Abstract
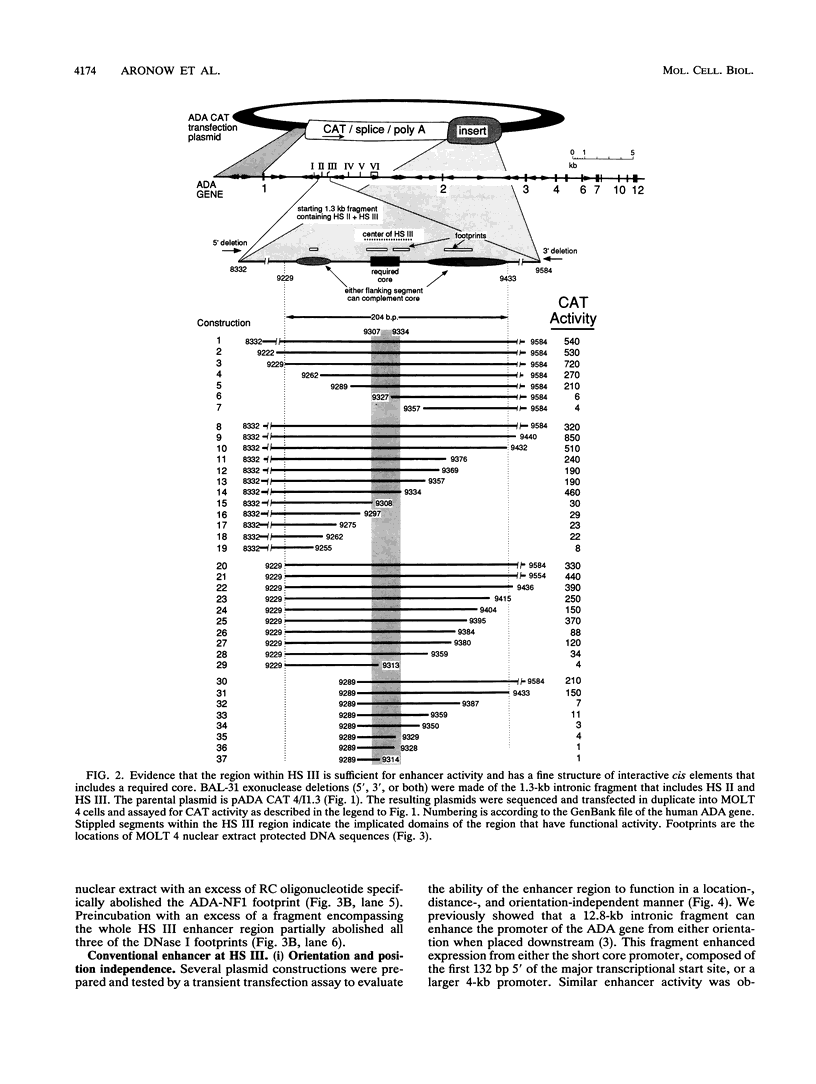
We previously observed that human ADA gene expression, required for the intrathymic maturation of T cells, is controlled by first-intron sequences. Used as a cis activator, the intron generates copy-dependent reporter expression in transgenic thymocytes, and we here dissect its critical determinants. Of six DNase I-hypersensitive sites (HS sites) in the intron, only HS III was a transfection-active classic enhancer in T cells. The enhancer contains a critical core region, ACATGGCAGTTGGTGGTGGAGGGGAACA, that interacts with at least two factors, ADA-NF1 and ADA-NF2. Activity of the core is strongly augmented by adjacent elements contained within a 200-bp domain corresponding to the limits of HS III hypersensitivity. These core-adjacent sequences include consensus matches for recognition by the AP-1, TCF-1 alpha, mu E, and Ets transcription factor families. In contrast, considerably more extensive sequences flanking the enhancer domain were required for position-independent and copy-proportional expression in transgenic mouse thymocytes. The additionally required upstream segment encompassed the nonenhancer HS II site. The required downstream segment, composed largely of Alu-repetitive DNA, was non-DNase I hypersensitive. Transgenes that lacked either segment were subject to strong positional effects. Among these variably expressing lines, the expression level correlated with the degree of hypersensitivity at HS III. This finding suggests that formation of hypersensitivity is normally facilitated by the flanking segments. These results delineate a complex thymic regulatory region within the intron and indicate that a series of interactions is necessary for the enhancer domain to function consistently within chromatin.
