ABSTRACT
Background
Insulinoma-associated protein 1 (INSM1) is a zinc finger transcriptional repressor with a limited spatial and temporal embryonic expression pattern in neuronal and neuroendocrine tissues. Interestingly, INSM1 activity is reactivated in neuroendocrine tumors such as small-cell lung cancer (SCLC), neuroblastoma, medulloblastoma, and retinoblastoma. Adenoviral constructs with the 1.7-kilobase pair INSM1 promoter-driven herpes simplex virus thymidine kinase (HSV-tk) gene could effectively suppress D283 Med subcutaneous xenograft tumor growth. Undesirably, sequences in the adenoviral backbone overrode promoter specificity in vivo. Incorporation of both the chicken β-globin HS4 insulator sequence and 2 copies of the mouse nicotinic acetylcholine receptor (nAchR) neuronal restrictive silencer element abolished the nonspecific activation of the INSM1 promoter in vivo.
Methods
The luciferase reporter gene was replaced with the HSV-tk suicide gene to generate the Ad-K5 virus. Both in vitro cell viability assays and in vivo tumor regression studies were used to determine the efficacy of the improved configuration INSM1 promoter adenoviral construct against a panel of neuroendocrine cell lines.
Results
In vitro cell viability assays with the Ad-K5 HSV-tk–expressing construct further reinforced that the Ad-K5 virus could eradicate SCLC, insulinoma, medulloblastoma, and neuroblastoma cells. Further, Ad-K5 virus treatment of a D283 Med subcutaneous xenograft tumor showed a superior antitumor effect over the control Ad-RSV (Rous sarcoma virus)-HSV-tk.
Conclusions
Improvements to the INSM1 promoter resulted in a stronger and more selective adenovirus. Treatment of a panel of neuroendocrine carcinomas with the Ad-K5 virus revealed enhanced antitumor activity over the RSV control, demonstrating its usefulness for the treatment of a variety of neuroendocrine tumors.
Keywords: Adenoviridae, herpes simplex virus thymidine kinase, INSM1 promoter, luciferase, neuroendocrine tumors
INTRODUCTION
Neuroendocrine carcinomas share a striking number of molecular gene signatures despite the fact that they arise in different organs throughout the body. Neuroendocrine forms of cancer affect both the pediatric and adult populations. Because of the aggressive nature of these forms of cancer, conventional treatment options such as chemotherapy and radiation can have deleterious side effects, especially in pediatric patients, with limited long-term benefit. Therefore, the development of new targeted treatment strategies is necessary, especially for aggressive forms of cancer such as small-cell lung cancer (SCLC), high-risk neuroblastoma, and medulloblastoma.
Currently, adenovirus remains the most frequently utilized virus for the delivery of cancer gene therapy. Adenovirus has many advantages as a choice for gene therapy: the ability to efficiently infect a wide variety of proliferating and nonproliferating cell types, the ability to generate the virus to high titers, the ability to accept a relatively large transgene, and the relative ease of manipulating the viral genome. One of the major limiting factors for use of adenoviral suicide gene therapy is the lack of targeting which results in liver toxicity. To avoid unwanted side effects, various strategies to target the adenovirus have been employed. One strategy is to transcriptionally regulate the expression of the transgene to a specific cell population. Unfortunately, the adenoviral sequences have been shown to interfere with the transcriptional control.1-4 One proven method to block interference of the adenoviral sequences with tissue-specific or inducible promoters is to include the chicken β-globin HS4 insulator element.1,2 Most targeted therapy takes advantage of a tumor-specific feature. Identification of a shared feature by a variety of neuroendocrine tumors would result in a rationally designed treatment that would selectively target and be effective against multiple forms of neuroendocrine carcinomas.
Insulinoma-associated protein 1 (INSM1) is a transcriptional repressor protein that is required for the development of the endocrine pancreas, adrenal glands, basal neuronal progenitor cells in the neocortex, and monoaminergic neurons in the hindbrain, as well as for transition of progenitors from apical to basal and neurogenic in the embryonic olfactory epithelium.5-10 INSM1 expression is restricted to early fetal development in neuronal and endocrine tissues.8,11,12 One striking feature of the INSM1 messenger RNA (mRNA) is despite its absence in normal adult tissues, it is strongly expressed in tumors of neuroendocrine origin such as SCLC, medulloblastoma, neuroblastoma, medullary thyroid carcinoma, insulinoma, retinoblastoma, pheochromocytoma, and pituitary tumors.12-14 Both a transgenic animal model and in vitro reporter gene assays clearly demonstrated that the spatial and temporal expression of INSM1 is regulated by the 1.7-kilobase pair (kbp) promoter region.13,15 Given its unique expression pattern, linkage of the 1.7-kbp region with the herpes simplex virus thymidine kinase (HSV-tk) suicide gene for delivery into tumor cells efficiently and selectively killed SCLC.16 Using adenovirus, the Ad-INSM1p-HSV-tk virus inhibited the growth of pediatric medulloblastoma, neuroblastoma, and retinoblastoma tumors.14 Disappointingly, biodistribution studies in mice using in vivo imaging of the Ad-INSM1 promoter luciferase2 constructs revealed off-target activity of the INSM1 promoter in the spleen, pancreas, kidney, and lung. Experiments revealed that the source of this off-target expression was due to regions within the adenoviral genome.17
Different approaches were designed, tested, and validated to prevent the nonspecific activation of the Ad-INSM1 promoter constructs and to restore the tissue selectivity in the adenovirus backbone. Incorporation of the chicken albumin β-globin insulator element upstream and 2 tandem copies of the mouse nicotinic acetylcholine receptor (nAchR) neuronal restrictive silencer elements (NRSEs) downstream relative to the INSM1 promoter region was successful to both reduce expression in nonneuronal cell lines and to modestly increase INSM1 promoter activity in INSM1-expressing tumor cell lines.17 The next logical step for verification of our improved adenoviral INSM1 promoter-driven cancer gene therapy was to incorporate the HSV-tk suicide gene into the HS4 insulator, 2×NRSE, INSM1 promoter adenoviral construct designated as Ad-K5. In vitro cell viability assays with SCLC, medulloblastoma, neuroblastoma, and insulinoma cell lines treated with Ad-K5 demonstrated a selective and efficient antitumor effect. The Ad-K5 construct was also injected into a D283 Med xenograft nude mouse tumor model and compared along with the strong viral Ad-RSV (Rous sarcoma virus)-HSV-tk construct. The Ad-K5 virus suppressed D283 Med tumor growth better than the Ad-RSV-HSV-tk virus. The data support the improvement of adenoviral INSM1-HSV-tk therapy as an alternative treatment option for multiple forms of rare neuroendocrine tumors.
