Abstract
Background
Vitamin K antagonist (VKA) therapy remains the most common method of stroke prevention in patients with atrial fibrillation. Time in therapeutic range (TTR) is a widely cited measure of the quality of VKA therapy. We sought to identify factors associated with TTR in a large, international clinical trial.
Methods and Results
TTR (international normalized ratio [INR] 2.0 to 3.0) was determined using standard linear interpolation in patients randomized to warfarin in the ROCKET AF trial. Factors associated with TTR at the individual patient level (i‐TTR) were determined via multivariable linear regression. Among 6983 patients taking warfarin, recruited from 45 countries grouped into 7 regions, the mean i‐TTR was 55.2% (SD 21.3%) and the median i‐TTR was 57.9% (interquartile range 43.0% to 70.6%). The mean time with INR <2 was 29.1% and the mean time with an INR >3 was 15.7%. While multiple clinical features were associated with i‐TTR, dominant determinants were previous warfarin use (mean i‐TTR of 61.1% for warfarin‐experienced versus 47.4% in VKA‐naïve patients) and geographic region where patients were managed (mean i‐TTR varied from 64.1% to 35.9%). These effects persisted in multivariable analysis. Regions with the lowest i‐TTRs had INR distributions shifted toward lower INR values and had longer inter‐INR test intervals.
Conclusions
Independent of patient clinical features, the regional location of medical care is a dominant determinant of variation in i‐TTR in global studies of warfarin. Regional differences in mean i‐TTR are heavily influenced by subtherapeutic INR values and are associated with reduced frequency of INR testing.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique identifier: NCT00403767.
Keywords: anticoagulants, arrhythmia, embolism, prevention, risk factors
Introduction
Both the efficacy and safety of warfarin anticoagulation in patients with atrial fibrillation (AF) are strongly dependent on the intensity of anticoagulation measured as the international normalized ratio (INR). The risk of ischemic stroke increases with INR levels <1.8, and the risk of intracranial hemorrhage increases sharply at INR levels >3.5.1 These findings support the standard “therapeutic” INR range of 2.0 to 3.0 for atrial fibrillation.2–4 A commonly used summary of the quality of warfarin anticoagulation is the linearly interpolated percent time in the therapeutic range (TTR).5–7 While many patient‐ and system‐level variables have been demonstrated to affect the INR, and there have been analyses of variation of average TTR at the institutional or geographic level,8–9 there are relatively few large studies assessing the impact of patient features on TTR at the level of the individual patient.10 In the current study, we explored individual and regional determinants of TTR among patients randomly allocated to warfarin in the global ROCKET AF (Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) double‐blind trial of rivaroxaban versus adjusted‐dose warfarin in patients with atrial fibrillation.11–12
Methods
The design, conduct, and main results of the ROCKET AF trial have been presented previously.11–12 In brief, rivaroxaban (20 mg daily; 15 mg daily in patients with creatinine clearance of 30 to 49 mL/min) was compared with adjusted‐dose warfarin (INR point target of 2.5, INR range 2.0 to 3.0) for the prevention of stroke or systemic embolism. Patients with electrocardiographically documented nonvalvular atrial fibrillation at moderate to high risk of stroke were recruited at 1178 participating sites in 45 countries. Elevated risk was indicated by a history of stroke, transient ischemic attack (TIA), or systemic embolism or ≥2 of the following: heart failure or left ventricular ejection fraction ≤35%, hypertension, age ≥75 years, or diabetes mellitus (CHADS2 score ≥2).13 The proportion of patients without prior ischemic stroke, TIA, or systemic embolism and ≤2 risk factors was limited to 10% of the cohort by region; the remainder required either prior thromboembolism or ≥3 risk factors. Investigators were chosen on the basis of performance in clinical trials and access to large clinical practices that included patients with atrial fibrillation. We do not have comprehensive information on recruiting physicians' specialty status. Warfarin dosing was managed by local physicians based on INR values generated by a standard point‐of‐care device (HemoSense, San Jose, CA). While physicians were reminded about the INR target of the trial and the need for monthly INR tests even when patients' anticoagulation status was stable,14 the study did not provide specific treatment algorithms for anticoagulation management. Patients with <6 weeks of exposure to vitamin K antagonist (VKA) medication immediately before entry into the trial were considered VKA naïve.
Statistical Analysis
For the current analyses, patients were included if they had been assigned to warfarin in the ROCKET AF trial and took ≥1 dose of warfarin and had ≥1 INR test. Daily INR values between tests were imputed using the Rosendaal technique,6 and individual patient‐level TTR (i‐TTR) was calculated as the proportion of daily values within a strict range of INR 2.0 to 3.0. This included time during the initiation of warfarin at the start of the trial and after temporary interruptions but did not include time during temporary interruptions of ≥7 days or any time after permanent discontinuation. Only 0.18% of inter‐INR test intervals were >8 weeks. Univariable relationships between baseline variables and i‐TTR were assessed with single‐predictor linear regression models. A multivariable model was developed using multiple linear regression in which a set of independent predictors was chosen in stepwise fashion from a set of candidate predictors. These candidates were age, sex, geographic region, body mass index, systolic and diastolic blood pressures, atrial fibrillation type, hypertension, diabetes, prior stroke or TIA, coronary artery disease, chronic obstructive pulmonary disease, peripheral artery disease, prior gastrointestinal bleed, liver disease, alcohol consumption in the past 12 months, CHADS2 score, estimated glomerular filtration rate (Modification of Diet in Renal Disease equation),15 hemoglobin, patient medications, and type of prior VKA experience. Only variable values at entry to the study were used. Multivariable models were developed both with and without a random effect for center; we report only the results for the models without a random effect for center, because both modeling approaches produced highly similar results. Regional and country mean i‐TTRs were unweighted averages of i‐TTR values for all individuals within the given region or country, respectively.
