ABSTRACT
Background
Multiple studies have demonstrated the important role of the nuclear factor kappa B (NF-κB) in cardiac pathology. However, these studies' conclusions differ regarding whether NF-κB is protective or detrimental for heart function. This disagreement is not surprising considering the complexity of NF-κB signaling that involves multiple components and regulation at several steps. Furthermore, NF-κB is a pleiotropic transcription factor that receives signals from multiple pathways, including the renin-angiotensin system (RAS) and cytokines, 2 important modulators of cardiac remodeling.
Methods
In this article, we review studies related to the role and mechanisms of NF-κB activation in the heart, particularly with regard to the RAS, inflammation, and diabetes. The objective of this review is to consolidate multiple, often contradictory, findings to develop a clear understanding of NF-κB signaling in the heart.
Conclusions
The studies we review demonstrate that NF-κB effects in the heart are mechanism specific and that NF-κB signaling is cyclical. Consequently, the timing of NF-κB measurement is critical, and studies focused on temporal changes in the NF-κB mechanism would help clarify its multiple roles in cardiac pathophysiology.
Keywords: Cytokines, diabetes mellitus, heart, NF-kappa B, renin-angiotensin system
INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in the modern world. The prevalence of CVD is on the rise because of the aging population and the increase in obesity and type 2 diabetes. Although the term CVD includes a variety of diseases with multiple causes, the common outcome of CVD is cardiac remodeling that leads to heart failure. Cardiac remodeling is associated with increased oxidative stress, inflammation, and activation of hormonal systems, all of which are involved in disease progression.1,2
Clinical and experimental studies have demonstrated a role of the renin-angiotensin system (RAS) in cardiac remodeling and heart failure. Chronic inflammation also has deleterious effects on cardiovascular function. However, these 2 mechanisms are not completely independent. The nuclear factor kappa B (NF-κB) family of transcription factors is the major mediator of inflammation. Additionally, NF-κB controls angiotensinogen (AGT) gene expression, the precursor of a bioactive peptide of the RAS, angiotensin II (AngII). AngII activates NF-κB, thereby generating a positive feedback loop. Tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine, also activates and is positively regulated by NF-κB. The observation that angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme (ACE) inhibitors reduce inflammation suggests that a part of their protective mechanism may be through the NF-κB pathway.3
Additionally, oxidative stress increases the expression of AGT through the activation of NF-κB in hepatocytes and vascular smooth muscle cells.4 AngII in turn activates NF-κB through the production of reactive oxygen species (ROS), thereby generating a feedback loop.5
Clinical and experimental studies have shown important roles of both the RAS and inflammation in diabetic cardiomyopathy. NF-κB regulates both pathways, suggesting that it may be a major hub of cellular activity in diabetes. NF-κB signaling mechanisms are complex and produce a wide variety of sometimes opposite biological effects in diverse cell types and organs.5 The effects of NF-κB in the heart are likewise diverse, with some studies pointing to cardioprotective effects and others demonstrating cardioprotection by blocking NF-κB activity.6-14 In this article, we review different mechanisms that activate NF-κB in the heart and demonstrate how crosstalk between inflammation and the RAS, through NF-κB, might influence disease progression.
NF-κB SIGNALING PATHWAYS
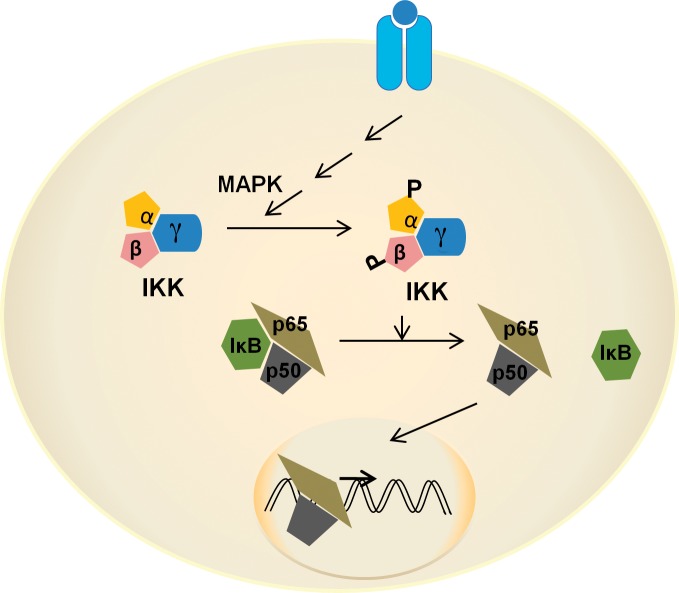
The NF-κB family consists of 5 members: p50/p105 (NF-κB1), p52/p100 (NF-κB2), p65 (RelA), RelB, and c-Rel. The member proteins form homo- or heterodimers, of which the p50/p65 heterodimer is the most abundant and is responsible for the majority of NF-κB canonical transcriptional activity (Figure 1). Homodimers of p50 subunits have been associated with inhibitory transcriptional activity.15 Generally, NF-κB dimers associate with an inhibitory-κB (IκB-α) protein that keeps the dimer in the cytoplasm in an inactive state. NF-κB activation begins with the activation of an IκB kinase (IKK) complex that consists of catalytic subunits IKK-α and IKK-β and the scaffolding subunit IKK-γ (the NF-κβ essential modifier [NEMO]). Several mitogen-activated protein (MAP) kinases that also include NF-κB–inducing kinase (NIK) activate IKK through the phosphorylation of IKK-α and IKK-β. IKK-β has higher activity than IKK-α for IκB-α and is considered important in the canonical pathway.5 In the canonical pathway of NF-κB activation, IκB-α is phosphorylated at serine residue (Ser)32,36 and/or Tyr42 and separated from the p50/p65 dimer, allowing the dimer to translocate to the nucleus and bind to cognate DNA sequences.