METHODS
Cell Cultures
The NCI-H69, NCI-H1155, NCI-H727, DMS53, U87MG, IMR-32, SK-N-SH, SK-N-BE(2), Y79, WERI-Rb1, HeLa, PANC-1, BEAS, RIN, D283 Med, and HepG2 cell lines were obtained from American Type Culture Collections (ATCC, Manassas, VA). Ad-293 cells were purchased from Agilent Technologies and were maintained according to ATCC's or Agilent's instructions. All the cells lines were cultured in Royal Parks Memorial Institute (RPMI) 1640 or Dulbecco's modified eagle's media (DMEM) containing 10% fetal bovine serum (FBS; Atlanta Biologicals) and 100 U/mL penicillin and 100 μg/mL streptomycin in a 37°C incubator with 5% CO2. All cell culture media was obtained from Mediatech (Manassas, VA) unless otherwise noted.
In Vitro Adenoviral Transduction Assays
To compare the activity of the adenovirus constructs, the Ad-HS4insINSM1p2×NRSE-luciferase2 (Ad-M3) and the original Ad-INSM1p-luciferase2 construct (Ad-C) were transduced at multiplicity of infection (MOI) 100 with various INSM1-expressing and nonexpressing cell lines. Cells were seeded at 100,000 cells/well in a 24-well dish on the same day as virus transduction. Forty-eight hours post–virus infection, the cells were washed and collected in 1× phosphate-buffered saline. The cells were assayed for luciferase activity using the ONE-Glo Luciferase Assay System (Promega, Madison, WI). Twenty-five microliters of the cell suspension was mixed in a 1:1 ratio with ONE-Glo luciferase reagent (Promega) and incubated at room temperature for 10 minutes in a white 96-well plate. The luciferase activity was measured using a TopCount NXT microplate scintillation and luminescence reader (Packard Instrument Company, Meriden, CT). Data were graphed as the mean of the relative luciferase activity. All graphs represent the mean relative light units (RLU). Transductions were done in triplicate on at least 2 occasions.
Generation of Adenoviral Constructs and Lysates
For the in vivo studies, the HS4ins-INSM1p-2×NRSE HSV-tk construct designated Ad-K5 was subcloned into the pShuttle plasmid backbone (Agilent Technologies, Santa Clara, CA). Positive clones were verified by restriction digestion and DNA sequence analysis (Davis Sequencing, Davis CA). The Ad-M3 construct was previously described17 and included the INSM1 promoter construct with the luciferase2 gene (Promega, derived from the pGL4.1 plasmid). Generation of the Ad-RSV-HSV-tk virus was previously described.14 The final round of amplification was done with forty 150-cm tissue culture dishes, and the viral lysate was purified on a cesium chloride (CsCl) gradient. The viral supernatant was dialyzed to remove the CsCl before use. The final viral amplification was titered using the Adeno-X-Rapid Titer Kit (Clontech, Mountain View, CA) and stored in aliquots at −70° C.
Adenovirus-Treated In Vitro Cell Viability Assays
Cell lines NCI-H69, NCI-H1155, PANC-1, RIN, D283 Med, Y79, WERI-Rb1, HeLa, IMR-32, SK-N-SH, SK-N-BE(2), and HepG2 were seeded at 5,000-10,000 cells per well in a 96-well, white-wall, clear-bottom dish. Ad-RSV-LacZ virus (Vector Biolabs, Philadelphia, PA) or Ad-K5 was transduced at MOI 0, 10, 25, 50, 100, and 200. Twenty-four hours post–virus infection, the cells were treated with 100 μM ganciclovir (GCV, Cytovene IV, Genentech Inc., San Francisco, CA) and incubated for an additional 5 days. The cells were assayed for viability using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer's instructions. The plates were read on the TopCounter NXT scintillation and luminescence counter (Packard Instruments). All assays were performed in triplicate on at least 2 occasions. Data are graphed relative to the MOI 0 set as control at 100%. The standard error of the mean was calculated for the experiments.