The relationship between geographic region and i‐TTR was further characterized using linear regression models with region as the only predictor within subgroups defined by prior VKA experience, both for i‐TTR and for i‐TTR excluding the first 90 days of warfarin therapy. For the current analysis, we grouped the countries involved in the ROCKET AF trial into the following regions: East Asia (China, Hong Kong, Korea, Malaysia, Philippines, Singapore, Thailand, and Taiwan); India; Eastern Europe (Bulgaria, Czech Republic, Greece, Hungary, Lithuania, Poland, Romania, Russia, Turkey, and Ukraine); Western Europe and similar (Western Europe/similar: Australia, Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Great Britain, Israel, Italy, Netherlands, Norway, New Zealand, and Sweden); South Africa; Latin America (Argentina, Brazil, Chile, Colombia, Mexico, Peru, and Venezuela); and Canada/United States. These regional groupings were modified from those used in the primary trial report to provide more cultural and ethnic homogeneity.12 Analyses done with the original regional groupings reproduced the same patterns of regional effect on average i‐TTR although the overall R2 values for the multivariable models were modestly reduced (data not shown). We did not include terms for both region and race in the same multivariable models because the 2 were almost completely collinear. We summarize stroke risk using the CHADS2 score.13 Statistical significance of differences in the width of distributions of INR values across regions was assessed using the Miller jackknife technique.16
Human Subjects
All individuals enrolled in the study gave informed consent. All appropriate national regulatory authorities and ethics committees at participating centers approved the study. An international executive committee designed the study and takes responsibility for the accuracy and completeness of all analyses.
Results
Baseline Patient Features
The ROCKET AF trial recruited atrial fibrillation patients at high risk for ischemic stroke. In the subpopulation (n=6983) included in the current analysis, the mean age was 71 years (median 73 years), 61% were male, 52% had had a prior stroke or TIA, and the mean CHADS2 stroke risk score was 3.3. A total of 83% were white, 12% were Asian, and there was a small representation of other racial/ethnic groups. Enrolled patients came from a broad set of geographic regions: 38% from Eastern Europe, 19% from Canada/United States, 16% from Western Europe/similar, 13% from Latin America, and 10% from East Asia (Table1). Thirty‐seven percent of patients were VKA naïve. Of the 4387 patients taking VKAs before entry into the trial, 1334 were not taking warfarin (Table2). Detailed features of patients participating in the ROCKET AF trial, stratified by geographic region, are presented in Table S1.
Table 1.
Mean i‐TTR by Baseline Characteristics: Clinical and Demographic Features
| Baseline Variable | N (%) | i‐TTR (Mean %) | Univariable P Value |
|---|---|---|---|
| Age, y | <0.0001 | ||
| <73 | 3487 (50) | 53.6±20.9 | |
| ≥73 | 3496 | 56.8±21.5 | |
| Sex | <0.0001 | ||
| Male | 4242 (61) | 56.4±21.2 | |
| Female | 2741 | 53.3±21.3 | |
| Race | <0.0001 | ||
| White | 5829 (83) | 56.3±20.9 | |
| African American | 83 (1) | 51.9±21.0 | |
| Asian | 872 (12) | 48.3±22.4 | |
| American Indian/Alaskan | 10 | 51.2±19.1 | |
| Hawaiian/ Pacific Islander | 4 | 60.1±12.5 | |
| Other | 185 (3) | 52.6±21.7 | |
| Region | <0.0001 | ||
| East Asia | 727 (10) | 50.4±21.4 | |
| India | 130 (2) | 35.9±23.3 | |
| Eastern Europe | 2663 (38) | 49.7±21.2 | |
| Western Europe/similar | 1088 (16) | 63.2±18.5 | |
| South Africa | 124 (2) | 54.8±22.1 | |
| Latin America | 924 (13) | 55.2±20.0 | |
| Canada/ United States | 1327 (19) | 64.1±18.2 | |
| BMI, kg/m2 | 0.0003 | ||
| <28 | 3426 (49) | 54.3±21.4 | |
| ≥28 | 3553 | 56.0±21.1 | |
| Systolic BP, mm Hg | 0.0005 | ||
| <130 | 2675 (38) | 56.8±20.8 | |
| ≥130 | 4300 | 54.2±21.5 | |
| AF type | 0.79 | ||
| Persistent | 5648 (81) | 55.1±21.5 | |
| Paroxysmal | 1239 (18) | 55.3±20.5 | |
| New onset/ diagnosis | 96 (1) | 56.5±19.5 | |
| Hypertension | 0.055 | ||
| Absence | 649 | 56.7±21.1 | |
| Presence | 6334 (91) | 55.0±21.3 | |
| Diabetes | 0.61 | ||
| Absence | 4230 | 55.3±21.3 | |
| Presence | 2753 (39) | 55.0±21.2 | |
| Prior stroke or TIA | 0.087 | ||
| Absence | 3338 | 55.6±21.2 | |
| Presence | 3645 (52) | 54.8±21.3 | |
| Congestive heart failure | <0.0001 | ||
| Absence | 2642 | 59.0±20.7 | |
| Presence | 4340 (62) | 52.9±21.2 | |
| eGFR (MDRD),15 mL/min per 1.73 m2 | 0.016 | ||
| <68 | 3431 (49) | 55.3±21.2 | |
| ≥68 | 3548 | 55.0±21.4 | |
| Baseline hemoglobin, g/L | 0.0052 | ||
| <10.0 | 2469 (35) | 53.5±21.5 | |
| ≥10.0 | 4510 | 56.1±21.1 | |
| CAD | <0.0001 | ||
| Absence | 5294 | 54.6±21.3 | |
| Presence | 1689 (24) | 57.0±21.0 | |
| COPD | 0.053 | ||
| Absence | 6259 | 55.4±21.3 | |
| Presence | 719 (10) | 53.7±21.1 | |
| PAD | 0.047 | ||
| Absence | 6558 | 55.1±21.3 | |
| Presence | 425 (6) | 57.2±20.7 | |
| Prior GI bleed | <0.0001 | ||
| Absence | 6713 | 55.0±21.3 | |
| Presence | 270 (4) | 60.7±19.3 | |
| Liver disease | 0.012 | ||
| Absence | 6616 | 55.3±21.2 | |
| Presence | 367 (5) | 52.5±21.9 | |
| Alcohol consumption (past 12 mo) | <0.0001 | ||
| Abstinent | 4516 (65) | 53.2±21.4 | |
| Light | 2117 (30) | 58.6±20.5 | |
| Moderate | 302 (4) | 62.1±19.9 | |
| Heavy | 47 (1) | 48.8±22.2 | |
| CHADS2 score* | <0.0001 L | ||
| 1* | 2 | 33.3±47.1 | 0.0015 Q |
| 2 | 920 (13) | 59.3±19.7 | |
| 3 | 3094 (44) | 55.1±21.3 | |
| 4 | 1963 (28) | 54.3±21.7 | |
| 5 | 852 (12) | 53.6±21.3 | |
| 6 | 152 (2) | 53.4±21.5 | |
Continuous predictors are split at median for summarizing TTR but tested as continuous. Missing data occurred in fewer than 0.2% of records for any variable. Race: for purposes of testing, race groups were white, Asian, and all others. i‐TTR indicates individual patient‐level time in therapeutic range; BMI, body mass index; BP, blood pressure; AF, atrial fibrillation; TIA, transient ischemic attack; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral artery disease; GI, gastrointestinal.
