Y, et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J Immunol. 2010 Sep 1;185(5):2730–2736. doi: 10.4049/jimmunol.0903839. Epub 2010 Jul 26. [DOI] [PubMed] [Google Scholar]
- 34.Cannons JL, Qi H, Lu KT, et al. Optimal germinal center responses require a multistage T cell: B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010 Feb 26;32(2):253–265. doi: 10.1016/j.immuni.2010.01.010. Epub 2010 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007 Dec 15;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 36.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009 May 11;206(5):1001–1007. doi: 10.1084/jem.20090313. Epub 2009 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Zhu T, Cai G, et al. Elevated circulating CD4+ ICOS+ Foxp3+ T cells contribute to overproduction of IL-10 and are correlated with disease severity in patients with systemic lupus erythematosus. Lupus. 2011 May;20(6):620–627. doi: 10.1177/0961203310392431. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of plasma cell generation from human germinal center B cells. J Leukoc Biol. 2009 Dec;86(6):1311–1318. doi: 10.1189/jlb.0409268. Epub 2009 Sep 17. [DOI] [PubMed] [Google Scholar]
- 39.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005 Dec 15;175(12):7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 40.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. Immunol. 2007 Nov 1;179(9):5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 41.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008 Jul 18;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. Epub 2008 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suto A, Kashiwakuma D, Kagami S, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008 Jun 9;205(6):1369–1379. doi: 10.1084/jem.20072057. Epub 2008 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eto D, Lao C, DiToro D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011 doi: 10.1371/journal.pone.0017739. Mar 14;6(3):e17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhordain P, Albagli O, Lin RJ, et al. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartholdy B, Matthias P. Transcriptional control of B cell development and function. Gene. 2004 Feb 18;327(1):1–23. doi: 10.1016/j.gene.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Kusam S, Toney LM, Sato H, Dent AL. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J Immunol. 2003 Mar 1;170(5):2435–2441. doi: 10.4049/jimmunol.170.5.2435. [DOI] [PubMed] [Google Scholar]
- 47.Ichii H, Sakamoto A, Hatano M, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002 Jun;3(6):558–563. doi: 10.1038/ni802. Epub 2002 May 20. [DOI] [PubMed] [Google Scholar]
- 48.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997 Apr 25;276(5312):589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 49.Ye BH, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997 Jun;16(2):161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 50.Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002 Jul 1;169(1):424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 51.Haynes NM, Allen CD, Lesley R, et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007 Oct 15;179(8):5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 52.Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004 Sep;5(9):943–952. doi: 10.1038/ni1100. Epub 2004 Aug 1. [DOI] [PubMed] [Google Scholar]
- 53.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002 Jan;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 54.Lee SK, Rigby RJ, Zotos D, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011 Jul 4;208(7):1377–1388. doi: 10.1084/jem.20102065. Epub 2011 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000 Dec 4;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009 Apr;10(4):375–384. doi: 10.1038/ni.1704. Epub 2009 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010 Sep;237(1):72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 58.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010 Oct;31(10):377–383. doi: 10.1016/j.it.2010.07.001. Epub 2010 Aug 31. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Li L, Jung J, et al. The distinct roles of T cell-derived cytokines and a novel follicular dendritic cell-signaling molecule 8D6 in germinal center-B cell differentiation. J Immunol. 2001 Jul 1;167(1):49–56. doi: 10.4049/jimmunol.167.1.49. [DOI] [PubMed] [Google Scholar]
- 60.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 61.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009 Sep 18;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. Epub 2009 Jul 23. [DOI] [PubMed] [Google Scholar]
- 62.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009 Aug 21;325(5943):1001–1005. doi: 10.1126/science.1176676. Epub 2009 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009 Aug 21;325(5943):1006–1010. doi: 10.1126/science.1175870. Epub 2009 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008 Feb;9(2):166–175. doi: 10.1038/ni1552. Epub 2007 Dec 23. [DOI] [PubMed] [Google Scholar]
- 65.Luzina IG, Atamas SP, Storrer CE, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001 Oct;70(4):578–584. [PubMed] [Google Scholar]
- 66.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005 May 26;435(7041):452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 67.Linterman MA, Rigby RJ, Wong RK, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009 Mar 16;206(3):561–576. doi: 10.1084/jem.20081886. Epub 2009 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bubier JA, Sproule TJ, Foreman O, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1518–1523. doi: 10.1073/pnas.0807309106. Epub 2009 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pisitkun P, Deane JA, Difilippantonio MJ, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006 Jun 16;312(5780):1669–1672. doi: 10.1126/science.1124978. Epub 2006 May 18. [DOI] [PubMed] [Google Scholar]
- 70.Rankin AL, Guay H, Herber D, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012 Feb 15;188(4):1656–1667. doi: 10.4049/jimmunol.1003871. Epub 2012 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Behar SM, Lustgarten DL, Corbet S, Scharff MD. Characterization of somatically mutated S107 VH11-encoded anti-DNA autoantibodies derived from autoimmune (NZB x NZW)F1 mice. J Exp Med. 1991 Mar 1;173(3):731–741. doi: 10.1084/jem.173.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002 Sep 20;297(5589):2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 73.Platt AM, Gibson VB, Patakas A, et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol. 2010 Aug 1;185(3):1558–1567. doi: 10.4049/jimmunol.1001311. Epub 2010 Jul 2. [DOI] [PubMed] [Google Scholar]
- 74.Hu YL, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009 Feb 1;182(3):1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 75.Kasagi S, Kawano S, Okazaki T, et al. Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice. J Immunol. 2010 Mar 1;184(5):2337–47. doi: 10.4049/jimmunol.0901652. Epub 2010 Feb 5. [DOI] [PubMed] [Google Scholar]