Abstract
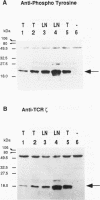
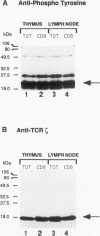
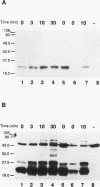
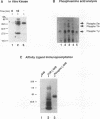
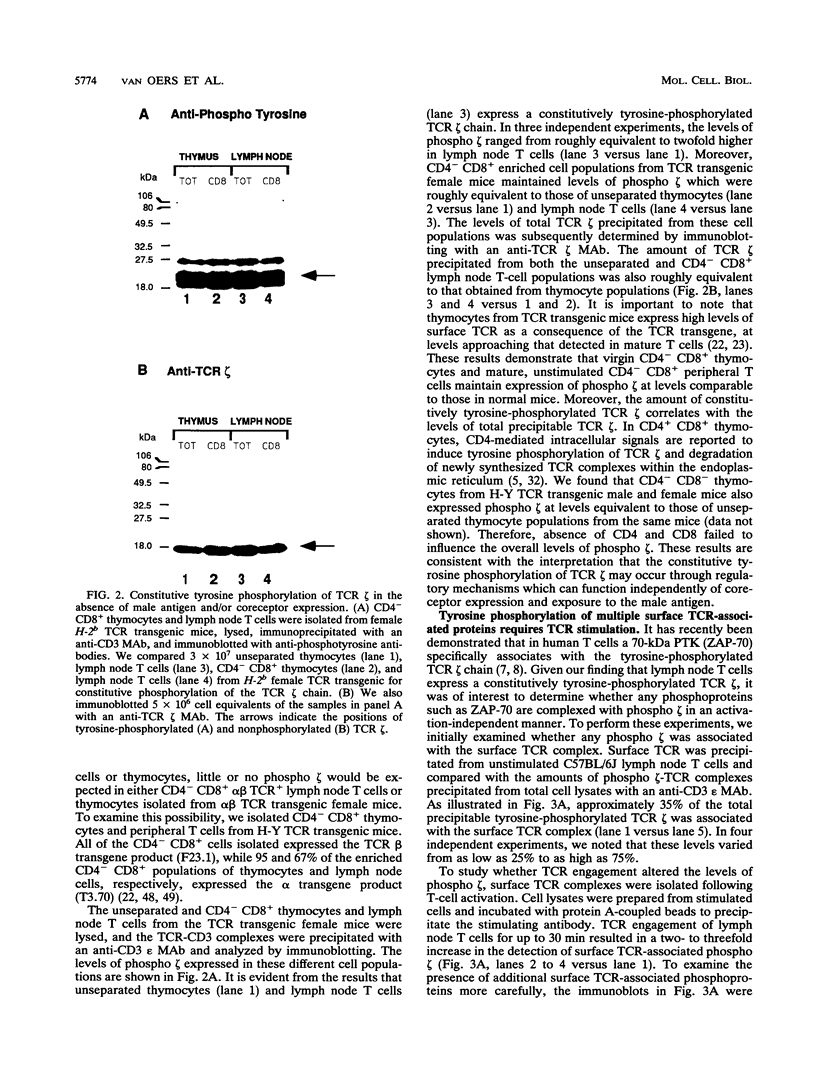
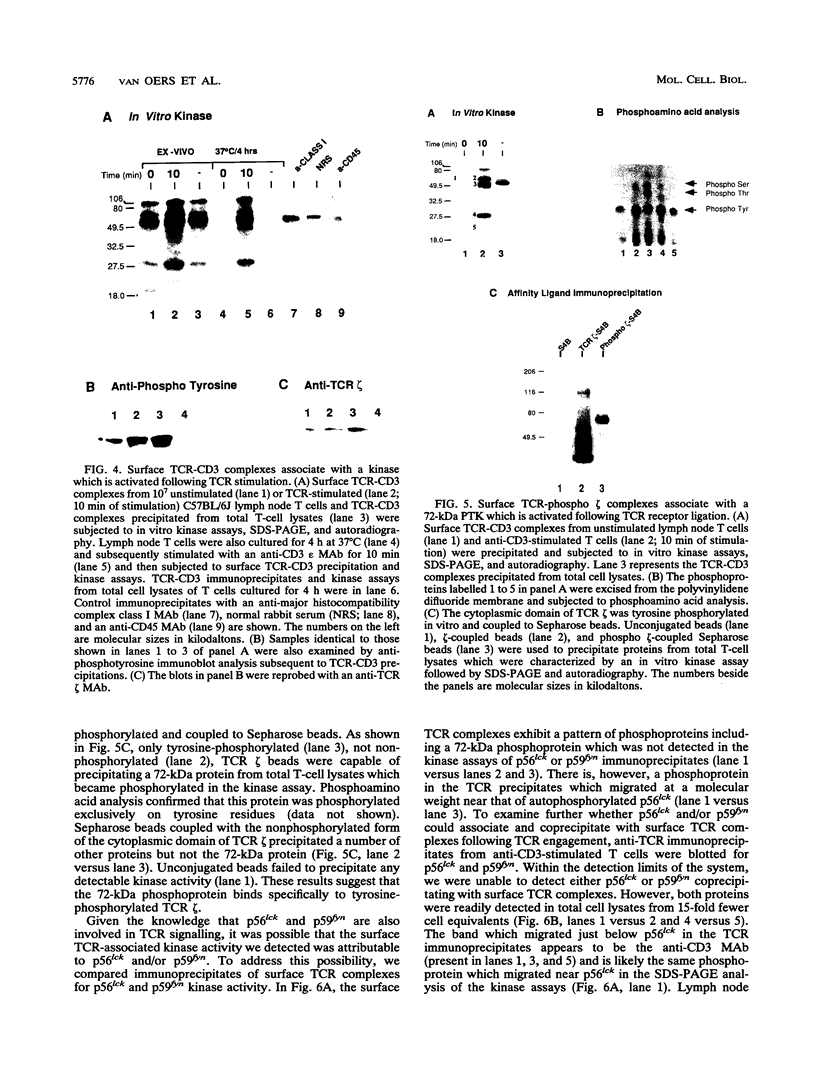
The T-cell receptor (TCR) zeta subunit is an important component of the TCR complex, involved in signal transduction events following TCR engagement. In this study, we showed that the TCR zeta chain is constitutively tyrosine phosphorylated to similar extents in thymocytes and lymph node T cells. Approximately 35% of the tyrosine-phosphorylated TCR zeta (phospho zeta) precipitated from total cell lysates appeared to be surface associated. Furthermore, constitutive phosphorylation of TCR zeta in T cells occurred independently of antigen stimulation and did not require CD4 or CD8 coreceptor expression. In lymph node T cells that constitutively express tyrosine-phosphorylated TCR zeta, there was a direct correlation between surface TCR-associated protein tyrosine kinase (PTK) activity and expression of phospho zeta. TCR stimulation of these cells resulted in an increase in PTK activity that coprecipitated with the surface TCR complex and a corresponding increase in the levels of phospho zeta. TCR ligations also contributed to the detection of several additional phosphoproteins that coprecipitated with surface TCR complexes, including a 72-kDa tyrosine-phosphorylated protein. The presence of TCR-associated PTK activity also correlated with the binding of a 72-kDa protein, which became tyrosine phosphorylated in vitro kinase assays, to tyrosine phosphorylated TCR zeta. The cytoplasmic region of the TCR zeta chain was synthesized, tyrosine phosphorylated, and conjugated to Sepharose beads. Only tyrosine-phosphorylated, not nonphosphorylated, TCR zeta beads were capable of immunoprecipitating the 72-kDa protein from total cell lysates. This 72-kDa protein is likely the murine equivalent of human PTK ZAP-70, which has been shown to associate specifically with phospho zeta. These results suggest that TCR-associated PTK activity is regulated, at least in part, by the tyrosine phosphorylation status of TCR zeta.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Miceli M. C., Parnes J. R., Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991 Mar 7;350(6313):62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Alexander-Miller M. A., Burke K., Koszinowski U. H., Hansen T. H., Connolly J. M. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993 Jul 1;151(1):1–10. [PubMed] [Google Scholar]
- Baniyash M., Hsu V. W., Seldin M. F., Klausner R. D. The isolation and characterization of the murine T cell antigen receptor zeta chain gene. J Biol Chem. 1989 Aug 5;264(22):13252–13257. [PubMed] [Google Scholar]
- Behlke M. A., Henkel T. J., Anderson S. J., Lan N. C., Hood L., Braciale V. L., Braciale T. J., Loh D. Y. Expression of a murine polyclonal T cell receptor marker correlates with the use of specific members of the V beta 8 gene segment subfamily. J Exp Med. 1987 Jan 1;165(1):257–262. doi: 10.1084/jem.165.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., McCarthy S. A., Maguire J. E., Nakayama T., Singer D. S., Klausner R. D., Singer A. Novel post-translational regulation of TCR expression in CD4+CD8+ thymocytes influenced by CD4. Nature. 1990 Mar 15;344(6263):247–251. doi: 10.1038/344247a0. [DOI] [PubMed] [Google Scholar]
