ABSTRACT
Background
The expression of transforming growth factor beta (TGF-β) and Smad3 regulates extracellular matrix homeostasis and inflammation in aortic aneurysms. The expression of Smad3 depends on signaling by angiotensin II (AngII) receptor pathways through TGF-β receptor–dependent and –independent pathways.
Methods
To determine the expression of AngII type 1 (AT1R) and type 2 receptors (AT2R), TGF-β, and Smad3 in thoracic aortic aneurysms, we performed immunohistochemistry testing on tissue and cultured cells derived from subjects with Marfan syndrome (MFS) and bicuspid aortic valve (BAV) malformation and from normal aortas of subjects who were organ donors.
Results
MFS and BAV aneurysm tissue showed enhanced accumulation of TGF-β and Smad3 in vascular smooth muscle cells (VSMCs) and in inflammatory cells in the subintimal layer and tunica media. The normal aortic wall exhibited minimal TGF-β and Smad3 staining. Cultured VSMCs from MFS and BAV samples showed nuclear Smad3 and strong cytoplasmic TGF-β expression in the cytoplasmic vesicles. In control cells, Smad3 was located mainly in the cytoplasm, and weak cytoplasmic TGF-β was distributed with a pattern similar to that of the aneurysm-derived cells. Compared to normal aorta cells, AT1R and AT2R expression was increased in both aneurysm types. Treatment of cultured VSMCs with the AT1R antagonist losartan caused both reduced TGF-β vesicle localization and nuclear expression of Smad3.
Conclusions
Increased TGF-β and Smad3 expression in aneurysm tissue and cultured VSMCs is consistent with aberrant TGF-β expression and the activation of Smad3 signaling. Losartan-mediated reduction in TGF-β expression and the cytoplasmic localization of Smad3 support a role for AT1R antagonism in the inhibition of aneurysm progression.
Keywords: Aneurysm, aorta, immunohistochemistry, Smad3 protein, transforming growth factor beta, vascular smooth muscle
INTRODUCTION
Aortic aneurysm is characterized by extracellular matrix breakdown and vascular smooth muscle cell (VSMC) apoptosis with varying degrees of vascular repair and inflammatory cell infiltration. Environmental, genetic, and hemodynamic factors all contribute to the complex pathophysiology of aortic aneurysm disease.1 Recent interest in the cytokine transforming growth factor beta (TGF-β) as a possible pathogenetic factor in aneurysm disease has followed from studies of the role of TGF-β in the extracellular regulation of fibrillin and in the development of the mouse model of Marfan syndrome (MFS).2,3
TGF-β is a family of multifunctional growth factors that influences proliferation, apoptosis, cell cycle arrest, differentiation, and matrix secretion.4 Three isoforms of TGF-β (TGF-β 1-3) are expressed in human subjects. Alteration in the level of TGF-β activity is associated with various connective tissue diseases. The loss of organization of microfibrils from defective fibrillin-1 associated with mutations in the FBN1 gene—regardless of the nature of the mutation—markedly changes the targeting and sequestration of latent TGF-β. Altered extracellular TGF-β availability may have significant effects on connective tissue homeostasis and on the activation of signaling pathways downstream of TGF-β receptors.
Smad proteins mediate the intracellular signaling of TGF-β.5 The binding of TGF-β to its receptors activates Smad signaling pathways that regulate matrix-associated protein expression.6 The phosphorylation of Smad2 and Smad3 results in the formation of heterooligomeric complexes with Smad4. The complexes translocate to the nucleus where transcription of target genes, including the Smad7 gene, is regulated. Smad7 is an inhibitory enzyme that associates with the activated TGF-β type I receptor and inhibits the activation of Smad2 and Smad3 by competing with receptor interaction.7
The effects of TGF-β signaling are highly sensitive to the level of Smad gene expression. A lack of Smad3 is associated with reduced matrix deposition but enhanced neointimal hyperplasia in response to vascular injury in Smad3-null mice, suggesting a role in cell proliferation and extracellular matrix secretion.8 Exogenous TGF-β administration results in the phosphorylation and nuclear translocation of Smad3.9 Whether altered TGF-β signaling in aneurysm disease is associated with the abnormal regulation of Smad expression remains unclear.