Figure 1.
Canonical nuclear factor kappa B (NF-κB) signaling. In the canonical pathway, phosphorylation of IκB kinase α/β by mitogen-activated protein kinase (MAPK) is followed by phosphorylation of IκB-α that occurs in an inactive complex with p50/p65. Phosphorylated IκB-α is released and degraded in the cytoplasm. The active heterodimer of p50/p65 enters the nucleus to regulate expression of multiple genes. IκB, inhibitory kappa B; IKK, inhibitory kappa B kinase; P, phosphorylation.
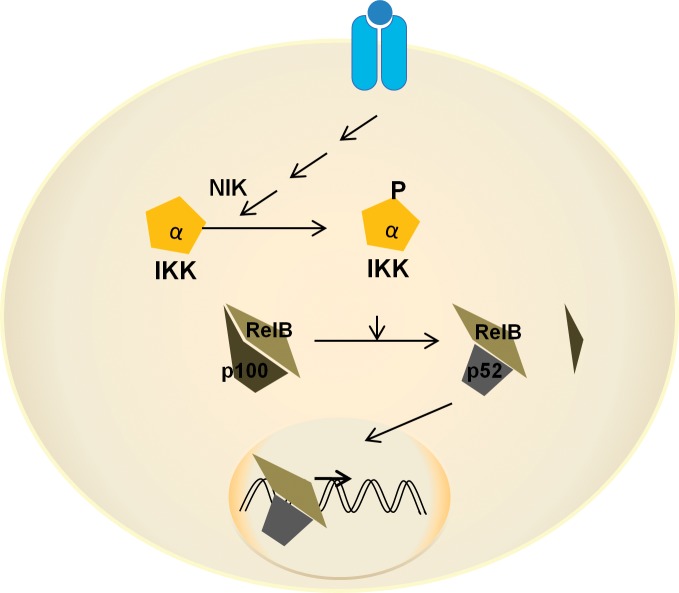
The noncanonical pathway involves the activation of NIK, resulting in the release of the p52/RelB heterodimer from inhibition (Figure 2).5 In yet another mechanism relevant to AngII-mediated NF-κB activation, Ser536 phosphorylation of p65 results in IκB-α–independent stimulation of p65 transcriptional activity.16 Recently, a new mechanism of NF-κB activation through monomethylation of p65 at K37 by histone H3-lysine 4 methyltransferase, SET7/9, has been described.17,18 Methylation of p65 was induced by TNF-α and interleukin-1 beta (IL-1β) and was responsible for the expression of about 25% of NF-κB–responsive genes in TNF-α–stimulated monocytes.18 Receptor-for-advanced-glycation-endproducts ligands induced inflammatory genes in monocytes. The expression of the inflammatory genes was inhibited by small interfering RNA-mediated SET7/9 knockdown, suggesting a role of this NF-κB activation mechanism in diabetes.18
Figure 2.
Noncanonical nuclear factor kappa B (NF-κB) signaling. In the noncanonical pathway, NIK has an important role in the phosphorylation and activation of IκB kinase α (IKK-α). IKK-α phosphorylates p100 that exists in an inactive complex with RelB. Phosphorylated p100 is partially cleaved to generate the active form p52. The p52/RelB heterodimer enters the nucleus and regulates the expression of several genes distinct from those activated by the canonical pathway. IKK, inhibitory kappa B kinase; NIK, nuclear factor kappa B–inducing kinase.