In Vivo Adenovirus Antitumor Efficacy
Eight-week-old male Nu/Nu mice (National Cancer Institute, Frederick, MD) were injected with 1×107 D283 Med tumor cells subcutaneously in the hind flanks and established tumors for 3 days. The 100 mm3 tumors were injected with 1×109 infectious unit (IFU) virus particles of the Ad-K5, Ad-RSV-HSV-tk, or Ad-M3 virus on 2 consecutive days to determine the efficacy of the cancer gene therapy. The animals received daily intraperitoneal injections of GCV at 50 mg/kg body weight. Reduction of the tumor mass was measured manually by caliper and the volume calculated. The animals' tumors were followed for 2 weeks. All animal experiments were performed in accordance with the approved protocol from the Research Institute for Children at Children's Hospital, New Orleans Institutional Animal Care and Use Committee.
RESULTS
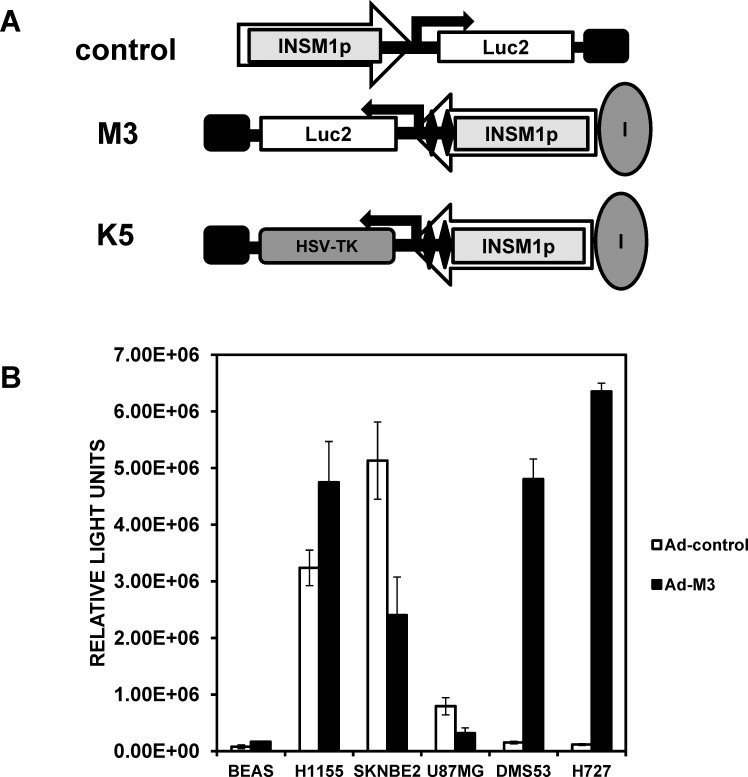
To take advantage of the limited expression of INSM1 in neuroendocrine tumors, we designed and tested an INSM1 promoter transcriptionally targeted cancer gene therapy for the treatment of various neuroendocrine cancers. Unexpectedly, in vivo biodistribution studies in mice revealed that sequences in the adenoviral genome resulted in expression of the INSM1 promoter-driven luciferase activity in the spleen, lung, and pancreas.17 Through methodical design and testing, we abolished nonspecific activation of luciferase activity in non-tumor-bearing mice through inclusion of 2 tandem copies of the NRSE element and the chicken β-globin HS4 insulator (Figure 1A).17 Next, we evaluated the improved specificity Ad-M3 construct in a panel of neuroendocrine cell lines. The Ad-M3 construct was transduced into INSM1-negative BEAS and U87MG cell lines and into INSM1-positive NCI-H1155, SK-N-BE(2), DMS53, and NCI-H727 cell lines, along with the original Ad-INSM1p-luciferase2 (Ad-C) construct. The luciferase activity from the Ad-M3 construct was significantly lower in the U87MG and BEAS cell lines than in the INSM1-expressing cell lines NCI-H1155, DMS53, NCI-H727, and SK-N-BE(2) (Figure 1B). Despite the lower activity, the Ad-C virus had an ∼800,000 RLU reading in the U87MG cells, and the Ad-M3 was reduced by 2.5-fold (Figure 1B). In the BEAS cells, both the Ad-C and Ad-M3 virus luciferase activity was low compared to all the other cell types. Intriguingly, an extremely low activity of the Ad-C virus was detected in the INSM1-positive DMS53 (∼150,000 RLU) and NCI-H727 (∼100,000 RLU) cells compared even to the BEAS normal bronchial epithelial cells (Figure 1B). In contrast, the Ad-M3 virus activity was increased by 31-fold in DMS53 and 54-fold in the NCI-H727 cell lines, demonstrating the impact the promoter improvements made to the overall activity. The NCI-H1155 cells showed a 1.5-fold increase, and the SK-N-BE(2) cells showed a 2-fold decrease in luciferase activity with the Ad-M3 virus (Figure 1B). Despite the measured decrease in SK-N-BE(2) cells with the Ad-M3 construct, SK-N-BE(2) cells still had a relative luciferase level comparable to the other INSM1-positive cell lines. Therefore, the in vitro Ad-M3 specificity and activity were enhanced through incorporation of the insulator and 2×NRSE elements with the INSM1 promoter region.
Figure 1.
Luciferase assays with Ad-C vs Ad-M3 virus in nonneuronal and neuroendocrine cell lines. (A) Schematic diagram of the various insulinoma-associated protein 1 (INSM1)-containing adenovirus constructs. The orientation of the INSM1 promoter transgene cassette with respect to the adenovirus E1A enhancer region is indicated by the large arrowheads. The large gray ovals represent the HS4 insulator element, the black diamonds indicate the 2× neuronal restrictive silencer elements (NRSEs), the small black rectangle denotes the SV40 poly-A tail, and the black direction arrows designate the start of transcription for the luciferase2 or herpes simplex virus thymidine kinase genes. (B) Ad-C virus and Ad-M3 virus multiplicity of infection (MOI) 100 were transduced into human INSM1-negative BEAS (bronchial epithelial) and U87MG (glioblastoma) cells and into the human INSM1-positive cell lines SK-N-BE(2) (neuroblastoma), NCI-H1155 (non–small-cell lung cancer with neuroendocrine differentiation), NCI-H727 (carcinoid), and DMS53 (small-cell lung cancer); incubated for 48 hours; and assayed for luciferase activity using the ONE-Glo luciferase reagent. All data represent the average of at least 3 experiments performed in triplicate. The luciferase activity is reported as relative light units measured on a TopCount NXT scintillation and luminescence reader.