Full text
PDF



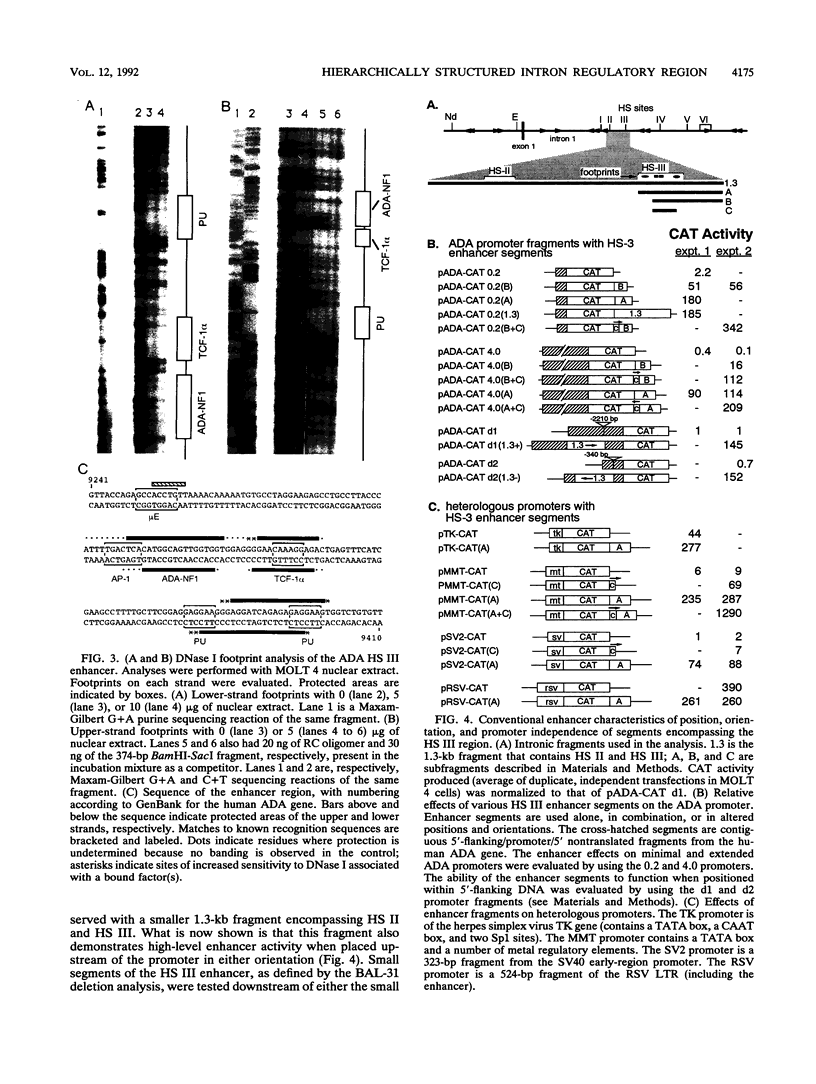
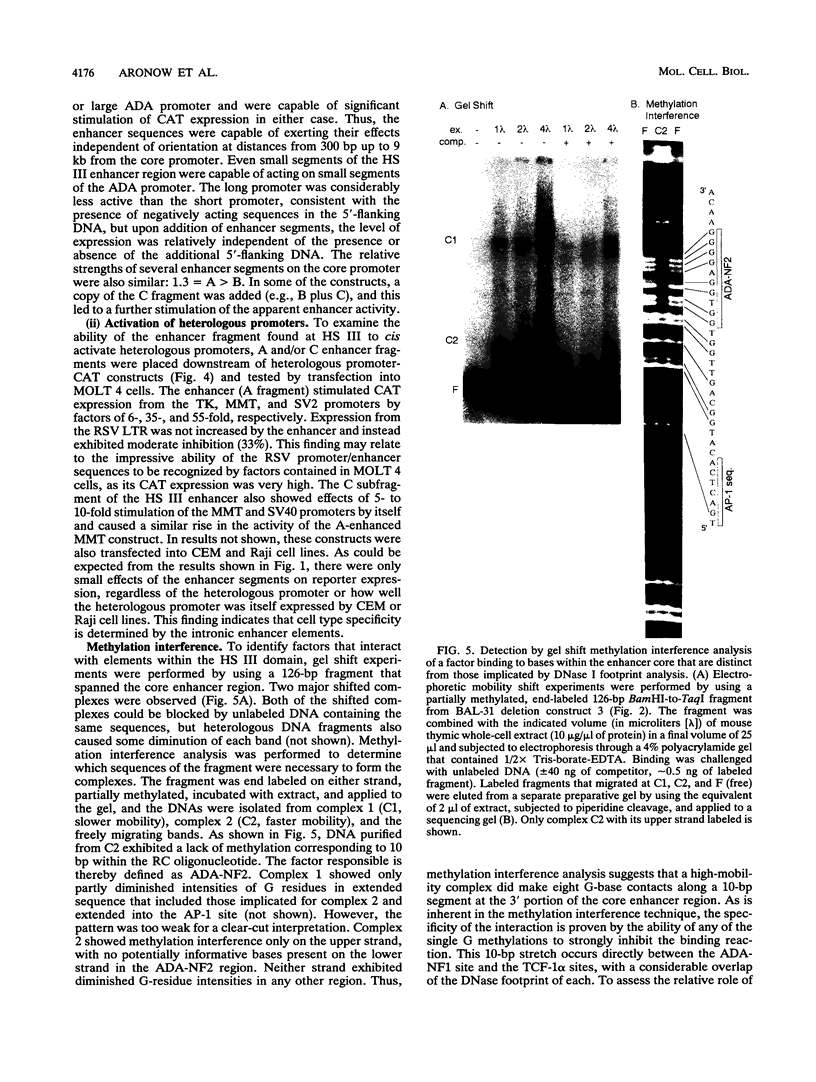
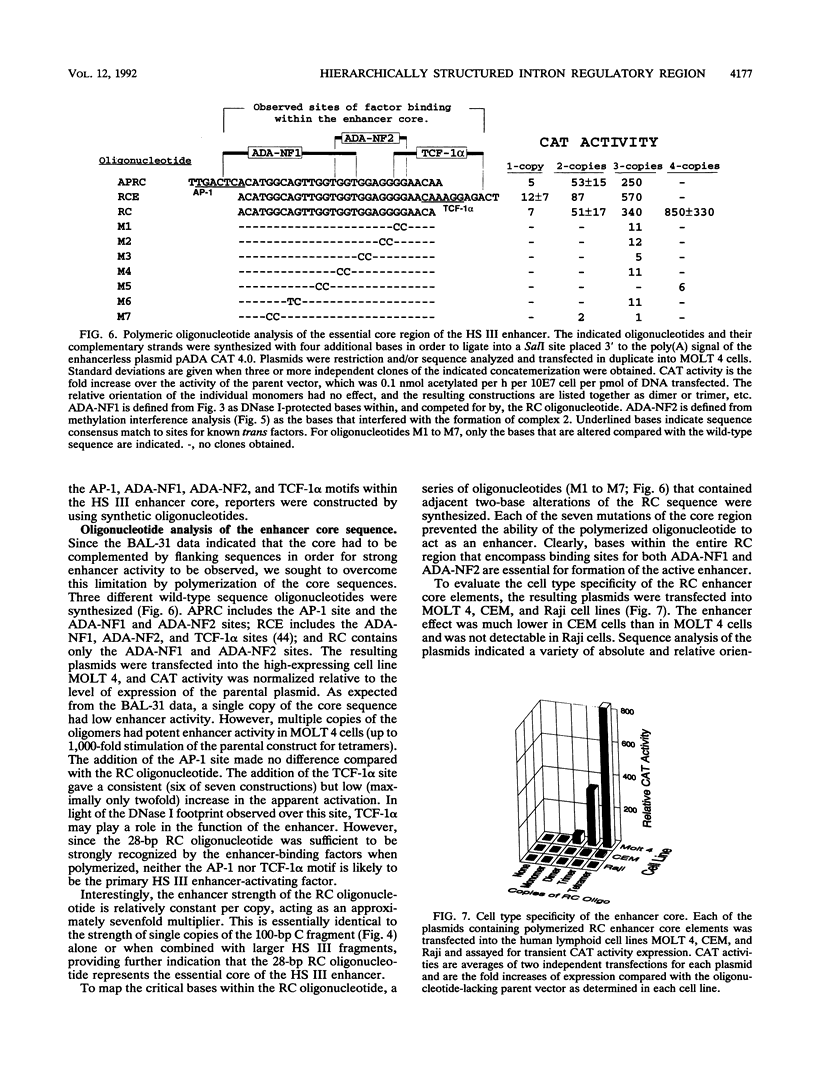


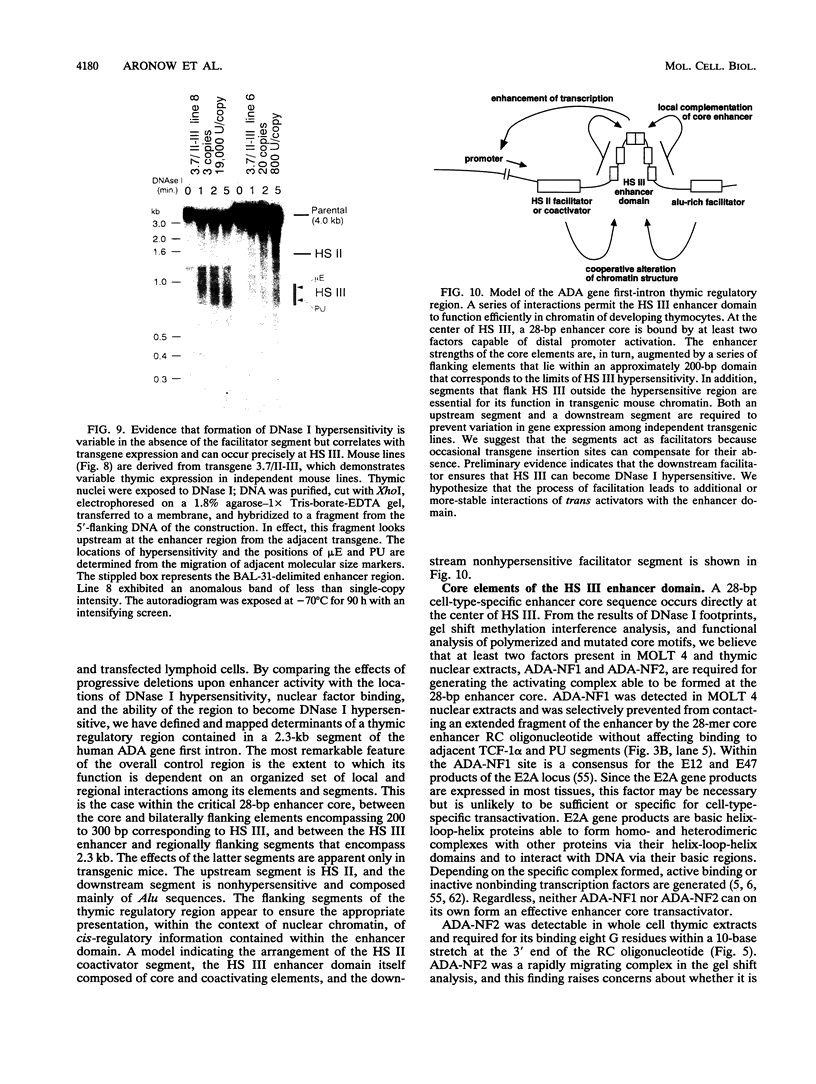





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Hutton J. J. Adenosine deaminase messenger RNAs in lymphoblast cell lines derived from leukemic patients and patients with hereditary adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1649–1660. doi: 10.1172/JCI110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Aronow B., Lattier D., Silbiger R., Dusing M., Hutton J., Jones G., Stock J., McNeish J., Potter S., Witte D. Evidence for a complex regulatory array in the first intron of the human adenosine deaminase gene. Genes Dev. 1989 Sep;3(9):1384–1400. doi: 10.1101/gad.3.9.1384. [DOI] [PubMed] [Google Scholar]
- Beckmann H., Su L. K., Kadesch T. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev. 1990 Feb;4(2):167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Komschlies K. L., Fujiwara S., Fisher R. J., Mathieson B. J., Gregorio T. A., Young H. A., Kasik J. W., Ozato K., Papas T. S. Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol. 1989 Jan 15;142(2):672–678. [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzy M. S., Schulte-Wissermann H., Gilbert E., Horowitz S. D., Pellett J., Hong R. Thymic morphology in immunodeficiency diseases: results of thymic biopsies. Clin Immunol Immunopathol. 1979 Jan;12(1):31–51. doi: 10.1016/0090-1229(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Boulet A. M., Scott M. P. Control elements of the P2 promoter of the Antennapedia gene. Genes Dev. 1988 Dec;2(12A):1600–1614. doi: 10.1101/gad.2.12a.1600. [DOI] [PubMed] [Google Scholar]