Acetylation of p50 has been identified as yet another novel mechanism of NF-κB activation that was demonstrated as essential for cardiac protection against ischemia/reperfusion (I/R) injury.19 In addition to the NF-κB subunits that translocate to the nucleus following activation, the cytoplasmic signal integrators—IKK subunits, NIK, and IκB-α—undergo nucleocytoplasmic shuttling, suggesting that these proteins might have independent nuclear functions.20
NF-κB subunits are ubiquitously expressed and activated by multiple mechanisms and by different stimuli in diverse cell types, resulting in a wide variety of sometimes opposite biological effects. The NF-κB canonical pathway is the most extensively studied mechanism. The canonical pathway regulates expression of about 200 genes, including genes coding for transcription factors.5 NF-κB thus may have direct as well as indirect effects through these transcription factors. Direct effects further consist of early and late response genes. Some of the proteins coded by NF-κB regulatory genes are inhibitors of NF-κB signaling, such as IκB-α, and produce a negative feedback control. Other genes produce proteins that are involved in generating signals that activate NF-κB pathways, such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression.21 Information about the gene expression profile activated by the noncanonical NF-κB pathway is limited. However, this expression profile appears to be distinct from the canonical pathway.5 Similarly, the effects of the acetylation of p50 and the methylation and phosphorylation at different sites of p65 are incompletely understood.
ROLE OF NF-κB IN CARDIAC PATHOPHYSIOLOGY
NF-κB regulates multiple cellular processes, including cell maturation, survival, and proliferation, and systemic processes such as inflammation. NF-κB is activated by stress and inflammatory stimuli that include cytokines, vasoactive peptides, and viral oncogenes. NF-κB activation is generally transient because of negative feedback regulation. Although a short activation of NF-κB might be beneficial, a prolonged activation may be detrimental by promoting chronic inflammation. Thus, the duration as well as the timing and cellular context—ie, the cell type and the stimulus—may determine the outcome of NF-κB signaling in the heart. Several studies have demonstrated a role of NF-κB signaling in different cardiac pathological states. Transaortic coarctation, a model of pressure overload, increased NF-κB activity in the mouse heart that developed oxidative stress and cardiac hypertrophy.22 Adenovirus-mediated intramyocardial gene transfer of a dominant negative form of Akt or cytoplasmic superoxide dismutase attenuated NF-κB activity and cardiac hypertrophy, suggesting a role of NF-κB in pressure overload.22 In Dahl salt-sensitive hypertensive rats, a high-salt diet for 18 weeks increased NF-κB activity in the heart and increased expression of TNF-α, interleukin-6 (IL-6), and IL-1β.23 Similarly, in spontaneously hypertensive rats (SHRs), cardiac expression of IL-6 and IL-1β increased as did the expression and activity of p65 NF-κB.24 In the following sections, we categorize studies based on the NF-κB subunits to determine whether different NF-κB activation mechanisms produce different outcomes in the context of heart diseases.
Role of the NF-κB p50 Subunit
In mice deficient for p50 NF-κB, p65 expression and nuclear localization were increased following the stimulation of mouse embryonic fibroblasts with lipopolysaccharide, resulting in increased IL-6 secretion compared to wild-type (WT) controls.25 Further, p50 deficiency increased cardiac remodeling and systolic dysfunction in response to myocardial ischemia. These studies suggest that p50 has a protective role in inflammation and cardiac remodeling and that p65 can function without the need for heterodimerization with p50, as in the canonical pathway.25 However, in another study on p50 knockout mice, coronary artery ligation–induced ventricular dilatation over 8 weeks was less in knockout animals than in WT mice.26 In an I/R model, protection of the heart by a histone deacetylase inhibitor, trichostatin A (TSA), was accompanied by increased nuclear localization of p50 NF-κB. TSA treatment resulted in increased acetylation of p50.19 In hearts from p50-deficient mice, TSA did not protect against I/R injury, suggesting an essential role of p50 in cardioprotection.19 In a human study, the p50 NF-κB (NF-κB1) promoter polymorphism (ATTG1)—associated with lower expression of p50—was correlated with heart failure in patients. The ATTG1 genotype was associated with enhanced cardiac remodeling and impaired heart function.27 Overexpression of p50 in H9c2 cells, a rat cardiomyoblast cell line, repressed expression of the c-Rel subunit of NF-κB; c-Rel has been shown to stimulate cardiac hypertrophy and fibrosis.28 Confirming these in vitro observations, hearts from p50 knockout mice were larger than WT animals' hearts and had increased c-Rel expression.28 The above studies largely show a protective role of p50 in the myocardium that may be associated with an inhibitory effect on gene transcription caused by a lack of a transactivation domain.