In Vitro Cell Viability Assays with Ad-K5
The next phase for development of a neuroendocrine-specific cancer gene therapy was to convert the reporter gene containing the Ad-M3 virus into a killer virus. Because of size constraints, the luciferase2 gene and HSV-tk suicide genes could not be incorporated into the same virus. Therefore, the luciferase2 gene in the Ad-M3 virus was replaced by the HSV-tk gene, resulting in Ad-HS4insINSM1p-2×NRSE-HSV-tk and named Ad-K5 (Figure 1A). We first determined if the Ad-K5 virus could selectively eliminate INSM1-expressing neuroendocrine cells. Cells were seeded in a 96-well dish and infected with MOI 0, 10, 25, 50, 100, 200 of Ad-RSV-LacZ or Ad-K5 virus. Twenty-four hours postinfection, the cells were treated with 100 μM GCV and incubated for an additional 5 days. At the end of the experiment, the cells were lysed in CellTiter-Glo reagent, incubated, and read on a luminescence plate reader. Results are shown in Figure 2.
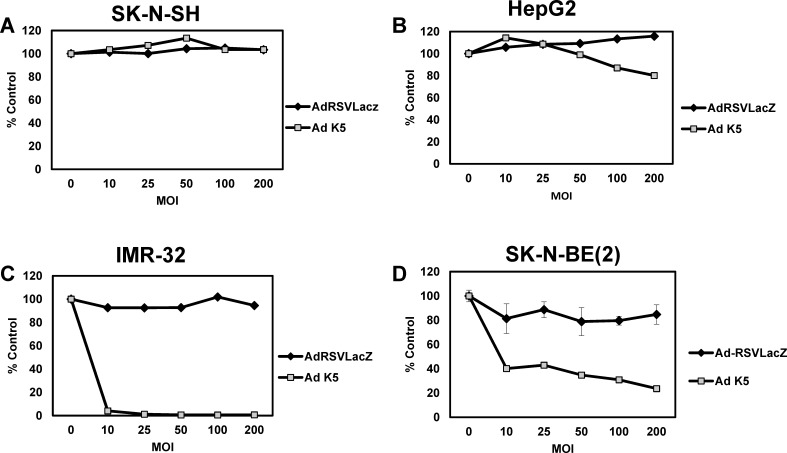
Figure 2.
Ad-K5 vs Ad-RSV-LacZ virus treatment of neuroblastoma cells. INSM1-negative (A) SK-N-SH (neuroblastoma) and (B) HepG2 (hepatocellular carcinoma) and INSM1-positive (C) IMR-32 (neuroblastoma) and (D) SK-N-BE(2) (neuroblastoma) were seeded at 5,000-10,000 cells/well in a 96-well dish and transduced with multiplicity of infection 0, 10, 25, 50, 100, and 200 of Ad-RSV-LacZ (negative control) and Ad-K5 virus. Twenty-four hours post–virus transduction, the cells were treated with 100 μM ganciclovir. The cells were incubated an additional 5 days and assayed for viability using the CellTiter-Glo assay. The graphs represent the average of 2 experiments performed in triplicate.
Because of the reduced activity observed in the SK-N-BE(2) cells with the Ad-M3 virus, we first tested two INSM1-positive neuroblastoma cell lines: SK-N-BE(2) and IMR-32. Treatment of the IMR-32 cells at MOI 10 caused 100% cell death of the tumor cells (Figure 2C). The SK-N-BE(2) cells did not respond as robustly; at MOI 200, the cytotoxic effect reached a maximum of 80% (Figure 2D). In comparison, the INSM1-negative SK-N-SH neuroblastoma cells and HepG2 hepatocellular carcinoma cells (Figures 2A and 2B) were completely resistant to virus treatment.
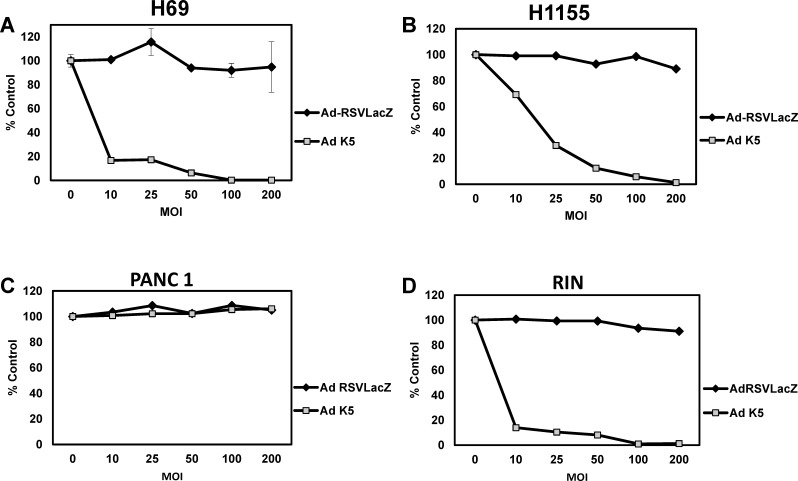
Next, we analyzed NCI-H69 (SCLC), NCI-H1155 (non–small-cell lung cancer with neuroendocrine differentiation [NSCLC-NE]), and RIN (rat insulinoma) cells for the ability of the Ad-K5 virus to destroy these cells. Both the NCI-H69 and NCI-H1155 cell viability was reduced in a dose-dependent manner (Figures 3A and 3B). Complete cell destruction was observed at MOI 100 in the NCI-H69 cells and at MOI 200 in the NCI-H1155 cells. The RIN cells were particularly sensitive to the Ad-K5 virus. The cells were decreased by 90% at MOI 10 and by 100% at MOI 100 (Figure 3D). The PANC-1 (pancreatic adenocarcinoma) cells served as a pancreas control because off-target activity of the original Ad-C virus was detected in the mouse liver, pancreas, lung, and spleen. In the PANC-1 human adenocarcinoma cells, the cell viability was not affected by the Ad-K5 virus prodrug/enzyme therapy.