- Boyer B. B., Kozak L. P. The mitochondrial uncoupling protein gene in brown fat: correlation between DNase I hypersensitivity and expression in transgenic mice. Mol Cell Biol. 1991 Aug;11(8):4147–4156. doi: 10.1128/mcb.11.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper S. A., Tilghman S. M. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 1989 Apr;3(4):537–546. doi: 10.1101/gad.3.4.537. [DOI] [PubMed] [Google Scholar]
- Chechik B. E., Schrader W. P., Minowada J. An immunomorphologic study of adenosine deaminase distribution in human thymus tissue, normal lymphocytes, and hematopoietic cell lines. J Immunol. 1981 Mar;126(3):1003–1007. [PubMed] [Google Scholar]
- Chinsky J. M., Ramamurthy V., Fanslow W. C., Ingolia D. E., Blackburn M. R., Shaffer K. T., Higley H. R., Trentin J. J., Rudolph F. B., Knudsen T. B. Developmental expression of adenosine deaminase in the upper alimentary tract of mice. Differentiation. 1990 Feb;42(3):172–183. doi: 10.1111/j.1432-0436.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Choi T., Huang M., Gorman C., Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991 Jun;11(6):3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis P., Antoniou M., Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J. 1990 Jan;9(1):233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Elgin S. C. Boundary functions in the control of gene expression. Trends Genet. 1991 Oct;7(10):335–340. doi: 10.1016/0168-9525(91)90424-o. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Emerson B. M., Lewis C. D., Felsenfeld G. Interaction of specific nuclear factors with the nuclease-hypersensitive region of the chicken adult beta-globin gene: nature of the binding domain. Cell. 1985 May;41(1):21–30. doi: 10.1016/0092-8674(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P., Hurst J., Collis P., Grosveld F. DNaseI hypersensitive sites 1, 2 and 3 of the human beta-globin dominant control region direct position-independent expression. Nucleic Acids Res. 1990 Jun 25;18(12):3503–3508. doi: 10.1093/nar/18.12.