Role of the NF-κB p65 Subunit
In a heart failure model of coronary ligation in mice, p65 and p50 subunits of NF-κB were translocated to the nucleus for up to 24 hours of infarction. However, only p65 was chronically activated in the myocardium as determined by DNA binding and electrophoresis mobility shift assay (EMSA).13 Transgenic mice overexpressing phosphorylation-resistant IκB-α mutant showed improved survival, cardiac remodeling, and function and reduced inflammation and apoptosis.13 These studies suggested that persistent activation of p65 contributed to cardiac remodeling in myocardial infarction. In vitro studies using adult rat cardiomyocytes showed increased p65 nuclear translocation following cyclical stretch for 24 hours that resulted in vascular endothelial growth factor secretion.29 Phenylephrine activation of NF-κB in H9c2 cells was caused by increased phosphorylation of the p65 subunit and was associated with atrial natriuretic factor promoter activity, suggesting a role of NF-κB in phenylephrine-induced hypertrophy.14 Similar phosphorylation of p65 and its involvement in the development of cardiac hypertrophy were demonstrated in SHRs.14 Recently, a direct physical interaction between p65 NF-κB and the nuclear factor of activated T cells (NFAT) transcription factor was demonstrated in cardiomyocytes, resulting in synergistic activation of NFAT transcriptional activity.30 Further, NFAT activity was reduced in the hearts of cardiac-specific p65 knockout mice that also showed attenuated pressure overload–induced cardiac hypertrophy.31 The calcineurin-NFAT pathway is known as a regulator of pathological cardiac remodeling, suggesting that activation of p65 has an adverse role in cardiac pathophysiology.31 Similarly, expression of G protein–coupled receptor kinase 5 (GRK5), which is increased in heart failure patients, is regulated by the binding of p65.32 Inhibition of NF-κB by the regulator of G-protein signaling homology domain of GRK5 reduced cardiac mass in SHRs and prevented phenylephrine-induced cardiac hypertrophy in Wistar Kyoto rats.14 Recently, transgenic mice with cardiac-specific overexpression of phosphorylation-deficient triple mutant IκB-α showed attenuated nuclear translocation of p65 in response to various stimuli.33 We demonstrated that these mice are protected from right ventricular hypertrophy resulting from monocrotaline-induced pulmonary hypertension.34 Similarly, transgenic mice overexpressing myotrophin in the heart developed cardiac hypertrophy and heart failure, associated with increased NF-κB activity as determined by p65 DNA binding.6 Lentivirus-mediated delivery of p65 short hairpin RNA in the hearts of these animals attenuated NF-κB activity and regressed cardiac hypertrophy. In an inducible transgenic mouse model, with cardiomyocyte-specific expression of constitutively active IKK-β, inflammatory dilated cardiomyopathy and heart failure were observed.35 Significantly, the disease could be reversed by inactivating the transgene or in vivo expression of the IκB-α superrepressor, suggesting that IKK-activated NF-κB in cardiomyocytes was sufficient to cause cardiomyopathy and heart failure.35 Overexpression of p65 or IKK-β–enhanced p22(phox) gene promoter activity and NF-κB decoy oligodeoxynucleotides significantly downregulated messenger RNA and protein expression of p22(phox).21 NF-κB inhibitors reduced the NADPH-dependent superoxide production, suggesting that the regulation of NADPH oxidase by NF-κB is the likely mechanism whereby proinflammatory factors induce oxidative stress in the heart.21 These studies show a pathological role of p65 activation in the heart.
Role of Other NF-κB Subunits
To understand the role of the c-Rel subunit in cardiac diseases, immunohistochemical studies were performed in control and diseased human hearts from patients with end-stage ischemic or idiopathic dilated cardiomyopathy.28 c-Rel was primarily localized to cardiomyocyte nuclei in diseased tissue compared to low levels and cytoplasmic presence in controls, suggesting a pathological role of this subunit. Studies in animal models showed that the hearts of c-Rel knockout mice were significantly smaller than those in WT animals. Further, AngII-induced hypertrophy and fibrosis were significantly attenuated in c-Rel–deficient mice, suggesting that c-Rel is involved in normal and pathological heart growth.28 Cardiac-specific NEMO knockout mice developed progressive eccentric cardiac hypertrophy with extensive cardiac fibrosis.12 TNF-α–induced NF-κB activation was largely absent in these animals; however, increased expression of TNF-α and IL-6 was observed in NEMO knockout hearts. Increased expression of TNF-α and IL-6 was accompanied by oxidative stress and reduced expression of antioxidant genes SOD1 and SOD2. This study suggested a protective role of NEMO-mediated NF-κB signaling in the heart.12
DIABETIC CARDIOMYOPATHY, INFLAMMATION, THE RAS, AND NF-κB
Increased Cytokine Production in a Diabetic Heart
Given that diabetes represents a condition of chronic inflammation, the role of NF-κB signaling in diabetic cardiomyopathy is important. In db/db mice, no increase in cardiac cytokines was observed at 11 weeks of age (after about 4 weeks of diabetes); however, plasma levels of TNF-α were significantly elevated.36 A similar increase in plasma TNF-α and IL-6 were observed after 20 weeks of diabetes in db/db mice.37 In these animals, cardiac NF-κB p65 activity and p50 gene expression were significantly elevated. A high-fat diet fed to mice for 6 weeks increased their plasma and cardiac IL-6 and plasma TNF-α level in association with reduced glucose uptake and metabolism in the heart.38 Acute (4 hours) IL-6 infusion to mice in the same study reproduced a similar phenotype. Furthermore, diet-induced inflammation and defective cardiac glucose metabolism were prevented in IL-6 knockout mice.38 Hyperglycemia exacerbates endotoxin-induced inflammation and advanced glycation endproduct (AGE)–induced oxidative stress.36,39 In streptozotocin-induced diabetes models, increased cardiac levels of TNF-α, IL-6, and IL-1β were observed after 8 weeks of diabetes in mice; increased cardiac levels of TNF-α and IL-1β, but not interferon-gamma, were observed in rats after 6 weeks.40,41 Treatment with a p38 MAPK (MAP kinase) inhibitor reduced cardiac inflammation and improved systolic dysfunction but did not improve diastolic function in the diabetic mice, suggesting that factors other than cytokines may contribute to cardiac stiffness. However, an ARB attenuated both systolic and diastolic dysfunction by decreasing inflammation and normalizing matrix metalloproteinase activity in the heart.42 Pralnacasan, an interleukin-converting enzyme inhibitor that prevents IL-1β activation, and a low dose of atorvastatin that reduced inflammation without affecting cholesterol levels attenuated cardiac dysfunction in the rat model.41,43 Similar beneficial effects of statins in diabetic nephropathy have been reported.44 Another study using an anti–TNF-α antibody showed protection from cardiac dysfunction in diabetic rats.45 From these studies, an inverse correlation between cytokine levels and cardiac dysfunction in the diabetic heart is clear. Because cytokines activate NF-κB and NF-κB increases expression of cytokines, in the following section we review studies that link hyperglycemia, cytokines, the RAS, and NF-κB.