Figure 3.
Cell viability assay with small-cell lung cancer (SCLC) and insulinoma cell lines. INSM1-positive (A) NCI-H69 (SCLC), (B) NCI-H1155 (non–small-cell lung cancer with neuroendocrine differentiation), and (D) RIN (rat insulinoma) and INSM1-negative (C) PANC-1 (pancreatic adenocarcinoma) were seeded in a 96-well dish. The cells were transduced with Ad-RSV-LacZ or Ad-K5 at multiplicity of infection 0, 10, 25, 50, 100, and 200 for 24 hours. Following the initial 24-hour incubation, 100 μM ganciclovir was added to the wells and the cells were incubated for an additional 5 days. The cell viability was measured using the CellTiter-Glo assay. The graphs represent the average of 2 experiments performed in triplicate.
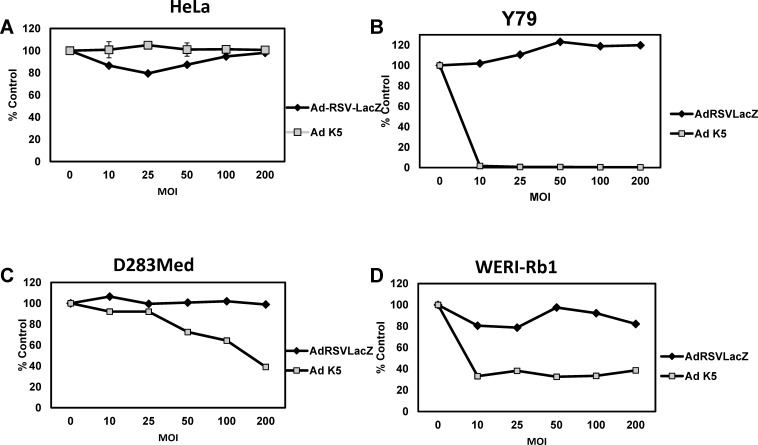
The last tumor cell lines tested were Y79 (retinoblastoma), WERI-Rb1 (retinoblastoma), and D283 Med (medulloblastoma). The Y79 cells were exquisitely sensitive to Ad-K5; the cells were completely abolished at MOI 10 (Figure 4B), whereas the highest cell death detected in the WERI-Rb1 cells was ∼80% at the highest MOI (Figure 4D). In contrast, D283 Med cells were the least susceptible to the therapy; the highest cell death achieved was 60% at MOI 200 (Figure 4C). HeLa (cervical carcinoma) cells are negative for INSM1 and are completely resistant to treatment with the Ad-K5 virus (Figure 4A). These data support the conclusion that the Ad-K5 virus can efficiently and selectively eliminate INSM1-positive neuroendocrine tumors, including SCLC, neuroblastoma, retinoblastoma, insulinoma, and medulloblastoma.
Figure 4.
In vitro cell viability assays in medulloblastoma and retinoblastoma cells. INSM1-nonexpressing (A) HeLa (cervical carcinoma), INSM1-expressing (B) Y79 (retinoblastoma), (C) D283 Med (medulloblastoma), and (D) WERI-Rb1 (retinoblastoma) were seeded in a 96-well dish. The cells were transduced with multiplicity of infection 0, 10, 25, 50, 100, and 200 Ad-RSV-LacZ or Ad-K5 virus. Twenty-four hours post–virus transduction, the cells were treated with 100 μM ganciclovir for an additional 5 days. Cell viability was measured using the CellTiter-Glo assay, and the data were graphed. Luciferase was measured on a TopCount NXT scintillation and luminescence reader. The graphs represent the average of 2 experiments performed in triplicate. Data are graphed relative to the 0 or no-virus-treated control.