CHADS2: linear (L) and quadratic (Q) models tested.
CHADS2=1: combined with CHADS2=2 for testing.
Table 2.
Mean i‐TTR by Baseline Characteristics: Medication Use
| Baseline Variable | N | i‐TTR Mean % | Univariable P Value |
|---|---|---|---|
| Prior VKA experience | <0.0001 | ||
| VKA naïve | 2596 (37) | 47.4±22.1 | |
| VKA experienced but warfarin naïve | 1334 (19) | 56.9±19.0 | |
| Warfarin experienced | 3053 (44) | 61.1±19.3 | |
| Aspirin | <0.0001 | ||
| Absence | 4949 (71) | 56.4±20.8 | |
| Presence | 2034 | 52.2±22.1 | |
| ACE inhibitor | 0.070 | ||
| Absence | 3223 | 55.7±21.3 | |
| Presence | 3760 (54) | 54.8±21.2 | |
| ACE inhibitor or ARB | 0.86 | ||
| Absence | 1808 | 55.3±21.4 | |
| Presence | 5175 (74) | 55.2±21.2 | |
| Amiodarone | <0.0001 | ||
| Absence | 6444 | 55.7±21.2 | |
| Presence | 539 (8) | 49.3±20.6 | |
| Digitalis | 0.0037 | ||
| Absence | 4278 | 55.8±21.3 | |
| Presence | 2705 (39) | 54.3±21.2 | |
| β‐Blocker | 0.0003 | ||
| Absence | 2411 | 53.9±21.8 | |
| Presence | 4572 (65) | 55.8±21.0 | |
| Loop diuretic | 0.75 | ||
| Absence | 4577 | 55.1±21.3 | |
| Presence | 2406 (34) | 55.3±21.3 | |
| Statin | <0.0001 | ||
| Absence | 3968 | 52.7±21.6 | |
| Presence | 3015 (43) | 58.4±20.3 | |
There were no missing data for this Table. i‐TTR indicates individual patient‐level time in therapeutic range; VKA, vitamin K antagonist; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker.
Univariable Associations With i‐TTR
The overall mean i‐TTR was 55.2% (SD 21.3%), and the median i‐TTR was 57.9% (interquartile range [IQR] 43.0% to 70.6%). The mean time with INR <2 was 29.1%, and the mean time with an INR >3 was 15.7%. When the definition of therapeutic range was expanded to INR 1.8 to 3.5, the mean time in this therapeutic range was 74.5% (SD 21.8%) and the median was 80.4% (IQR 65.9% to 89.9%). In univariable analysis, multiple patient features were associated with i‐TTR (Tables1 and 2). Younger patients, female patients, those with heart failure, and patients with higher CHADS2 scores had lower mean i‐TTR levels. Asian patients had a mean i‐TTR a full 8% lower than did white patients. Patients who reported light to moderate alcohol consumption had higher i‐TTR levels than did those who were alcohol abstinent. Prior experience with warfarin had a notably strong association with i‐TTR. Warfarin‐experienced patients had a mean i‐TTR of 61.1% compared with a mean of 47.4% for VKA‐naïve patients. Patients who had previously taken non–warfarin VKAs had a mean i‐TTR of 56.9%. Multiple other medications taken at entry into the study were statistically associated with i‐TTR. During follow‐up, 2287 patients had ≥1 hospitalizations. There was no decrease in mean i‐TTR among these hospitalized patients compared with those avoiding hospitalization during the trial (data not shown).
Association of Geographic Region and Mean i‐TTR
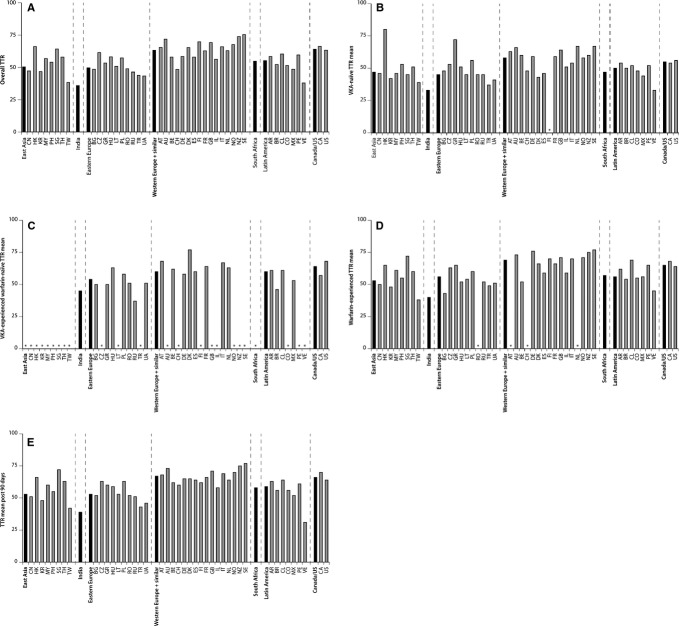
There was remarkable variation in mean i‐TTR across geographic regions, ranging from 36% in the small group of patients treated in India to 50% for patients treated in Eastern Europe and East Asia to 63% and 64%, respectively, for patients treated in Western Europe/similar and Canada/United States (Figure 1a). Across regions, there were marked differences in the proportion of patients naïve to VKAs, ranging from 9.7% of patients in Canada/United States to 53% in Eastern Europe. In Canada/United States, East Asia, and South Africa, the predominant VKA used before the trial was warfarin. But, in the remaining regions, many patients who were VKA experienced were still warfarin naïve (Table3). Nonetheless, the qualitative regional patterns of i‐TTR across regions persisted after stratification by patients' experience with VKAs, although the absolute differences in mean TTR were modestly reduced. Patients treated in Canada/United States and Western Europe/similar still had the highest TTRs, whether patients were experienced with warfarin or completely VKA naïve. The point estimates were modestly higher for Western Europe/similar versus Canada/United States in these stratified analyses (Table3). Country‐level patterns of mean i‐TTR stratified by warfarin experience at baseline are presented in Figure 1b through 1d.
Figure 1.