Activation of NF-κB by Hyperglycemia in the Heart
Increased p65 NF-κB activity was observed by EMSA in rat myocardium after 12 weeks of diabetes induced by streptozotocin.46 The accumulation of AGEs has been implicated in diabetic cardiomyopathy. In a study using glycated bovine serum albumin, increased oxidative stress was observed in neonatal rat cardiomyocytes in a time- and concentration-dependent manner and was associated with nuclear translocation of p65 NF-κB in a protein kinase C (PKC)-dependent manner.39 The exposure of neonatal rat cardiomyocytes to 25 mM glucose for 48 hours resulted in increased levels of total and phospho-NF-κB, both in the cell lysate and in the nucleus.47 This result was accompanied by increased expression of TNF-α. The effects of glucose on NF-κB and TNF-α could be inhibited by the PKC inhibitor Ro 31-8220, suggesting the involvement of the PKC/NF-κB pathway. Similarly, the exposure of H9c2 cells to 33 mM glucose caused increased IKK, IκB-α, and p65 phosphorylation and NF-κB–responsive luciferase activity.48
Activation of NF-κB by Proinflammatory Cytokines in the Heart
TNF-α was not cytotoxic and did not provoke apoptosis in normal myocytes.49 However, TNF-α caused a 2.2-fold increase in apoptosis in myocytes defective for NF-κB activation.49 In vitro studies in the human cardiac cell line AC16 and in vivo studies in cardiac-specific TNF-α transgenic mice showed that TNF-α activated NF-κB signaling, as determined by decreased levels of IκB-α, by increased nuclear levels of p65, and by EMSA.50 NF-κB activation resulted in decreased expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and an increase in glucose oxidation rate, the increased glucose oxidation rate representing a likely mechanism in inflammatory cardiomyopathy.50 In this regard, PGC-1α was reported to be decreased significantly at 2, 8, and 20 weeks of diabetes in the hearts of a streptozotocin-induced diabetic mouse model.51 TNF-α expression was increased significantly in the hearts of diabetic mice compared to controls at 2 and 20 weeks but not at 8 weeks. To compare acute versus chronic stimulation with TNF-α of NF-κB activity in the heart, mice acutely injected with TNF-α or cardiac-restricted TNF-α transgenic mice, respectively, were studied.52 Whereas both acute and chronic stimulation activated p50/p65 heterodimers, chronic TNF-α exposure additionally resulted in nuclear translocation of transcriptionally inactive p50 homodimers, suggesting that transcriptionally inactive p50 homodimers constituted an adaptive response to minimize the inflammatory consequences of chronic TNF-α stimulation.52 In vitro studies in H9c2 cells showed enhanced TNF-α expression in response to free fatty acids.51
Activation of NF-κB by AngII in the Heart
Experimental and clinical studies have demonstrated a significant role of the RAS in diabetic cardiomyopathy. The following studies provided evidence that AngII activates NF-κB in the heart, thereby suggesting NF-κB as a central pathway in diabetic cardiomyopathy. In rat neonatal ventricular myocytes, AngII activated NF-κB signaling as determined by nuclear localization and DNA binding assay of the p65 subunit.53 A specific inhibitor of PKC prevented p65 translocation to the nucleus, suggesting involvement of this pathway. Similar activation of a PKC-dependent canonical NF-κB pathway by AngII in a time- and concentration-dependent manner was reported in adult feline cardiomyocytes and isolated hearts.54 Increased expression of TNF-α accompanied NF-κB activation. In adult rat cardiomyocytes, both AngII and TNF-α induced NF-κB DNA binding activity at 30 minutes of stimulation, although TNF-α was significantly more potent than AngII.10 Using supershift assay, the study determined that AngII-induced NF-κB DNA binding activity largely consisted of p50. Interestingly, AngII did not activate NF-κB in cardiac fibroblasts.10 In another study with isolated adult rat cardiomyocytes, AngII increased NF-κB p50 expression in a dose-dependent manner when applied not only to intact cells but also to isolated nuclei.55 AngII-induced p50 expression could be partially blocked by AT1 and AT2 antagonists and more completely by the combination of both. Several studies have described intracellular or intracrine actions of AngII.56-59 Cardiomyocyte-restricted expression of the NF-κB superrepressor IκB-α delta N (ΔNMHC) in mice attenuated AngII-induced cardiac hypertrophy, suggesting a requirement of NF-κB in AngII response.10 In the rat embryonic cardiomyocyte cell line H9c2, AngII induced NADPH oxidase-activated p50 binding to the cardiac SCN5A sodium channel promoter, resulting in decreased transcriptional activity.60 Increased NF-κB DNA binding was observed in the hearts of rats double transgenic for human renin and AGT; the binding was prevented by pyrrolidine dithiocarbamate treatment.61 Increased NF-κB activity in SHRs was prevented by captopril treatment, likely through inhibition of the RAS.24 These studies showed that AngII activates NF-κB signaling in cardiomyocytes.