In Vivo Suicide Gene Therapy in D283 Med Tumor-Bearing Mice
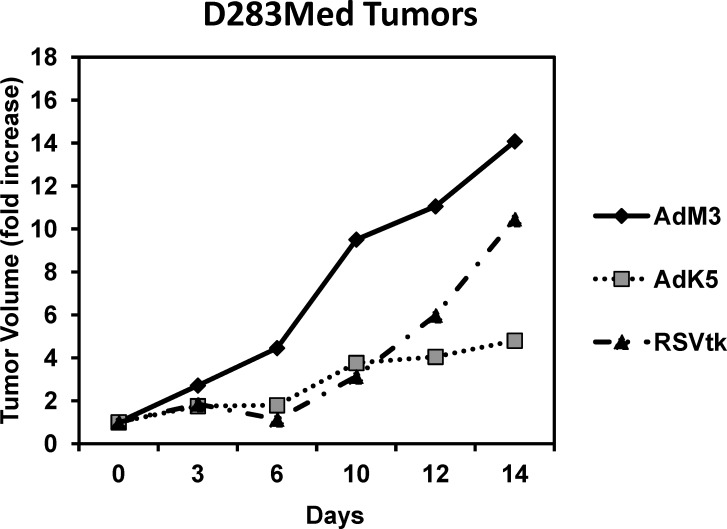
The final validation for our improved suicide gene therapy was to test the in vivo efficacy using a xenograft tumor model. We chose to use the D283 Med cells because we have previous experience generating tumors in nude mice with these cells although they were one of the least responsive cell lines in the in vitro studies. Animals were injected with 1×107 cherry fluorescent-labeled D283 Med cells. When the tumors reached 100 mm3, the animals were divided into groups. Animals were given two 1×109 IFU intratumoral injections of adenovirus 1 day apart. The adenoviruses that were compared were the Ad-M3 (INSM1 promoter-driven luciferase reporter construct [negative control]), Ad-RSV-HSV-tk (strong positive control), and the Ad-K5 virus. Twenty-four hours post–virus administration, the animals were given daily 50 mg/kg intraperitoneal injections of GCV. The tumor volumes were measured manually with a caliper every other day. The change in tumor volume was plotted over a 2-week period. In the Ad-M3 negative control group, the tumor grew by 14-fold compared to the start of treatment (Figure 5, solid line). The Ad-RSV-HSV-tk positive control virus did not eliminate the tumor mass but did inhibit tumor growth compared to the negative Ad-M3 control group. At the 2-week mark, the Ad-RSV-HSV-tk tumors had increased by 10-fold, a 4-fold reduction compared to the Ad-M3 group (Figure 5, dashed line). Encouragingly, although the tumor mass was not eliminated by the Ad-K5 therapy, the growth of the tumor was significantly reduced compared to both the negative control Ad-M3 and the positive control Ad-RSV-HSV-tk groups. The volume of the Ad-K5–treated tumor increased by 4.8-fold at the end of 2 weeks, a 10-fold reduction compared to the Ad-M3 tumors (Figure 5, dotted line). Therefore, these data show a clear benefit from the use of the improved INSM1 promoter-driven HSV-tk suicide gene therapy in an in vivo tumor model.
Figure 5.
In vivo efficacy of the Ad-K5 virus in the treatment of D283 Med tumors. Subcutaneous D283 Med cherry fluorescent tumors were established by injection of 1×107 cells into the hind flanks of male athymic Nu/Nu mice. Three days following tumor cell injection, the animals received 2 consecutive 1×109 infectious unit intratumoral injections of Ad-M3 (negative control, solid line), Ad-RSV-HSV-tk (positive control, dashed line), or Ad-K5 (dotted line) virus. Twenty-four hours postinjection, the animals were given daily intraperitoneal 50 mg/kg injections of ganciclovir. The tumor volumes were measured manually with a caliper every other day for 2 weeks and the tumor volume calculated. The graph shows the fold change in tumor volume relative to the starting tumor volume during the 2 weeks.
DISCUSSION
The expression profile of the INSM1 gene makes it ideal to be exploited for the development of a neuroendocrine cancer-restricted gene therapy. INSM1 expression is transient during early development in the brain, pancreas, adrenal glands, thymus, thyroid, intestine, eye, olfactory bulb, and pituitary.8,11,12 Despite the lack of expression in adult tissue, the INSM1 gene is reactivated in neuroendocrine tumors where embryonic INSM1 expression is detected such as retinoblastoma, neuroblastoma, medulloblastoma, medullary thyroid carcinoma, SCLC, carcinoid, pituitary tumor, insulinoma, and pheochromocytoma.12-14 Validation of the specificity and efficacy of the INSM1 promoter-driven HSV-tk gene therapy in a DNA form in SCLC16 and delivered as an adenovirus for the treatment of neuroblastoma, retinoblastoma, and medulloblastoma has been demonstrated.14 Despite these successes, long-term in vivo bioluminescence studies with the Ad-INSM1p-luciferase2 (Ad-C) viral construct revealed nonspecific activation of luciferase activity in the abdominal region of mice. Following intravenous injection of the Ad-C virus, tissue distribution studies showed the highest expression of luciferase activity in the spleen, lung, and pancreas of the nude mice.17 Multiple strategies were used to reestablish the INSM1 promoter specificity in the adenovirus. Inclusion of the chicken β-globin HS4 insulator upstream of the INSM1 promoter and a tandem copy repeat of the NRSE element downstream restored promoter specificity in vivo.17 In the current study, we extended the observations made with the improved HS4 insulator INSM1p-2×NRSE luciferase2 construct (Ad-M3), first in a panel of INSM1-negative BEAS and U87MG cell lines and then in INSM1-positive NCI-H1155, SK-N-BE(2), DMS53, and NCI-H727 cell lines. Interestingly, using the original Ad-C construct, luciferase activity was relatively high in the U87MG cells (∼800,000 RLU). Incorporation of the insulator and NRSE elements (Ad-M3) into the adenovirus construct reduced the luciferase activity to 60% less than the Ad-C. Surprisingly, in both the DMS53 and NCI-H727 INSM1-expressing cells, Ad-C activity was low (∼100,000 RLU), 8-fold less than in the U87MG INSM1-negative cells. However, the Ad-M3 activity was 31-fold higher and 54-fold higher in DMS53 and NCI-H727, respectively, than the Ad-C virus activity, showing a significant benefit to the new promoter configuration. SK-N-BE(2) cells were the only cells that did not have a marked benefit from the Ad-M3 virus. In SK-N-BE(2) cells, the activity of the Ad-M3 virus was 50% lower than the Ad-C virus. These data reinforce the advantage of incorporation of the HS4 insulator and NRSE sequences to not only prevent off-target activity of the INSM1 promoter but also to enhance INSM1 promoter activity in a cell type–specific manner.