In a mouse model of MFS, aortic aneurysms were associated with increased TGF-β signaling. TGF-β antagonists—including the TGF-β-neutralizing antibody and the AT1R blocker losartan—prevented the development of aneurysms. AT1R blockade also partially reversed noncardiovascular manifestations of MFS, such as impaired alveolar septation and muscle regeneration.3,10 The mechanism by which AT1R antagonism influences TGF-β signaling is still unknown. The essential role of Smad3 in angiotensin II (AngII)-induced vascular fibrosis and atherosclerosis development supports the importance of interactions between TGF-β signaling, the Smad proteins, and AngII receptor activation.11
We provide evidence for altered TGF-β/Smad3 signaling in human thoracic aortic aneurysms associated with MFS and with bicuspid aortic valve (BAV) malformation, and we examine the effects of AT1R blocking using losartan in aneurysm-derived VSMCs.
METHODS
Tissue Collection
Normal thoracic aortic tissue was collected from organ donors—3 males, 2 females; age 40 ± 11 years (mean ± standard deviation [SD])—through the Queenslanders Donate organization at the Princess Alexandra Hospital in Brisbane, Australia. Ascending aortic aneurysm samples were collected from MFS patients (3 males, 2 females; age 46 ± 24 years) and BAV patients (3 males, 2 females; age 65 ± 13 years) undergoing elective aortic aneurysm repair surgery. Subjects with MFS satisfied the revised diagnostic criteria of De Paepe et al.12 We used the presence of BAV on ultrasound, together with enlarged ascending aortic diameter, as criteria for the diagnosis of BAV-associated aneurysm. The Institutional Ethics Committee approved the study, and subjects or relatives gave informed consent.
After collection, specimens were incubated in Dulbecco's Modified Eagle Medium (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (Invitrogen) and antibiotics (penicillin, streptomycin, and fungizone) (Invitrogen) and were processed within 24 hours.
Immunohistochemical Preparation
Aortic specimens were fixed in buffered formalin for at least 24 hours, processed, and embedded in paraffin. Expression and localization of AT1R, AT2R, TGF-β 1-3, and Smad3 were analyzed using immunohistochemistry on 4-μm-thick cross sections of aorta. Primary antibodies used were AT1R and AT2R (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), TGF-β 1-3 (1:75; Genzyme, Cambridge, MA), and Smad3 (1:50; Cell Signaling Technology, Beverly, MA). Tissue was incubated overnight in a humidified chamber at 4°C with a primary antibody. A secondary antibody (1:100) and ABC solution (1:100; Vectastain Universal Elite ABC Kit, Vector Laboratories, Burlingame, CA) were applied for 1 hour followed by 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO) for 5-10 minutes. Sections were counterstained with hematoxylin, dehydrated in ethanol and xylene, and mounted permanently in DePeX (BDH Chemicals, Kilsyth, Victoria, Australia). Positive staining was identified as areas of dark brown color. Negative controls were processed in the same manner but without the primary antibody.
VSMC Culture
To establish cultured VSMCs, the tunica adventitia was separated from the tunica media under aseptic conditions in the cell culture hood following methods published previously.13 VSMCs from passages 1 to 6 were used in this study, and cells were plated on 13-mm round coverslips. Prior to the experiments, VSMCs were incubated in serum-free media for 24 hours. Losartan treatment (10 μM) was applied for 48 hours, and the media was changed every 24 hours. VSMCs were processed for immunohistochemical study as described in the previous paragraph for tissue samples.