CONCLUSIONS
In the heart, NF-κB signaling has been extensively studied, yet we are far from a complete understanding of the role of this pathway in different cardiac pathological states. Studies using genetic models have provided significant information regarding the role of the individual components of the NF-κB signaling machinery. However, because of the multiplicity of interactions of these components, a composite picture of the effects of the addition or deletion of individual components on the overall NF-κB mechanism is lacking. Further, most studies investigating the activation of NF-κB in the heart have examined only 1 or 2 mechanisms, providing an incomplete understanding of the pathways involved. The presentation of 1 or 2 mechanisms as representing all NF-κB activity has likely led to confusion regarding protective or detrimental effects of this pathway in the heart. The studies we reviewed demonstrate that the effects of NF-κB in the heart are mechanism specific. Additionally, NF-κB signaling is cyclical in nature and autoregulated by continuous feedback mechanisms. Therefore, the timing of the NF-κB measurement is critical, and studies determining temporal changes in NF-κB mechanisms would provide useful information. In this review, we were careful to identify the specific pathways studied in the heart and hope that doing so can help clarify the multiple roles of NF-κB in cardiac pathophysiology.
ACKNOWLEDGMENT
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010 Oct 29;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007 Jun 26;115(25):3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 3.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002 Aug 1;22(8):1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 4.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000 Sep;212(1-2):155–169. [PubMed] [Google Scholar]
- 5.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6(2):111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Young D, Maitra RK, et al. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol. 2008 Jan 18;375(3):637–649. doi: 10.1016/j.jmb.2007.10.006. Epub 2007 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Guo Y, Tan W, et al. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007 Oct 2;116(14):1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. Epub 2007 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Yin R, Ernest R, et al. Liver X receptors are negative regulators of cardiac hypertrophy via suppressing NF-kappaB signalling. Cardiovasc Res. 2009 Oct 1;84(1):119–126. doi: 10.1093/cvr/cvp180. Epub 2009 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young D, Popovic ZB, Jones WK, Gupta S. Blockade of NF-kappaB using IkappaB alpha dominant-negative mice ameliorates cardiac hypertrophy in myotrophin-overexpressed transgenic mice. J Mol Biol. 2008 Sep 5;381(3):559–568. doi: 10.1016/j.jmb.2008.05.076. Epub 2008 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freund C, Schmidt-Ullrich R, Baurand A, et al. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005 May 10;111(18):2319–2325. doi: 10.1161/01.CIR.0000164237.58200.5A. Epub 2005 May 3. [DOI] [PubMed] [Google Scholar]
- 11.Zelarayan L, Renger A, Noack C, et al. NF-kappaB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res. 2009 Dec 1;84(3):416–424. doi: 10.1093/cvr/cvp237. Epub 2009 Jul 20. [DOI] [PubMed] [Google Scholar]
- 12.Kratsios P, Huth M, Temmerman L, et al. Antioxidant amelioration of dilated cardiomyopathy caused by conditional deletion of NEMO/IKKgamma in cardiomyocytes. Circ Res. 2010 Jan 8;106(1):133–144. doi: 10.1161/CIRCRESAHA.109.202200. Epub 2009 Oct 22. [DOI] [PubMed] [Google Scholar]
- 13.Hamid T, Guo SZ, Kingery JR, et al. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011 Jan 1;89(1):129–138. doi: 10.1093/cvr/cvq274. Epub 2010 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorriento D, Santulli G, Fusco A, et al. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-kappaB-dependent hypertrophic gene expression. Hypertension. 2010 Oct;56(4):696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. Epub 2010 Jul 26. [DOI] [PubMed] [Google Scholar]
- 15.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-α suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol. 2010 Oct;299(4):C750–C759. doi: 10.1152/ajpcell.00078.2010. Epub 2010 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR. RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs. Circ Res. 2006 Sep 29;99(7):723–730. doi: 10.1161/01.RES.0000244015.10655.3f. Epub 2006 Sep 7. [DOI] [PubMed] [Google Scholar]