The next phase of testing for the cancer gene therapy was to exchange the luciferase2 reporter gene for the HSV-tk suicide gene and establish the in vitro toxicity in various neuroendocrine tumor cell lines. Tumor-specific activation of a nontoxic prodrug into a toxic agent inside the tumor cell is an attractive approach to target cancer cells. Theoretically, dose escalation could be done without increasing collateral damage to normal surrounding tissues. The key to the success for this therapy is to exquisitely limit the activation of the toxin to the tumor cells. One way to target the toxin to the tumor cell is through transcriptional regulation of the suicide transgene. Other laboratories have developed tissue-specific promoter-driven adenovirus-based cancer therapies; examples include the prostate-specific antigen promoter or probasin promoter for prostate cancer, the hTERT promoter for telomerase-positive cancer cells, and the midkine promoter for neuroblastoma.18 However, one drawback to the promoters used thus far is that they are also active, although at a much lower level, in normal cells. Experiments were carried out with an in vitro cell viability assay to determine the effectiveness of the Ad-K5 virus versus a nonlethal control virus, Ad-RSV-LacZ. Following infection with an increasing MOI of Ad-K5 virus and subsequent treatment with a fixed concentration of GCV, the cells were assayed. We analyzed numerous neuroendocrine cell types to demonstrate the feasibility of our adenovirus for the treatment of a variety of neuroendocrine carcinoma types. In the cell viability assays, the IMR-32 (neuroblastoma), H69 (SCLC), Y79 (retinoblastoma), and RIN (rat insulinoma) achieved a 90%-100% cell death rate at MOI 10, while the NCI-H1155 reached 100% at MOI 200. The INSM1-negative cell lines HepG2, SK-N-SH, PANC-1, and HeLa were completely refractory to the viral therapy even at MOI 200, showing that the Ad-K5 virus retained the expected selective activity profile. The best antitumor activity achieved in the D283 Med and WERI-Rb1 cell lines was 60%, while the SK-N-BE(2) reached 80%. Although the virus did not completely kill all of the tested cell lines, it maintained its specificity. The difference in the ability of the Ad-K5 virus to completely eliminate the different tumor cells is most likely associated with the activity level of the INSM1 promoter in the individual carcinoma cells. While previous studies revealed a measured benefit from inclusion of the NRSE and insulator in SCLC, neuroblastoma, and retinoblastoma cells, in vitro reporter gene assays showed a 50% decrease in the Ad-M3 constructs in D283 Med cells,17 explaining the inability of the Ad-K5 virus to completely kill the cells even at high MOI. Another limiting factor to the success of the adenovirus therapy is the ability of the adenovirus to gain access to the tumor cells. Certain tumor cells have been shown to downregulate expression of the coxsackie and adenovirus receptor on their cell surfaces, limiting the amount of the adenovirus that could gain access inside the tumor.19,20 Despite these limiting factors, our Ad-M3 viral construct shows strong activity in vitro in a wide array of neuroendocrine tumors.
The final test to validate the new virus was in an in vivo nude mouse D283 Med xenograft model. Previous studies using a single intratumoral injection of Ad-INSM1-HSV-tk virus showed an inhibition of tumor growth but not a regression of the tumor mass.14 The reason for the observed in vitro and in vivo discrepancy was due to the lack of dispersion of the virus throughout the tumor mass.14 In this report, the Ad-M3 (negative control), the Ad-RSV-HSV-tk (positive control), and Ad-K5 virus were given as 2 consecutive intratumoral injections followed by daily GCV injections. The animals were followed for 14 days. In the Ad-M3 group, the tumors grew 14 times their pretreatment size, and the Ad-RSV-HSV-tk tumors grew to 10-fold their starting size, while the Ad-K5 tumors grew at a much reduced rate, reaching only 4 times their initial size. These data are encouraging and prove that this approach is a viable option for the treatment of neuroendocrine carcinomas. However, the data show that additional improvements are necessary for this therapy to be efficacious as a single agent in the treatment of neuroendocrine carcinoma. Our future approaches include using multiple injections spread over several days to ensure a better distribution of the virus throughout the tumor. However, the presence of preexisting immunity to the adenovirus would preclude this type of approach in humans.21 Therefore, our next strategy to achieve better distribution of the virus within the tumor is to generate an INSM1 promoter-controlled conditionally replicating virus. Because of the highly selective regulation that is demonstrated, incorporation of the INSM1-controlled E1A gene would target replication and expansion of the virus for better dispersion within the tumor with limited side effects.
CONCLUSIONS
Sensitive in vitro bioluminescence imaging assays revealed off-target activity of the Ad-INSM1 promoter-luciferase2 virus in mice.17 Methods to protect the INSM1 promoter sequence from the interfering adenoviral sequences resulted in a more selective and enhanced INSM1 promoter activity in a panel of INSM1-expressing neuroendocrine carcinoma cells. Exchange of the luciferase2 gene with the HSV-tk gene demonstrated that the Ad-K5 virus could efficiently and exclusively eliminate SCLC, high-risk neuroblastoma, medulloblastoma, and retinoblastoma tumor cells in vitro. Finally, Ad-K5 treatment of D283 Med subcutaneous tumors in nude mice demonstrated a strong antitumor response that outperformed by 5-fold the Ad-RSV-HSV-tk virus.