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K., van den Elsen P., Bier E., Maxam A., Terhorst C. A T cell-specific enhancer is located in a DNase I-hypersensitive area at the 3' end of the CD3-delta gene. EMBO J. 1988 Aug;7(8):2401–2407. doi: 10.1002/j.1460-2075.1988.tb03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Chesa P. G., Nishimura H., Rettig W. J., Maccari J. E., Endo T., Seravalli E., Seki T., Silver J. Regulation of Thy-1 gene expression in transgenic mice. Cell. 1987 Jul 31;50(3):445–452. doi: 10.1016/0092-8674(87)90498-3. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosseye S., Diebold N., Griscelli C., Nezelof C. Severe combined immunodeficiency disease: a pathological analysis of 26 cases. Clin Immunol Immunopathol. 1983 Oct;29(1):58–77. doi: 10.1016/0090-1229(83)90007-7. [DOI] [PubMed] [Google Scholar]
- Gottesdiener K. M., Karpinski B. A., Lindsten T., Strominger J. L., Jones N. H., Thompson C. B., Leiden J. M. Isolation and structural characterization of the human 4F2 heavy-chain gene, an inducible gene involved in T-lymphocyte activation. Mol Cell Biol. 1988 Sep;8(9):3809–3819. doi: 10.1128/mcb.8.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner-Maniscalco V., Kuritsky L., Rosen F. S. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981 Dec;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Yang L. H., Morle G., Leiden J. M. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. doi: 10.1073/pnas.86.17.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Karpinski B. A., Yang L. H., Cacheris P., Morle G. D., Leiden J. M. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol Cell Biol. 1989 Jun;9(6):2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991 Mar 8;64(5):941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Lang G., Wotton D., Owen M. J., Sewell W. A., Brown M. H., Mason D. Y., Crumpton M. J., Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988 Jun;7(6):1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattier D. L., States J. C., Hutton J. J., Wiginton D. A. Cell type-specific transcriptional regulation of the human adenosine deaminase gene. Nucleic Acids Res. 1989 Feb 11;17(3):1061–1076. doi: 10.1093/nar/17.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey C. H., Bodine D. M., Nienhuis A. W. Mechanism of DNase I hypersensitive site formation within the human globin locus control region. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1143–1147. doi: 10.1073/pnas.89.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. D., Sylwestrowicz T. A., Granger S., Massaia M., Franks R., Janossy G., Hoffbrand A. V. Distribution of terminal deoxynucleotidyl transferase and purine degradative and synthetic enzymes in subpopulations of human thymocytes. J Immunol. 1982 Oct;129(4):1430–1435. [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Meyer K. B., Sharpe M. J., Surani M. A., Neuberger M. S. The importance of the 3'-enhancer region in immunoglobulin kappa gene expression. Nucleic Acids Res. 1990 Oct 11;18(19):5609–5615. doi: 10.1093/nar/18.19.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzina S., Hanscombe O., Whyatt D., Grosveld F., Philipsen S. Hypersensitive site 4 of the human beta globin locus control region. Nucleic Acids Res. 1991 Apr 11;19(7):1413–1419. doi: 10.1093/nar/19.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Copeland N. G., Jenkins N. A., Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991 Nov;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher J., Tilghman S. M. Dominant negative regulation of the mouse alpha-fetoprotein gene in adult liver. Science. 1990 Dec 21;250(4988):1732–1735. doi: 10.1126/science.1702902. [DOI] [PubMed] [Google Scholar]