A, Overall distribution of mean i‐TTR (%) by country (gray bars), grouped by region (black bars). B, Distribution of mean i‐TTR (%) by country (gray bars), grouped by region (black bars), for patients naïve to vitamin K antagonist therapy at baseline. C, Distribution of mean i‐TTR (%) by country (gray bars), grouped by region (black bars), for patients experienced with vitamin K antagonist therapy but naïve to warfarin treatment at baseline. D, Distribution of mean i‐TTR (%) by country (gray bars), grouped by region (black bars), for warfarin‐experienced patients at baseline. E, Distribution of overall mean i‐TTR (%) by country (gray bars), grouped by region (black bars), excluding the first 90 days in the trial. TTR indicates time in therapeutic range at individual patient level; East Asia: CN, China; HK, Hong Kong; KR, Korea; MY, Malaysia; PH, Philippines; SG, Singapore; TH, Thailand; TW, Taiwan; Eastern Europe: BG, Bulgaria; CZ, Czech Republic; GR, Greece; HU, Hungary; LT, Lithuania; PL, Poland; RO, Romania; RU, Russia; TR, Turkey; UA, Ukraine; Western Europe/similar: AT, Austria; AU, Australia; BE, Belgium; CH, Switzerland; DE, Germany; DK, Denmark; ES, Spain; FI, Finland; FR, France; GB, Great Britain; IL, Israel; IT, Italy; NL, Netherlands; NO, Norway; NZ, New Zealand; SE, Sweden; Latin America: AR, Argentina; BR, Brazil; CL, Chile; CO, Colombia; MX, Mexico; PE, Peru; VE, Venezuela.
Table 3.
Regional Mean i‐TTR by Prior VKA Experience
| N | i‐TTR, mean % | SE | Median (25th, 75th) | Parameter Estimate | P Value | |
|---|---|---|---|---|---|---|
| VKA naïve | ||||||
| East Asia | 356 | 47.3 | 1.1 | 49 (34, 63) | −7.75 | 0.0005 |
| India | 87 | 32.6 | 2.5 | 29 (13, 49) | −22.46 | <0.0001 |
| Eastern Europe | 1414 | 45.2 | 0.6 | 47 (31, 61) | −9.90 | <0.0001 |
| Western Europe/similar | 233 | 57.8 | 1.3 | 62 (48, 72) | 2.72 | 0.25 |
| South Africa | 29 | 46.5 | 4.8 | 47 (26, 64) | −8.57 | 0.054 |
| Latin America | 348 | 50.1 | 1.1 | 54 (37, 64) | −5.00 | 0.025 |
| Canada/United States | 129 | 55.1 | 1.8 | 58 (46, 70) | Ref | |
| VKA experienced but warfarin naïve* | ||||||
| East Asia | 0 | |||||
| India | 20 | 45.5 | 5.1 | 47 (28, 63) | −14.72 | 0.0006 |
| Eastern Europe | 619 | 53.6 | 0.8 | 55 (43, 68) | −6.53 | <0.0001 |
| Western Europe/similar | 399 | 60.1 | 0.9 | 63 (50, 73) | Ref | |
| South Africa | 0 | |||||
| Latin America | 293 | 60.1 | 1.0 | 62 (50, 72) | −0.08 | 0.96 |
| Canada/United States | 3 | 64.1 | 3.8 | 65 (57, 70) | ||
| Warfarin experienced | ||||||
| East Asia | 371 | 53.3 | 1.1 | 56 (41, 68) | −11.83 | <0.0001 |
| India | 23 | 39.9 | 4.6 | 42 (27, 52) | −25.25 | <0.0001 |
| Eastern Europe | 630 | 55.9 | 0.7 | 58 (45, 70) | −9.16 | <0.0001 |
| Western Europe/similar | 456 | 68.7 | 0.7 | 70 (60, 79) | 3.61 | 0.64 |
| South Africa | 95 | 57.3 | 2.1 | 63 (46, 71) | −7.76 | <0.0001 |
| Latin America | 283 | 56.4 | 1.2 | 59 (45, 71) | −8.75 | <0.0001 |
| Canada/United States | 1195 | 65.1 | 0.5 | 67 (55, 78) | Ref | |
i‐TTR indicates individual patient‐level time in therapeutic range; VKA, vitamin K antagonist.
Because there are only 3 patients in this group in North America, Western Europe is used as the reference instead. There are no patients in this group in East Asia or South Africa.
By 90 days, patients newly started on warfarin should have achieved a relatively stable dose. Analyses excluding the first 90 days of follow‐up resulted in improved regional mean i‐TTRs, particularly in regions with a high proportion of patients naïve to warfarin. Despite these changes, the regional‐ (Table4) and country‐level (Figure1e) differences persisted. When we further stratified these analyses starting at 90 days by patients' previous exposure to warfarin and VKAs, the regional TTR patterns were preserved (data not shown).
Table 4.
Regional Mean i‐TTR After First 90 Days of Follow‐up
| Region | N | i‐TTR, mean % | SD | Median (25th, 75th) | Parameter Estimate | P Value |
|---|---|---|---|---|---|---|
| East Asia | 677 | 53.3 | 21.7 | 56 (40, 67) | −12.52 | <0.0001 |
| India | 115 | 39.5 | 25.2 | 42 (21, 56) | −26.38 | <0.0001 |
| Eastern Europe | 2462 | 53.0 | 21.5 | 55 (40, 68) | −12.82 | <0.0001 |
| Western Europe/similar | 1019 | 66.6 | 17.7 | 69 (58, 79) | 0.76 | 0.37 |
| South Africa | 115 | 57.6 | 21.1 | 59 (46, 74) | −8.19 | <0.0001 |
| Latin America | 875 | 59.0 | 20.0 | 61 (48, 74) | −6.84 | <0.0001 |
| Canada/United States | 1244 | 65.8 | 18.7 | 68 (56, 79) | Ref |
i‐TTR indicates individual patient‐level time in therapeutic range.
Multivariable Associations With i‐TTR
In multiple linear regression models of mean i‐TTR, many features remained statistically significant (Table5). Particularly strong effects were seen for patients who were VKA naïve (−9.1%, compared with warfarin experienced), women (−9.3%), patients with heart failure (−3.2%), patients with chronic obstructive pulmonary disease (−2.9%), patient using amiodarone at study entry (−3.6%), and across categories of use of alcohol. Even after adjustment for all other significant features (but leaving out race because of collinearity with region), the effect of geographic region remained remarkable. With Canada/United States patients as the referent group, the mean i‐TTR was an absolute 8.6% lower for patients in Eastern Europe, 8.7% lower in East Asia, 4.7% lower in Latin America, and essentially the same for patients treated in Western Europe/similar. The small sample of patients in India had a particularly low average i‐TTR. Although there were several strong determinants of mean i‐TTR, the overall multivariable model R2 was only 16%. By percent of variance explained (ie, partial R2), the strongest risk factors were VKA experience (3.7%) and geographic region (4.3%).