- 17.Ea CK, Baltimore D. Regulation of NF-kappaB activity through lysine monomethylation of p65. Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):18972–18977. doi: 10.1073/pnas.0910439106. Epub 2009 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Reddy MA, Miao F, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008 Sep 26;283(39):26771–26781. doi: 10.1074/jbc.M802800200. Epub 2008 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LX, Zhao Y, Cheng G, et al. Targeted deletion of NF-kappaB p50 diminishes the cardioprotection of histone deacetylase inhibition. Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2154–H2163. doi: 10.1152/ajpheart.01015.2009. Epub 2010 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birbach A, Gold P, Binder BR, et al. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem. 2002 Mar 29;277(13):10842–10851. doi: 10.1074/jbc.M112475200. Epub 2002 Jan 18. [DOI] [PubMed] [Google Scholar]
- 21.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem. 2007 Oct-Dec;113(4-5):163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 22.Hingtgen SD, Li Z, Kutschke W, Tian X, Sharma RV, Davisson RL. Superoxide scavenging and Akt inhibition in myocardium ameliorate pressure overload-induced NF-κB activation and cardiac hypertrophy. Physiol Genomics. 2010 Apr 1;41(2):127–136. doi: 10.1152/physiolgenomics.00202.2009. Epub 2010 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seymour EM, Bennink MR, Watts SW, Bolling SF. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappaB activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010 May;55(5):1179–1185. doi: 10.1161/HYPERTENSIONAHA.109.149393. Epub 2010 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vázquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm (Lond) 2010 May 12;7:21. doi: 10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmers L, van Keulen JK, Hoefer IE, et al. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res. 2009 Mar 13;104(5):699–706. doi: 10.1161/CIRCRESAHA.108.189746. Epub 2009 Jan 24. [DOI] [PubMed] [Google Scholar]
- 26.Frantz S, Hu K, Bayer B, et al. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J. 2006 Sep;20(11):1918–1920. doi: 10.1096/fj.05-5133fje. Epub 2006 Jul 12. [DOI] [PubMed] [Google Scholar]
- 27.Santos DG, Resende MF, Mill JG, et al. Nuclear Factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med Genet. 2010 Jun 9;11:89. doi: 10.1186/1471-2350-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaspar-Pereira S, Fullard N, Townsend PA, et al. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am J Pathol. 2012 Mar;180(3):929–939. doi: 10.1016/j.ajpath.2011.11.007. Epub 2011 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029055. e29055. Epub 2011 Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Chen Y, Auger-Messier M, Molkentin JD. Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res. 2012 Apr 13;110(8):1077–1086. doi: 10.1161/CIRCRESAHA.111.260729. Epub 2012 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkins BJ, Dai YS, Bueno OF, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004 Jan 9;94(1):110–118. doi: 10.1161/01.RES.0000109415.17511.18. Epub 2003 Dec 1. [DOI] [PubMed] [Google Scholar]
- 32.Islam KN, Koch WJ. Involvement of nuclear factor κB (NF-κB) signaling pathway in regulation of cardiac G protein-coupled receptor kinase 5 (GRK5) expression. J Biol Chem. 2012 Apr 13;287(16):12771–12778. doi: 10.1074/jbc.M111.324566. Epub 2012 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown M, McGuinness M, Wright T, et al. Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol. 2005 Jul;289(1):H466–H476. doi: 10.1152/ajpheart.00170.2004. Epub 2005 Feb 4. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Wei C, Thomas CM, et al. Cardiac-specific genetic inhibition of nuclear factor-κB prevents right ventricular hypertrophy induced by monocrotaline. Am J Physiol Heart Circ Physiol. 2012 Apr 15;302(8):H1655–H1666. doi: 10.1152/ajpheart.00756.2011. Epub 2012 Jan 13. [DOI] [PubMed] [Google Scholar]
- 35.Maier HJ, Schips TG, Wietelmann A, et al. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2012 Jul 17;109(29):11794–11799. doi: 10.1073/pnas.1116584109. Epub 2012 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Raptis M, Black E, Stan M, Amar S, Graves DT. Influence of diabetes on the exacerbation of an inflammatory response in cardiovascular tissue. Endocrinology. 2004 Nov;145(11):4934–4939. doi: 10.1210/en.2004-0737. Epub 2004 Jul 29. [DOI] [PubMed] [Google Scholar]