- Vidal M., Morris R., Grosveld F., Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990 Mar;9(3):833–840. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. L., Fischer W. H., Jones K. A. A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev. 1991 Apr;5(4):656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- Waterman M. L., Jones K. A. Purification of TCF-1 alpha, a T-cell-specific transcription factor that activates the T-cell receptor C alpha gene enhancer in a context-dependent manner. New Biol. 1990 Jul;2(7):621–636. [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Wilson R. B., Kiledjian M., Shen C. P., Benezra R., Zwollo P., Dymecki S. M., Desiderio S. V., Kadesch T. Repression of immunoglobulin enhancers by the helix-loop-helix protein Id: implications for B-lymphoid-cell development. Mol Cell Biol. 1991 Dec;11(12):6185–6191. doi: 10.1128/mcb.11.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. A novel, inducible and T cell-specific enhancer located at the 3' end of the T cell receptor alpha locus. EMBO J. 1989 Mar;8(3):729–733. doi: 10.1002/j.1460-2075.1989.tb03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte D. P., Wiginton D. A., Hutton J. J., Aronow B. J. Coordinate developmental regulation of purine catabolic enzyme expression in gastrointestinal and postimplantation reproductive tracts. J Cell Biol. 1991 Oct;115(1):179–190. doi: 10.1083/jcb.115.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wustmann G., Szidonya J., Taubert H., Reuter G. The genetics of position-effect variegation modifying loci in Drosophila melanogaster. Mol Gen Genet. 1989 Jun;217(2-3):520–527. doi: 10.1007/BF02464926. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oosterwegel M., Dooijes D., Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991 Jan;10(1):123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]