Table 5.
Predictors of i‐TTR (%) in Warfarin Patients by Multiple Linear Regression Modeling*: Geographical Site of Recruitment Grouped as Regions
| Baseline Characteristic | Multivariable | |||
|---|---|---|---|---|
| Parameter Estimate | F | P Value | Partial R2 | |
| VKA experience | ||||
| Warfarin experienced | Ref | 129.71 | <0.0001 | 0.0374 |
| VKA experienced/warfarin naïve | −2.221 | |||
| VKA naïve | −9.138 | |||
| Region | ||||
| Canada/United States | Ref | 49.24 | <0.0001 | 0.0426 |
| Western Europe/similar | 0.680 | |||
| Latin America | −4.692 | |||
| South Africa | −8.347 | |||
| Eastern Europe | −8.598 | |||
| East Asia | −8.680 | |||
| India | −20.559 | |||
| CHF | −3.172 | 37.05 | <0.0001 | 0.0053 |
| Female | −9.324 | 22.74 | <0.0001 | 0.0033 |
| COPD | −2.859 | 13.34 | 0.0003 | 0.0019 |
| eGFR, 10 mL/min per 1.73 m2 | 0.0030 | |||
| Linear | −6.474 | 11.36 | 0.0008 | |
| Quadratic | 0.169 | 4.77 | 0.029 | |
| Hemoglobin, 2 g/L | 0.0029 | |||
| Linear | −8.794 | 11.17 | 0.0008 | |
| Quadratic | 0.276 | 5.02 | 0.025 | |
| Systolic BP | −0.0453 | 9.39 | 0.0022 | 0.0014 |
| BMI | 0.0022 | |||
| Linear | 0.677 | 7.38 | 0.0066 | |
| Quadratic | −0.0086 | 5.13 | 0.024 | |
| Diabetes | −1.208 | 5.77 | 0.016 | 0.0008 |
| Alcohol consumption, 12 mo | ||||
| Abstinent | Ref | 5.76 | 0.0006 | 0.0025 |
| Light | 1.772 | |||
| Moderate | 3.012 | |||
| Heavy | −4.921 | |||
| Medications at entry to the trial | ||||
| Amiodarone | −3.608 | 16.42 | <0.0001 | 0.0024 |
| Statin | 1.682 | 11.36 | 0.0008 | 0.0016 |
| Aspirin | −1.111 | 4.10 | 0.043 | 0.0006 |
i‐TTR indicates individual patient‐level time in therapeutic range; VKA, vitamin K antagonist; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; BP, blood pressure; BMI, body mass index.
Variation in i‐TTR Across Countries
There was substantial variation in i‐TTR across the 45 countries in ROCKET AF, ranging from a mean of 36% to 75%. Substitution of individual countries for geographic regions in the multiple linear regression model led to an increase in the overall model R2 from 16% to 19% (Table6). Even within regions, there was considerable variability across countries (Figure1). Of particular interest, the mean TTR was 47% in China and 38% in Taiwan but 66% in Hong Kong and 64% in Singapore. Ninety‐nine percent of the patients in all 4 of these regions were identified as being of Asian race. When we substituted patient's race for patient's region in the multivariable model, the overall model R2 deteriorated to 12.8% (Table7).
Table 6.
Predictors of i‐TTR (%) in Warfarin Patients by Multiple Linear Regression Modeling: Geographical Site of Recruitment Grouped as Countries
| Baseline Characteristic | Multivariable | |||
|---|---|---|---|---|
| Parameter Estimate | F | P Value | Partial R2 | |
| VKA experience | 74.94 | <0.0001 | 0.0217 | |
| Warfarin experienced | Ref | |||
| VKA experienced/warfarin naïve | −7.399 | |||
| VKA naïve | −2.153 | |||
| CHF | −2.373 | 20.19 | <0.0001 | 0.0029 |
| Country | 13.73 | <0.0001 | 0.0876 | |
| United States | Ref | |||
| Argentina | −1.486 | |||
| Australia | 7.531 | |||
| Austria | 5.945 | |||
| Belgium | −3.014 | |||
| Brazil | −7.721 | |||
| Bulgaria | −9.669 | |||
| Canada | 2.801 | |||
| Chile | −0.302 | |||
| China | −11.081 | |||
| Colombia | −8.315 | |||
| Czech Republic | −1.641 | |||
| Denmark | 1.548 | |||
| Finland | 4.294 | |||
| France | 1.180 | |||
| Germany | −1.600 | |||
| Greece | −7.210 | |||
| Hong Kong | 3.003 | |||
| Hungary | −3.170 | |||
| India | −21.495 | |||
| Israel | −4.492 | |||
| Italy | 3.818 | |||
| Korea | −14.885 | |||
| Lithuania | −10.173 | |||
| Malaysia | −4.867 | |||
| Mexico | −10.713 | |||
| Netherlands | −0.160 | |||
| New Zealand | 9.905 | |||
| Norway | 4.564 | |||
| Peru | −2.116 | |||
| Philippines | −3.744 | |||
| Poland | −1.884 | |||
| Romania | −10.103 | |||
| Russia | −10.903 | |||
| Singapore | 2.828 | |||
| South Africa | −7.806 | |||
| Spain | −2.821 | |||
| Sweden | 12.009 | |||
| Switzerland | −10.068 | |||
| Taiwan | −20.772 | |||
| Thailand | −3.865 | |||
| Turkey | −16.503 | |||
| Ukraine | −13.930 | |||
| United Kingdom | 6.014 | |||
| Venezuela | −20.635 | |||
| COPD | −2.669 | 11.91 | 0.0006 | 0.0017 |
| Female | −6.275 | 10.24 | 0.0014 | 0.0015 |
| Diabetes | −1.598 | 10.22 | 0.0014 | 0.0015 |
| BMI | 0.0022 | |||
| Linear | 0.744 | 9.14 | 0.0025 | |
| Quadratic | −0.010 | 6.58 | 0.010 | |
| Systolic BP | −0.037 | 6.28 | 0.012 | 0.0009 |
| Hemoglobin, 2 g/L | 0.0014 | |||
| Linear | −4.654 | 3.11 | 0.078 | |
| Quadratic | 0.109 | 0.78 | 0.38 | |
| eGFR, 10 mL/min per 1.73 m2 | 0.0013 | |||
| Linear | −3.478 | 3.26 | 0.071 | |
| Quadratic | 0.073 | 0.90 | 0.34 | |
| Alcohol consumption, 12 mo | 2.41 | 0.065 | 0.0010 | |
| Abstinent | Ref | |||
| Light | 0.838 | |||
| Moderate | 1.624 | |||
| Heavy | −5.340 | |||
| Medications at entry to the trial | ||||
| Amiodarone | −2.616 | 8.74 | 0.0031 | 0.0013 |
| Statin | 0.818 | 2.63 | 0.11 | 0.0004 |
| Aspirin | −0.619 | 1.25 | 0.26 | 0.0002 |
i‐TTR indicates individual patient‐level time in therapeutic range; VKA, vitamin K antagonist; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate.