ACKNOWLEDGMENTS
This work was performed in conjunction with the Diana Helis Henry Medical Research Foundation and supported by the Research Institute for Children, Children's Hospital, New Orleans, Louisiana and Louisiana State University Health Sciences Center.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Systems-Based Practice.
REFERENCES
- 1.Martin-Duque P, Jezzard S, Kaftansis L, Vassaux G. Direct comparison of the insulating properties of two genetic elements in an adenoviral vector containing two different expression cassettes. Hum Gene Ther. 2004 Oct;15(10):995–1002. doi: 10.1089/hum.2004.15.995. [DOI] [PubMed] [Google Scholar]
- 2.Steinwaerder DS, Lieber A. Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vitro and in vivo. Gene Ther. 2000 Apr;7(7):556–567. doi: 10.1038/sj.gt.3301139. [DOI] [PubMed] [Google Scholar]
- 3.Vassaux G, Hurst HC, Lemoine NR. Insulation of a conditionally expressed transgene in an adenoviral vector. Gene Ther. 1999 Jun;6(6):1192–1197. doi: 10.1038/sj.gt.3300910. [DOI] [PubMed] [Google Scholar]
- 4.Ye X, Liang M, Meng X, et al. Insulation from viral transcriptional regulatory elements enables improvement to hepatoma-specific gene expression from adenovirus vectors. Biochem Biophys Res Commun. 2003 Aug 8;307(4):759–764. doi: 10.1016/s0006-291x(03)01251-8. [DOI] [PubMed] [Google Scholar]
- 5.Farkas LM, Haffner C, Giger T, et al. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008 Oct 9;60(1):40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Gierl MS, Karoulias N, Wende H, Strehle M, Birchmeier C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 2006 Sep 1;20(17):2465–2478. doi: 10.1101/gad.381806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob J, Storm R, Castro DS, et al. Insm1 (IA-1) is an essential component of the regulatory network that specifies monoaminergic neuronal phenotypes in the vertebrate hindbrain. Development. 2009 Jul;136(14):2477–2485. doi: 10.1242/dev.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellitzer G, Bonné S, Luco RF, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006 Mar 22;25(6):1344–1352. doi: 10.1038/sj.emboj.7601011. Epub 2006 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum JN, Duggan A, García-Añoveros J. Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev. 2011 Feb 1;6:6. doi: 10.1186/1749-8104-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008 Feb;135(3):473–481. doi: 10.1242/dev.011783. Epub 2007 Dec 19. [DOI] [PubMed] [Google Scholar]
- 11.Breslin MB, Zhu M, Lan MS. NeuroD1/E47 regulates the E-box element of a novel zinc finger transcription factor, IA-1, in developing nervous system. J Biol Chem. 2003 Oct 3;278(40):38991–38997. doi: 10.1074/jbc.M306795200. Epub 2003 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto Y, De Silva MG, Toscani A, et al. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with “zinc-finger” DNA-binding motifs. J Biol Chem. 1992 Jul 25;267(21):15252–15257. [PubMed] [Google Scholar]
- 13.Lan MS, Russell EK, Lu J, Johnson BE, Notkins AL. IA-1, a new marker for neuroendocrine differentiation in human lung cancer cell lines. Cancer Res. 1993 Sep 15;53(18):4169–4171. [PubMed] [Google Scholar]
- 14.Wang HW, Breslin MB, Chen C, et al. INSM1 promoter-driven adenoviral herpes simplex virus thymidine kinase cancer gene therapy for the treatment of primitive neuroectodermal tumors. Hum Gene Ther. 2009 Nov;20(11):1308–1318. doi: 10.1089/hum.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Notkins AL, Lan MS. Molecular characterization of the promoter region of a neuroendocrine tumor marker, IA-1. Biochem Biophys Res Commun. 1997 Jul 30;236(3):776–781. doi: 10.1006/bbrc.1997.7054. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen N, Pedersen MW, Lan MS, Breslin MB, Poulsen HS. The insulinoma-associated 1: a novel promoter for targeted cancer gene therapy for small-cell lung cancer. Cancer Gene Ther. 2006 Apr;13(4):375–384. doi: 10.1038/sj.cgt.7700887. [DOI] [PubMed] [Google Scholar]
- 17.Akerstrom V, Chen C, Lan MS, Breslin MB. Modifications to the INSM1 promoter to preserve specificity and activity for use in adenoviral gene therapy of neuroendocrine carcinomas. Cancer Gene Ther. 2012 Dec;19(12):828–838. doi: 10.1038/cgt.2012.66. Epub 2012 Oct 19. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi H, Hitt MM. Transcriptionally targeted adenovirus vectors. Curr Gene Ther. 2005 Aug;5(4):411–427. doi: 10.2174/1566523054546189. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Zinn KR, Barnett BG, et al. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur J Cancer. 2002 Sep;38(14):1917–1926. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 20.Krasnykh V, Dmitriev I, Navarro JG, et al. Advanced generation adenoviral vectors possess augmented gene transfer efficiency based upon coxsackie adenovirus receptor-independent cellular entry capacity. Cancer Res. 2000 Dec 15;60(24):6784–6787. [PubMed] [Google Scholar]
- 21.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997 Jan 1;8(1):37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]