Quantitative Analysis
The expression of AT1R, AT2R, TGF-β, and Smad3 was examined in normal aorta controls, in MFS and BAV aortic wall samples, and in cultured VSMCs. An image analysis system (AxioVision version 4.5, Carl Zeiss Microscopy, Jena, Germany) was used to quantitate the degree of specific staining for each antibody. Twenty alternate fields on each section were assessed. Expression was assessed as the percentage area of positive staining averaged over 20 fields at a magnification of 250×. In cultured VSMCs, the expression of AT1R was measured using a semiquantitative analysis scoring system (0=absent, 1=mild, 2=moderate, and 3=strong). The percentage of VSMC-positive TGF-β staining was calculated from 10 fields at a magnification of 125×. Similarly, the percentage of VSMCs showing staining for nuclear Smad3 was determined. Nuclear Smad3 localization was compared between losartan-treated and untreated aneurysm subjects (combined MFS and BAV) and controls from 10 fields at a magnification of 125×. Scoring was carried out by one investigator (MN) using the random selection of deidentified slides.
Statistical Analysis
Data are presented as the mean ± SD. The Mann-Whitney nonparametric test was used to compare groups, and P<0.05 was considered significant.
RESULTS
Increased Expression of Smad3 and TGF-β in the Tunica Media
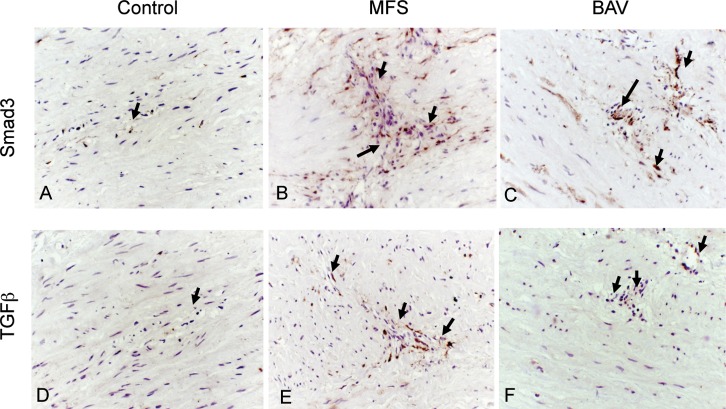
Increased expression of Smad3 and TGF-β was observed in tunica media VSMCs of MFS patients (2.1% ± 0.9% and 0.9% ± 0.6%, respectively; Figures 1B and 1E) and BAV patients (2.5% ± 1.3% and 0.9% ± 0.2%, respectively; Figures 1C and 1F) compared to control aortas (0.4 ± 0.2% and 0.1 ± 0.08%, respectively; Figures 1A and 1D; P<0.05). In the subintima and in areas of medial remodeling/degeneration where focal infiltration of chronic inflammatory cells and myofibroblasts were present, TGF-β and Smad3 expression was also enhanced (not shown).
Figure 1.
Increased accumulation of Smad3 (A-C) and transforming growth factor beta (TGF-β) (D-F) observed in the area of pathological remodeling/focal degeneration in the aortic wall of Marfan syndrome (MFS) (B, E) and bicuspid aortic valve (BAV) (C, F) aneurysms. Positive expression of Smad3 and TGF-β is identified as areas of dark brown color (arrows). The control aortic wall (A, D) showed weak to negligible expression (hematoxylin;magnification 250×).
Increased Expression of AT1R and AT2R in the Tunica Media
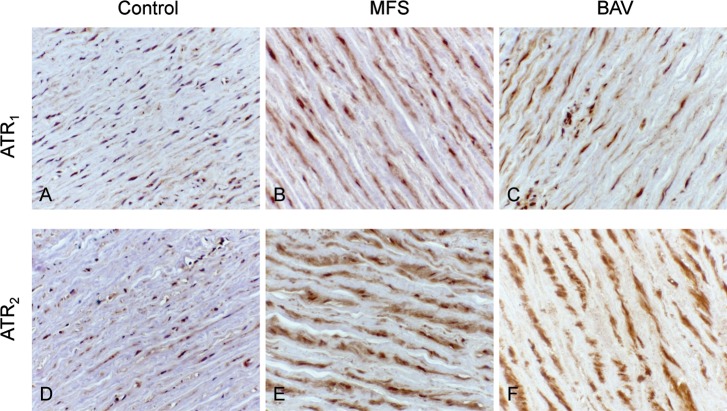
Increased expression of AT1R and AT2R was observed in the VSMCs of MFS (5.7% ± 1.7% and 8.3% ± 2.9%, respectively; Figures 2B and 2E) and BAV (5.8% ± 1.6% and 9.6% ± 2.2%, respectively; Figures 2C and 2F) aortic media compared to control aortic media (1.9% ± 0.4% and 3.3% ± 1.1%, respectively; Figures 2A and 2D; P<0.05).