Table 7.
Predictors of i‐TTR (%) in Warfarin Patients by Multiple Linear Regression Modeling Using Race in Model Rather Than Region
| Baseline Characteristic | Multivariable | |||
|---|---|---|---|---|
| Parameter Estimate | F | P Value | Partial R2 | |
| VKA experience | 239.37 | <0.0001 | 0.0690 | |
| Warfarin experienced | Ref | |||
| VKA experienced/warfarin naïve | −11.965 | |||
| VKA naïve | −4.149 | |||
| CHF | −4.942 | 92.90 | <0.0001 | 0.0132 |
| Female | −14.294 | 37.94 | <0.0001 | 0.0054 |
| Race | 20.47 | <0.0001 | 0.0088 | |
| White | Ref | |||
| Black | 1.924 | |||
| Asian | −5.919 | |||
| Other | −3.836 | |||
| eGFR, 10 mL/min per 1.73 m2 | 0.0048 | |||
| Linear | −11.123 | 24.26 | <0.0001 | |
| Quadratic | 0.331 | 14.26 | 0.0002 | |
| Hemoglobin, 2 g/L | 0.0043 | |||
| Linear | −15.327 | 24.42 | <0.0001 | |
| Quadratic | 0.559 | 15.92 | <0.0001 | |
| Systolic BP | −0.055 | 13.42 | 0.0003 | 0.0019 |
| Alcohol consumption, 12 mo | 10.09 | <0.0001 | 0.0044 | |
| Abstinent | Ref | |||
| Light | 2.427 | |||
| Moderate | 4.541 | |||
| Heavy | −3.944 | |||
| COPD | −2.305 | 8.44 | 0.0037 | 0.0012 |
| BMI | 0.0022 | |||
| Linear | 0.422 | 2.80 | 0.095 | |
| Quadratic | −0.004 | 1.13 | 0.29 | |
| Diabetes | −0.638 | 1.57 | 0.21 | 0.0002 |
| Medications at entry to the trial | ||||
| Amiodarone | −4.395 | 23.89 | <0.0001 | 0.0034 |
| Statin | 2.568 | 26.35 | <0.0001 | 0.0038 |
| Aspirin | −0.782 | 2.02 | 0.16 | 0.0003 |
i‐TTR indicates individual patient‐level time in therapeutic range; VKA, vitamin K antagonist; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; BP, blood pressure; COPD, chronic obstructive pulmonary disease; BMI, body mass index.
Distribution of INR Values and Management of Test Results
Geographic regional variation in mean i‐TTR levels was largely due to time at INR <2.0 (Figure2, Table8). For Canada/United States patients, the mean time at INR <2.0 was 19.9%. In contrast, for Eastern European patients, the mean time at INR <2.0 was 35.2%, and for patients in East Asia, the time at INR <2.0 was 37.1%. The differences in mean time at INR >3.0 were much smaller and contributed much less to the differences in i‐TTR across regions. If we define “dangerously” low as INR <1.7, the regional pattern persisted with patients in Canada/United States and Western Europe/similar dangerously low ≈8% of the time compared with 19.7% of the time in Eastern Europe and 18.8% in East Asia. Canada/US and Western Europe/similar centers were in the “expanded” therapeutic range of INR 1.8 to 3.5 a mean of 84% and 83% of the time, respectively. By contrast, patients in East Asia and Eastern Europe were in this expanded range ≈70% of the time.
Figure 2.
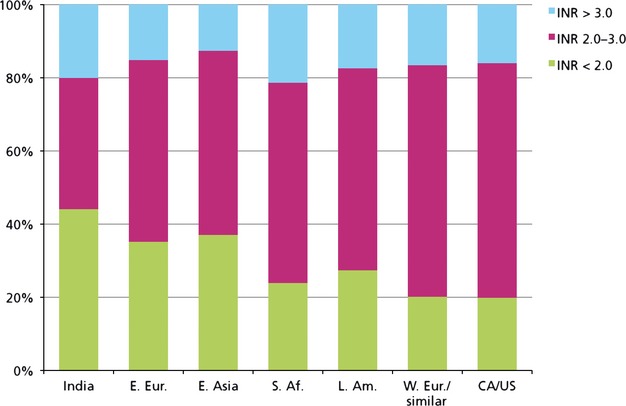
Distribution of times in therapeutic (INR 2.0 to 3.0), low, and high INR range by geographic region. INR indicates international normalized ratio.
Table 8.