- 37.Mariappan N, Elks CM, Sriramula S, et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010 Feb 1;85(3):473–483. doi: 10.1093/cvr/cvp305. Epub 2009 Sep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009 Nov;58(11):2536–2546. doi: 10.2337/db08-1361. Epub 2009 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Kho AL, Anilkumar N, et al. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006 Mar 7;113(9):1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. Epub 2006 Feb 27. [DOI] [PubMed] [Google Scholar]
- 40.Westermann D, Rutschow S, Van Linthout S, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006 Oct;49(10):2507–2513. doi: 10.1007/s00125-006-0385-2. Epub 2006 Aug 26. [DOI] [PubMed] [Google Scholar]
- 41.Westermann D, Van Linthout S, Dhayat S, et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes. 2007 Jul;56(7):1834–1841. doi: 10.2337/db06-1662. Epub 2007 May 1. [DOI] [PubMed] [Google Scholar]
- 42.Westermann D, Rutschow S, Jäger S, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007 Mar;56(3):641–646. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- 43.Van Linthout S, Riad A, Dhayat N, et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007 Sep;50(9):1977–1986. doi: 10.1007/s00125-007-0719-8. Epub 2007 Jun 23. [DOI] [PubMed] [Google Scholar]
- 44.Giunti S, Calkin AC, Forbes JM, et al. The pleiotropic actions of rosuvastatin confer renal benefits in the diabetic Apo-E knockout mouse. Am J Physiol Renal Physiol. 2010 Sep;299(3):F528–F535. doi: 10.1152/ajprenal.00127.2010. Epub 2010 Jun 16. [DOI] [PubMed] [Google Scholar]
- 45.Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007 Nov;102(6):500–507. doi: 10.1007/s00395-007-0673-0. Epub 2007 Oct 5. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Khan ZA, Cukiernik M, Chakrabarti S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am J Physiol Endocrinol Metab. 2003 Jun;284(6):E1089–E1097. doi: 10.1152/ajpendo.00540.2002. Epub 2003 Feb 11. [DOI] [PubMed] [Google Scholar]
- 47.Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG. The signal transduction pathway of PKC/NF-kappa B/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol. 2009 Feb 11;8:8. doi: 10.1186/1475-2840-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai KH, Wang WJ, Lin CW, et al. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. J Cell Physiol. 2012 Apr;227(4):1347–1357. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 49.Mustapha S, Kirshner A, De Moissac D, Kirshenbaum LA. A direct requirement of nuclear factor-kappa B for suppression of apoptosis in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H939–H945. doi: 10.1152/ajpheart.2000.279.3.H939. [DOI] [PubMed] [Google Scholar]
- 50.Palomer X, Alvarez-Guardia D, Rodríguez-Calvo R, et al. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res. 2009 Mar 1;81(4):703–712. doi: 10.1093/cvr/cvn327. Epub 2008 Nov 27. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Feng W, Xue W, et al. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009 Jun;58(6):1391–1402. doi: 10.2337/db08-1697. Epub 2009 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haudek SB, Bryant DD, Giroir BP. Differential regulation of myocardial NF kappa B following acute or chronic TNF-alpha exposure. J Mol Cell Cardiol. 2001 Jun;33(6):1263–1271. doi: 10.1006/jmcc.2001.1388. [DOI] [PubMed] [Google Scholar]
- 53.Rouet-Benzineb P, Gontero B, Dreyfus P, Lafuma C. Angiotensin II induces nuclear factor- kappa B activation in cultured neonatal rat cardiomyocytes through protein kinase C signaling pathway. J Mol Cell Cardiol. 2000 Oct;32(10):1767–1778. doi: 10.1006/jmcc.2000.1211. [DOI] [PubMed] [Google Scholar]
- 54.Kalra D, Sivasubramanian N, Mann DL. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation. 2002 May 7;105(18):2198–2205. doi: 10.1161/01.cir.0000015603.84788.47. [DOI] [PubMed] [Google Scholar]
- 55.Tadevosyan A, Maguy A, Villeneuve LR, et al. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010 Jul 16;285(29):22338–22349. doi: 10.1074/jbc.M110.121749. Epub 2010 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond) 2012 Sep;123(5):273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook JL, Re RN. Lessons from in vitro studies and a related intracellular angiotensin II transgenic mouse model. Am J Physiol Regul Integr Comp Physiol. 2012 Mar 1;302(5):R482–R493. doi: 10.1152/ajpregu.00493.2011. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012 Mar 1;302(5):R518–R530. doi: 10.1152/ajpregu.00525.2011. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol. 2012 Mar 1;302(5):R494–R509. doi: 10.1152/ajpregu.00487.2011. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shang LL, Sanyal S, Pfahnl AE, et al. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008 Jan;294(1):C372–C379. doi: 10.1152/ajpcell.00186.2007. Epub 2007 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller DN, Dechend R, Mervaala EM, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000 Jan;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]