Figure 2.
Increased expression (identified as dark brown color) of angiotensin II type 1 (AT1R) and type 2 receptors (AT2R) was observed in vascular smooth muscle cells of Marfan syndrome (MFS) (B, E) and bicuspid aortic valve (BAV) (C, F) aortic media compared to control aortic media (A, D) (hematoxylin;magnification 250×).
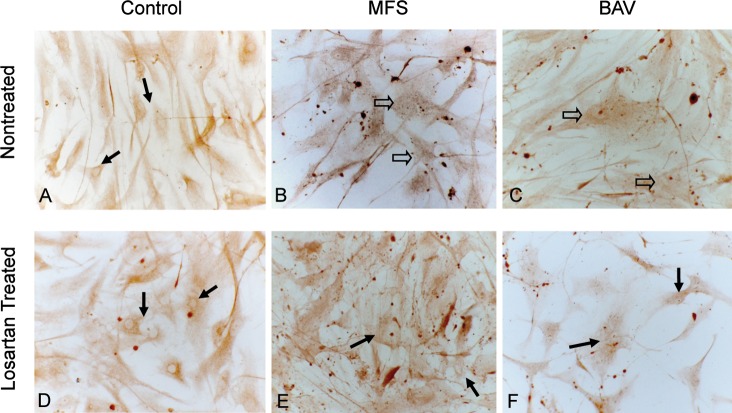
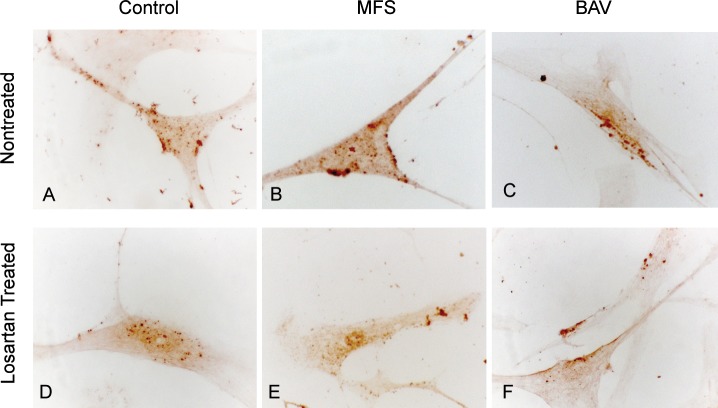
Smad3 Distribution in Cultured VSMCs
Smad3 was present in the cytoplasm of cultured VSMCs in all of the aneurysm and control groups. However, the percentage of nuclear Smad3 expression in MFS and BAV VSMCs was significantly higher compared to control (13.3% ± 3.8% vs 5.3% ± 2.7%; Figures 3A, 3B, and 3C; P<0.05). Losartan treatment resulted in decreased nuclear Smad3 expression in cultured MFS and BAV VSMCs (5.3% ± 2.7%) compared to untreated MFS and BAV VSMCs (13.3% ± 3.8%) and a similar level of nuclear Smad3 expression in losartan-treated control VSMCs (8.6% ± 4.1%, Figures 3D, 3E, and 3F) compared to untreated control VSMCs (Figure 3A).
Figure 3.
Smad3 expression is identified as dark brown areas of color in cultured aortic vascular smooth muscle cells (VSMCs). Nuclear accumulation is shown in Marfan syndrome (MFS) and bicuspid aortic valve (BAV) VSMCs (open arrows; B, C). Lack of Smad3 nuclear expression in the control (A) is indicated with arrows. Losartan treatment of MFS and BAV VSMCs reduced nuclear accumulation of nuclear Smad3 (E, F) (hematoxylin;magnification 250×).