i‐TTR, Time in Other INR Ranges, and INR Test Results and Frequency by Region
| Parameter | Region | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistic | Total | East Asia | India | EasternEurope | Western Europe/similar | South Africa | Latin America | Canada/United States | |
| i‐TTR | |||||||||
| i‐TTR (INR 2.0 to 3.0, %) | N (patients) | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 55.2±21.27 | 50.4±21.42 | 35.9±23.34 | 49.7±21.16 | 63.2±18.48 | 54.8±22.011 | 55.2±20.00 | 64.1±18.23 | |
| (25th, 75th percentile) | (43.0, 70.6) | (37.6, 64.8) | (18.6, 50.7) | (36.8, 64.8) | (53.7, 75.9) | (42.9, 70.6) | (44.5, 69.3) | (54.8, 77.0) | |
| Time above TR (INR >3.0, %) | N | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 15.7±13.13 | 12.6±12.83 | 20.0±21.34 | 15.1±12.61 | 16.5±13.20 | 21.3±18.10 | 17.4±12.95 | 16.0±12.28 | |
| (25th, 75th percentile) | (7.0, 21.5) | (3.7, 17.0) | (6.1, 25.5) | (6.2, 21.2) | (7.4, 22.7) | (8.7, 28.3) | (9.0, 23.5) | (8.3, 21.1) | |
| Time below TR (INR <2.0, %) | N | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 29.1±21.94 | 37.1±23.00 | 44.1±25.87 | 35.2±22.78 | 20.2±17.88 | 23.9±23.25 | 27.4±20.01 | 19.9±16.16 | |
| (25th, 75th percentile) | (13.6, 38.6) | (20.6, 51.0) | (25.2, 61.0) | (19.2, 46.3) | (8.7, 26.2) | (7.3, 32.9) | (14.2, 34.1) | (9.1, 26.7) | |
| Time dangerously out of range—low (INR <1.7) | N | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 14.8±19.10 | 18.8±19.92 | 30.9±25.73 | 19.7±21.58 | 8.0±13.19 | 11.9±18.61 | 13.8±17.77 | 7.7±11.74 | |
| (25th, 75th percentile) | (2.7, 18.7) | (5.6, 27.0) | (13.6, 48.0) | (5.2, 26.1) | (1.1, 9.5) | (1.1, 14.2) | (3.2, 15.9) | (1.2, 9.7) | |
| Time in INR range of 1.8 to 3.5 | N | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 74.5±21.75 | 69.9±22.37 | 51.8±27.30 | 68.8±22.90 | 82.7±17.19 | 75.6±23.62 | 74.8±20.36 | 83.6±15.64 | |
| (25th, 75th percentile) | (65.9, 89.9) | (59.2, 85.9) | (33.1, 72.2) | (58.6, 85.7) | (77.1, 94.1) | (69.9, 90.8) | (67.8, 88.4) | (78.2, 93.8) | |
| INR tests (multiple observations/patient) | N (tests) | 181 640 | 17 218 | 2415 | 64 644 | 30 439 | 3131 | 22 134 | 41 659 |
| Average INR | |||||||||
| Mean±SD | 2.4±0.87 | 2.3±0.83 | 2.4±1.21 | 2.4±0.93 | 2.5±0.80 | 2.5±0.86 | 2.4±0.92 | 2.5±0.78 | |
| Median | 2.3 | 2.2 | 2.1 | 2.2 | 2.4 | 2.4 | 2.3 | 2.4 | |
| (25th, 75th percentile) | (1.8, 2.8) | (1.7, 2.7) | (1.5, 2.8) | (1.7, 2.8) | (2.0, 2.9) | (1.9, 2.9) | (1.8, 2.8) | (2.0, 2.9) | |
| Average No. of tests/patient | Mean±SD | 26±13.3 | 24±10.8 | 19±11.5 | 24±11.6 | 28±13.9 | 25±10.9 | 24±10.6 | 31±17.0 |
| (25th, 75th percentile) | (18, 34) | (17, 32) | (8, 27) | (17, 32) | (19, 37) | (19, 33) | (17, 31) | (19, 43) | |
| Average No. of days between INR measurements | N | 6983 | 727 | 130 | 2663 | 1088 | 124 | 924 | 1327 |
| Mean±SD | 21±5.1 | 23±4.7 | 22±5.6 | 23±5.0 | 20±5.3 | 21±4.8 | 22±4.3 | 19±4.8 | |
| (25th, 75th percentile) | (19, 25) | (22, 26) | (20, 26) | (21, 26) | (16, 24) | (19, 24) | (20, 25) | (17, 23) | |
i‐TTR indicates individual patient‐level time in therapeutic range; INR, international normalized ratio.
The distributions of INR values confirmed that lower i‐TTRs resulted primarily from subtherapeutic INR levels (Table8). The median INR was 2.4 for patients in Canada/United States and Western Europe/similar but 2.2 for patients in Eastern Europe and East Asia and 2.3 for patients in Latin America. The distributions were narrower in Canada/United States and Western Europe/similar with IQRs of 0.9 INR unit compared with East Asia and Latin America with IQRs of 1.0 INR unit and Eastern Europe with an IQR of 1.1 INR units (all P<0.001)
We compared the average number of days between INR measurements (Figure3, Table8). Patients in Canada/United States and Western Europe had the most frequent INR tests at an average interval of 19 and 20 days, respectively. By contrast, patients in Eastern Europe and in East Asia had the least frequent INR testing with an average interval of 23 days (P<0.001). We extended this analysis to compare the time to subsequent INR after an extreme INR value. There was marked variation in median time to a follow‐up INR test after an INR ≤1.5, from 9 days in Western Europe/similar and Canada/United States to 26 and 27 days in Eastern Europe and East Asia, respectively (P<0.001). Similarly, the median time to a subsequent INR test after an INR ≥4.0 was 7 days in Western Europe/similar and Canada/United States versus 25 and 23 days in Eastern Europe and East Asia, respectively (P<0.001). Figure3 displays the distribution of mean inter‐test intervals as a function of initial INR test result. There is an inverted “U” pattern seen in all regions but inter‐test intervals were shortest in regions with the highest TTRs, regardless of initial test result.
Figure 3.
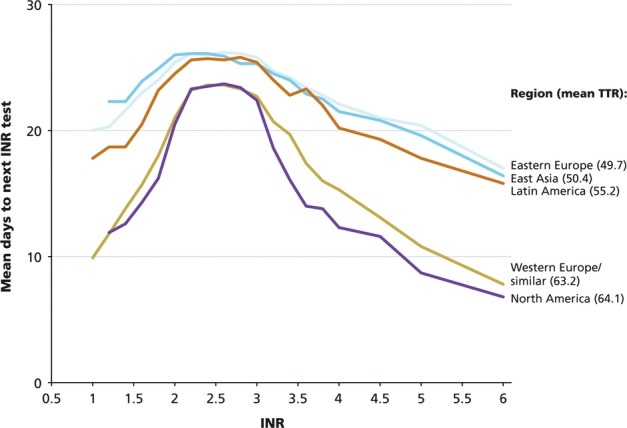
Inter‐INR test interval by value of first INR, stratified by geographic region. INR indicates international normalized ratio; TTR, time in therapeutic range.