- Carlow D. A., van Oers N. S., Teh S. J., Teh H. S. Deletion of antigen-specific immature thymocytes by dendritic cells requires LFA-1/ICAM interactions. J Immunol. 1992 Mar 15;148(6):1595–1603. [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., D'Adamio L., Howard F. D., Sieh M., Hussey R. E., Koyasu S., Reinherz E. L. CD3 eta and CD3 zeta are alternatively spliced products of a common genetic locus and are transcriptionally and/or post-transcriptionally regulated during T-cell development. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5202–5206. doi: 10.1073/pnas.88.12.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Davidson D., Chow L. M., Fournel M., Veillette A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J Exp Med. 1992 Jun 1;175(6):1483–1492. doi: 10.1084/jem.175.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. H., McDuffie M., Kappler J. W., Marrack P., Cambier J. C. Both immature and mature T cells mobilize Ca2+ in response to antigen receptor crosslinking. Nature. 1987 Nov 12;330(6144):179–181. doi: 10.1038/330179a0. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Huesmann M., Scott B., Kisielow P., von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991 Aug 9;66(3):533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Irving B. A., Chan A. C., Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med. 1993 Apr 1;177(4):1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Karnitz L., Sutor S. L., Torigoe T., Reed J. C., Bell M. P., McKean D. J., Leibson P. J., Abraham R. T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992 Oct;12(10):4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse K. P., Wiest D. L., Singer A. Subcellular localization of T-cell receptor complexes containing tyrosine-phosphorylated zeta proteins in immature CD4+CD8+ thymocytes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2438–2442. doi: 10.1073/pnas.90.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Teh H. S., Blüthmann H., von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988 Oct 20;335(6192):730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Lippincott-Schwartz J., Bonifacino J. S. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Koyasu S., McConkey D. J., Clayton L. K., Abraham S., Yandava B., Katagiri T., Moingeon P., Yamamoto T., Reinherz E. L. Phosphorylation of multiple CD3 zeta tyrosine residues leads to formation of pp21 in vitro and in vivo. Structural changes upon T cell receptor stimulation. J Biol Chem. 1992 Feb 15;267(5):3375–3381. [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992 Jan 3;255(5040):79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- Nakayama T., June C. H., Munitz T. I., Sheard M., McCarthy S. A., Sharrow S. O., Samelson L. E., Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+ cells by CD4. Science. 1990 Sep 28;249(4976):1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Samelson L. E., Nakayama Y., Munitz T. I., Sheard M., June C. H., Singer A. Ligand-stimulated signaling events in immature CD4+CD8+ thymocytes expressing competent T-cell receptor complexes. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9949–9953. doi: 10.1073/pnas.88.22.