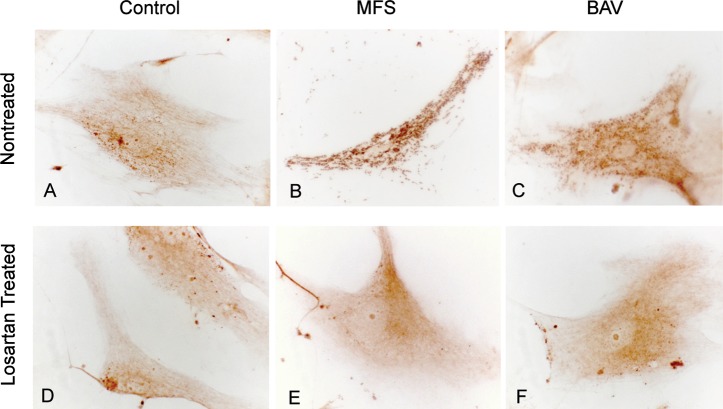
TGF-β Distribution in Cultured VSMCs
TGF-β expression distribution was mainly cytoplasmic. In MFS and BAV VSMCs, TGF-β expression was seen in numerous vesicles and areas of exocytosis, with some extracellular localization (Figures 4B and 4C). Control VSMCs had diffuse distribution of TGF-β expression within the cytoplasm (Figure 4A). Losartan treatment reduced TGF-β vesicle expression, and diffuse cytoplasmic expression was more prominent (Figures 4D, 4E, and 4F).
Figure 4.
Transforming growth factor beta (TGF-β) expression (brown color) in cultured aortic vascular smooth muscle cells (VSMCs). Increased expression of TGF-β is shown in VSMCs from Marfan syndrome (MFS) and bicuspid aortic valve (BAV) samples where vesicular localization is also increased (B, C). Losartan treatment resulted in decreased expression and diffuse localization of intracellular TGF-β (E, F) similar to control VSMCs (A, D) (hematoxylin;magnification 500×).
AT1R Expression in Cultured VSMCs
AT1R expression was increased in cultured MFS and BAV VSMCs (2.4% ± 0.3%, Figures 5B and 5C) compared to control VSMCs (1.5% ± 0.5%, Figure 5A). Losartan treatment reduced AT1R in cultured MFS and BAV VSMCs (1.4% ± 0.2%, Figures 5E and 5F) to the same level as losartan-treated control VSMCs (1.4% ± 0.4%, Figure 5D).
Figure 5.
Angiotensin II type 1 receptor (AT1R) expression (brown color) in cultured aortic vascular smooth muscle cells (VSMCs). Increased expression of AT1R in cultured Marfan syndrome (MFS) and bicuspid aortic valve (BAV) VSMCs (B, C) was found. Losartan treatment reduced AT1R in cultured MFS and BAV VSMCs (E, F) to the same level as losartan-treated control VSMCs (D) (hematoxylin;magnification 500×).
DISCUSSION
Our study revealed increased expression of Smad3 and TGF-β in VSMCs in the tunica media of subjects with MFS and BAV aneurysms compared to control subjects. We also showed enhanced accumulation of TGF-β and Smad3 in myofibroblasts, VSMCs, and chronic inflammatory cells in the subintima and tunica media. Altered expression of AT1R and AT2R has been implicated in the development of aortic aneurysms. In this study, we found significantly increased AT1R and AT2R expression in VSMCs in the tunica media of MFS and BAV aneurysms as previously reported.14 The new activation of AT1R is believed to be responsible for the pathophysiological actions of AngII, such as the regulation of cell proliferation, inflammation, and fibrosis. The role of the AT2R receptor has not been completely defined but is associated with the inhibition of cell growth and inflammatory cell recruitment, as well as the blocking of AT1R actions.15,16 The identification of increased expression of AT2R in this study may indicate a negative feedback mechanism that counteracts the effects of AT1R activation. In addition, the activation of AT2R independently attenuates tissue remodeling by exerting inhibitory effects on AngII-induced synthesis of the extracellular matrix and mitogenesis by the withdrawal of growth factors and resulting programmed cell death. The constant high expression of AT2R may be inhibitory to the repair process, leading to a slow response to injury and the progression of aortic aneurysm.
