Abstract
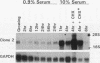
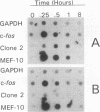
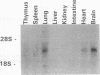
Early-growth-response genes, also known as immediate-early genes, play important roles in regulating cell proliferation. We have identified a new type of early-growth-response gene product, a 77,811-Da putative serine/threonine kinase, which is highly inducible by serum and phorbol ester. mRNA encoding this putative kinase is markedly elevated within 1 h after treatment with mitogen, and this induction is synergistically increased by cycloheximide. Dexamethasone blocks serum induction of the kinase mRNA, as does transformation by v-Ki-ras. The kinase mRNA was detected in mouse brain, lung, and heart. This new putative kinase, which we term Snk, for serum-inducible kinase, showed similarity in its proposed catalytic domain to many other protein kinases; however, no other kinase showed enough sequence similarity with Snk to suggest the existence of a common function. Hence, Snk represents a new type of protein kinase involved in the early mitogenic response whose activity is transcriptionally and posttranscriptionally regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson A., Sabelli P. A., Dickinson J. R., Cole D., Richardson M., Kreis M., Shewry P. R., Halford N. G. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8602–8605. doi: 10.1073/pnas.88.19.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol. 1989 Nov;9(11):5034–5044. doi: 10.1128/mcb.9.11.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Teich N. M. Identification of a 39,000-dalton protein in cells transformed by the FBJ murine osteosarcoma virus. Virology. 1982 Jan 15;116(1):221–235. doi: 10.1016/0042-6822(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Fletcher B. S., Kujubu D. A., Perrin D. M., Herschman H. R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992 Mar 5;267(7):4338–4344. [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K. Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family. Proc Natl Acad Sci U S A. 1987 Jan;84(2):388–392. doi: 10.1073/pnas.84.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Erikson E., Blenis J., Maller J. L., Erikson R. L. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci U S A. 1988 May;85(10):3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kujubu D. A., Herschman H. R. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992 Apr 25;267(12):7991–7994. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., Herschman H. R. Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1(3):263–270. [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion M. K., Sadowski H. B., Winn V., Young D. A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [PubMed] [Google Scholar]
- Politis A. D., Sivo J., Driggers P. H., Ozato K., Vogel S. N. Modulation of interferon consensus sequence binding protein mRNA in murine peritoneal macrophages. Induction by IFN-gamma and down-regulation by IFN-alpha, dexamethasone, and protein kinase inhibitors. J Immunol. 1992 Feb 1;148(3):801–807. [PubMed] [Google Scholar]
- Poon M., Megyesi J., Green R. S., Zhang H., Rollins B. J., Safirstein R., Taubman M. B. In vivo and in vitro inhibition of JE gene expression by glucocorticoids. J Biol Chem. 1991 Nov 25;266(33):22375–22379. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Kasper C. B. Quantitation of mRNAs specific for the mixed-function oxidase system in rat liver and extrahepatic tissues during development. Arch Biochem Biophys. 1989 May 15;271(1):10–20. doi: 10.1016/0003-9861(89)90250-6. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J. N., Faller D. V. P21 v-ras inhibits induction of c-myc and c-fos expression by platelet-derived growth factor. Mol Cell Biol. 1988 Dec;8(12):5080–5085. doi: 10.1128/mcb.8.12.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]