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Singer A., Hsi E. D., Samelson L. E. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989 Oct 19;341(6243):651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Ueda Y., Yamada H., Shores E. W., Singer A., June C. H. In vivo calcium elevations in thymocytes with T cell receptors that are specific for self ligands. Science. 1992 Jul 3;257(5066):96–99. doi: 10.1126/science.1621102. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Frank S. J., Robey F. A., Weissman A. M., Klausner R. D. Biochemical characterization of the eta chain of the T-cell receptor. A unique subunit related to zeta. J Biol Chem. 1989 Sep 5;264(25):14812–14817. [PubMed] [Google Scholar]
- Park D. J., Rho H. W., Rhee S. G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D., Griswold-Prenner I., Rosner M. R., Fitch F. W. Multiple components of the T cell antigen receptor complex become tyrosine-phosphorylated upon activation. J Biol Chem. 1993 Feb 25;268(6):4488–4493. [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Romeo C., Amiot M., Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992 Mar 6;68(5):889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Davidson W. F., Morse H. C., 3rd, Klausner R. D. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature. 1986 Dec 18;324(6098):674–676. doi: 10.1038/324674a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrist J. P., Karnitz L., Abraham R. T. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem. 1991 Jul 5;266(19):12135–12139. [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Kishi H., Scott B., Borgulya P., von Boehmer H., Kisielow P. Early deletion and late positive selection of T cells expressing a male-specific receptor in T-cell receptor transgenic mice. Dev Immunol. 1990;1(1):1–10. doi: 10.1155/1990/18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kishi H., Scott B., Von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989 Mar 1;169(3):795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Zúiga-Pflücker J. C., Bolen J. B., Kruisbeek A. M. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J Exp Med. 1989 Nov 1;170(5):1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Morin P., Tian Q. S., Daley J., Blue M. L., Schlossman S. F., Anderson P. Expression and tyrosine phosphorylation of the T cell receptor zeta-subunit in human thymocytes. J Immunol. 1991 Feb 15;146(4):1142–1148. [PubMed] [Google Scholar]
- Wange R. L., Kong A. N., Samelson L. E. A tyrosine-phosphorylated 70-kDa protein binds a photoaffinity analogue of ATP and associates with both the zeta chain and CD3 components of the activated T cell antigen receptor. J Biol Chem. 1992 Jun 15;267(17):11685–11688. [PubMed] [Google Scholar]
- Watts J. D., Wilson G. M., Ettenhadieh E., Clark-Lewis I., Kubanek C. A., Astell C. R., Marth J. D., Aebersold R. Purification and initial characterization of the lymphocyte-specific protein-tyrosyl kinase p56lck from a baculovirus expression system. J Biol Chem. 1992 Jan 15;267(2):901–907. [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Weiss A., Koretzky G., Schatzman R. C., Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Kirberg J., Rocha B. An unusual lineage of alpha/beta T cells that contains autoreactive cells. J Exp Med. 1991 Nov 1;174(5):1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]