Cultured MFS and BAV VSMCs showed strong TGF-β expression in numerous intracytoplasmic and extracytoplasmic vesicles and in localized areas of the extracellular space. Nuclear localization of Smad3 was found in cultured MFS and BAV VSMCs. Treatment with the AT1R antagonist losartan resulted in a decreased concentration of TGF-β in intracellular and extracellular vesicles, as well as a reduced nuclear Smad3 concentration. Increased expression of TGF-β and Smad3 in aneurysm tissue and cultured VSMCs is consistent with aberrant TGF-β regulation and with activation of the Smad3 signaling pathway.
We found increased expression of Smad3 and TGF-β colocalized in areas of degeneration where focal VSMC apoptosis and matrix metalloproteinase (MMP) 2 activation are present.13 Previous investigators have found a relationship between increased expression of TGF-β1 and reduced VSMC density (because of VSMC apoptosis and reduced proliferative ability) in association with aortic dilatation in abdominal aortic aneurysm patients.17 Our findings suggest the involvement of Smad3 and TGF-β in pathological remodeling of the aortic wall. Losartan treatment reduced expression of TGF-β and Smad3 in VSMCs derived from MFS and BAV aneurysms, suggesting the potential value of this treatment in subjects with thoracic aneurysm disease and consistent with its effect in the mouse model of MFS.
Despite the uncertain role of increased Smad3 expression in aneurysm development, others have reported an antiproliferative role for Smad2 and a role for Smad3 in cellular apoptosis.17 Constitutive expression of Smad3 in the presence of TGF-β induced cellular apoptosis in human lung epithelial cells.18
AT1R antagonism blocks the effects of TGF-β.19 It prevents abdominal aortic aneurysm progression independently of blood pressure reduction by inhibiting proteolysis, apoptosis, and inflammation in aortic tissue.20 Thus, the activation of TGF-β determined by increased expression in the present study seems to be associated with aneurysm development in MFS and BAV subjects. The primary effect of TGF-β is profibrotic and antiinflammatory, and increased TGF-β is usually associated with an increased ratio of the concentration of tissue inhibitors of metalloproteinases (TIMPs) compared to the concentration of MMPs. This model is opposite to the apparent situation in aortic aneurysm where there is matrix degeneration and a decreased TIMP/MMP ratio. To complicate the issue further, increased TGF-β expression heals established abdominal aortic aneurysms,21 while emphysema in MFS is thought to be the result of reduced deposition of TGF-β in the extracellular space in the lung parenchyma.10 On the other hand, inhibition of TGF-β expression was associated with the prevention of aneurysm development in a mouse model of MFS.3 Whether differences in the mechanisms underlying the formation of abdominal aortic and thoracic aneurysms in MFS and BAV patients are caused by differences in the level of TGF-β is unclear. Overall, these disparate results suggest that tissue expression levels of TGF-β may not indicate aneurysm risk. Further study is needed to clarify the downstream pathway of TGF-β signaling, including the role of the Smad pathways.
The activation of AT1R can sustain its effect by interacting with Smad3 independently of its action on TGF-β.8,11 Nuclear translocation of Smad3 is associated with the transcription of target genes, including Smad7. Smad7 is an inhibitory enzyme that associates with the activated TGF-β type I receptor and interferes with the activation of Smad2 and Smad3 by competing with receptor interaction.22 Further studies are needed to clarify these effects.
CONCLUSIONS
This study showed substantial altered expression of TGF-β and Smad3 and AngII receptors in human MFS and BAV thoracic aneurysms, as well as the potential beneficial effects of the AT1R blocker losartan.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Prince Charles Hospital cardiac surgeons, the Department of Anatomical Pathology at Prince Charles Hospital, patients participating in the study, the Queenslanders Donate organization at the Princess Alexandra Hospital in Brisbane, and the families and relatives of organ donors.
Footnotes
Funding: This project was partly funded by the Prince Charles Hospital Foundation.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat Clin Pract Cardiovasc Med. 2007 Aug;4(8):418–427. doi: 10.1038/ncpcardio0937. [DOI] [PubMed] [Google Scholar]
- 2.Kaartinen V, Warburton D. Fibrillin controls TGF-beta activation. Nat Genet. 2003 Mar;33(3):331–332. doi: 10.1038/ng0303-331. [DOI] [PubMed] [Google Scholar]
- 3.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006 Apr 7;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. Epub 2006 May 4. [DOI] [PubMed] [Google Scholar]
- 5.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997 Dec 4;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 6.Van Linthout S, Seeland U, Riad A, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008 Jul;103(4):319–327. doi: 10.1007/s00395-008-0715-2. Epub 2008 Mar 17. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Yokote K, Fujimoto M, et al. Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res. 2005 Apr 29;96(8):904–912. doi: 10.1161/01.RES.0000163980.55495.44. Epub 2005 Mar 24. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Vita J, Sánchez-López E, Esteban V, Rupérez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005 May 17;111(19):2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. Epub 2005 May 9. [DOI] [PubMed] [Google Scholar]
- 9.Saika S, Miyamoto T, Ishida I, et al. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol. 2002 Dec;86(12):1428–1433. doi: 10.1136/bjo.86.12.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterner-Kock A, Thorey IS, Koli K, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002 Sep 1;16(17):2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Huang XR, Canlas E, et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006 Apr 28;98(8):1032–1039. doi: 10.1161/01.RES.0000218782.52610.dc. Epub 2006 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996 Apr 24;62(4):417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003 Sep 9;108(Suppl 1):II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 14.Nataatmadja M, West J, West M. Angiotensin II type 2 receptor expression and CD34 progenitor cell co-localization in aortic aneurysm. [American Heart Association Abstract 1563]. Circulation. 2007 116:II_325. [Google Scholar]
- 15.Ruiz-Ortega M, Rupérez M, Esteban V, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006 Jan;21(1):16–20. doi: 10.1093/ndt/gfi265. Epub 2005 Nov 9. [DOI] [PubMed] [Google Scholar]
- 16.Su JZ, Fukuda N, Jin XQ, et al. Effect of AT2 receptor on expression of AT1 and TGF-beta receptors in VSMCs from SHR. Hypertension. 2002 Dec;40(6):853–858. doi: 10.1161/01.hyp.0000042096.17141.b1. [DOI] [PubMed] [Google Scholar]
- 17.Fukui D, Miyagawa S, Soeda J, Tanaka K, Urayama H, Kawasaki S. Overexpression of transforming growth factor beta1 in smooth muscle cells of human abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2003 Jun;25(6):540–545. doi: 10.1053/ejvs.2002.1857. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa K, Osada H, Masuda A, et al. Induction of apoptosis by Smad3 and down-regulation of Smad3 expression in response to TGF-beta in human normal lung epithelial cells. Oncogene. 1998 Oct 1;17(13):1743–1747. doi: 10.1038/sj.onc.1202052. [DOI] [PubMed] [Google Scholar]
- 19.Aquilina K, Hobbs C, Tucker A, Whitelaw A, Thoresen M. Do drugs that block transforming growth factor beta reduce posthaemorrhagic ventricular dilatation in a neonatal rat model? Acta Paediatr. 2008 Sep;97(9):1181–1186. doi: 10.1111/j.1651-2227.2008.00903.x. Epub 2008 Jul 9. [DOI] [PubMed] [Google Scholar]
- 20.Kaschina E, Schrader F, Sommerfeld M, et al. Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J Hypertens. 2008 Dec;26(12):2361–2373. doi: 10.1097/HJH.0b013e328313e547. [DOI] [PubMed] [Google Scholar]
- 21.Dai J, Losy F, Guinault AM, et al. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005 Aug 16;112(7):1008–1015. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 22.Nakao A, Okumura K, Ogawa H. Smad7: a new key player in TGF-beta-associated disease. Trends Mol Med. 2002 Aug;8(8):361–363. doi: 10.1016/s1471-4914(02)02376-6. [DOI] [PubMed] [Google Scholar]