Discussion
The linearly interpolated TTR has become a widely accepted measure of the quality of anticoagulation management.7 In the ROCKET AF trial, the mean i‐TTR for patients assigned to warfarin was 55%, lower than that reported for other recent trials of novel anticoagulants,17–19 with a wide range of i‐TTR values. The ROCKET AF trial enrolled patients at particularly high risk of stroke. One of these stroke risk factors, heart failure, was associated with an adjusted 3% decrease in average i‐TTR. However, other standard stroke risk factors in AF (ie, prior stroke/TIA, hypertension, and diabetes) had only modest, if any, univariate effects on i‐TTR and none was selected as a significant predictor of i‐TTR in our multivariable model. The 2 strongest determinants of i‐TTR (by partial R2) were not patient comorbidities but rather pretrial experience with warfarin and geographic site. Patients who were taking warfarin before entry into the trial had an adjusted absolute 9% higher mean i‐TTR than those who were VKA naïve. Interregional mean i‐TTR varied by as much as 21%, even after accounting for patient clinical features and experience with warfarin. Excluding the small number of patients treated in India, interregional differences in mean i‐TTR still spanned an absolute 8.7%. Excluding the first 90 days of warfarin treatment, to allow warfarin‐naïve patients to become warfarin‐experienced, still resulted in large interregional differences in mean TTR. When country was included in the model instead of region, the total variance explained increased. Large regional effects on TTR with similar geographic patterns have been observed in other recent trials.8,20
Poorer i‐TTR primarily reflected INR values below 2.0. The risk of stroke among patients with AF rises steeply the lower the INR value below 2.0.1 The TTR does not distinguish low from very low INR values. When we assessed time in dangerously low INR range (ie, INR <1.7) the regional differences persisted. When we focused specifically on the regional distributions of INR values, we found the lower the mean i‐TTR, the lower the mean INR. For East Asia, in particular, the IQR of INR values was nearly as narrow as that for the regions with higher TTRs, suggesting that physicians were implicitly targeting the low end of the target range of INR 2.0 to 3.0. The fact that the mean TTR for patients treated in Hong Kong and Singapore was much higher than the mean TTR in the remaining countries in East Asia indicates that medical care practices and not race determine anticoagulation control in this region.21 We found, as well, that regions with lower TTR values obtained INR tests less frequently. While follow‐up test intervals were shorter the more extreme the initial INR results, the intervals were still longer in regions with the lowest TTR. Rose et al have proposed as a quality indicator the time to follow‐up INR after an INR ≤1.5 or ≥4.0.22 We found longer test intervals after such extreme INR values in regions with lower mean TTRs.
We have used the distinctively large and geographically diverse cohort of warfarin‐treated patients in the ROCKET AF trial to add to our understanding of the determinants of TTR. Our study benefits from the high quality of information captured at study entry, close longitudinal follow‐up, and standardized INR testing with a single type of point‐of‐care device. There was comprehensive recording of clinically scheduled INR test results. As a consequence of the trial protocol, patients did not go for long periods (ie, >8 weeks) without an INR test, consistent with guideline recommendations.14 This is in contrast to usual clinical care where the distribution of test intervals is broader, adding uncertainty to the calculation of TTR.23
Our study has limitations. As always, our trial‐based results may not generalize completely to usual clinical care. Our regional findings reflect the care provided by a limited set of investigators in any geographic region. As such, we cannot claim that our findings are clearly representative of warfarin management in participating countries. However, it is notable that the regional pattern of our results is similar to those reported by the ACTIVE W trial for the countries where both trials recruited patients.8 In particular, in both the ROCKET AF and ACTIVE W trials the country with the highest i‐TTR was Sweden and a recent survey of anticoagulation in clinical care in Sweden reported a mean i‐TTR of 74%, nearly identical to the value of 75% observed in the ROCKET AF trial.24 We cannot account for the resource constraints or cultural norms underlying different regional practices in managing warfarin. The widely used Rosendaal measurement of i‐TTR uses linear interpolation to impute daily INR values. Other imputation approaches might reduce interregional differences in i‐TTR. Ultimately, while i‐TTR makes sense as a measure of the quality of warfarin management, its quantitative relationship to the net benefit of warfarin therapy is still uncertain.
In conclusion, we found that patient clinical features, such as heart failure, were significant but modest determinants of i‐TTR. Further, we were able to quantify the reduced control of warfarin anticoagulation faced by new users of the drug. However, our most notable finding was the striking influence of geographic region, presumably reflecting different levels of aggressiveness in achieving the INR point target of 2.5, different support systems to manage warfarin, and different regional barriers to frequent INR testing and warfarin dose changes. Such geographic variation in medical practice has become a truism of health care epidemiology.25–26 While our understanding of the determinants of i‐TTR remains incomplete, it is clear that the providers of care, and the systems within which they work, have a profound effect on the quality of anticoagulation.
Sources of Funding
The ROCKET AF trial was supported by research grants from Johnson & Johnson Pharmaceutical Research & Development (Raritan, NJ) and Bayer HealthCare AG (Leverkusen, Germany). The Duke Clinical Research Institute (Durham, NC) coordinated the trial and performed the statistical analyses for this manuscript, independent of the sponsors. Dr Singer was supported, in part, by the Eliot B. and Edith C. Shoolman fund of Massachusetts General Hospital (Boston, MA).
Disclosures
Dr Singer has served as a consultant to Bayer HealthCare, Boehringer Ingelheim, Bristol‐Myers Squibb, CSL Behring, Daiichi Sankyo, Johnson & Johnson, Pfizer, and Sanofi. He is a member of the executive committee of the ROCKET AF trial of rivaroxaban versus warfarin in patients with atrial fibrillation. Ms Hellkamp has no conflicts to report. Dr Piccini has received grants for clinical research from Johnson & Johnson and Boston Scientific and served as a consultant or on advisory boards for Medtronic, Forest Laboratories, Sanofi Aventis, and Johnson & Johnson. Dr Mahaffey has received the following: grant support: significant: AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Momenta Pharmaceuticals, Novartis, Portola, Pozen, Regado Biotechnologies, Sanofi‐Aventis, Schering‐Plough (now Merck), and The Medicines Company. Consulting fees: significant: AstraZeneca and Johnson & Johnson; modest: Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Ortho/McNeill, Pfizer, Polymedix, Sanofi‐Aventis, and Schering‐Plough (now Merck). Dr Lokhnygina has no conflicts to report. Dr Pan is an employee of Johnson & Johnson. Dr Halperin has received honoraria for serving on a steering committee for Johnson & Johnson and Bayer and fees for advisory activities for Boehringer Ingelheim, Bristol‐Myers Squibb, and Pfizer. Dr Becker has received research support from Bayer and Johnson & Johnson. Dr Breithardt has received honoraria for serving on a steering committee for Johnson & Johnson and Bayer and has received fees for serving on advisory boards for Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, and Sanofi‐Aventis. Dr Hankey has received honoraria for serving on trial executive committees for Johnson & Johnson, Bayer, and Sanofi‐Aventis and on trial adjudication committees and an advisory board for Boehringer Ingelheim. Dr Hacke has received honoraria for serving on an executive committee for Johnson & Johnson and Bayer and advisory board fees from Boehringer Ingelheim. Dr Nessel is an employee of Johnson & Johnson. Dr Patel has received honoraria for serving on the executive committee of the ROCKET AF trial of rivaroxaban versus warfarin in AF (Johnson & Johnson, Bayer) and has received consulting fees (Ortho McNeil Janssen, Bayer HealthCare) and served on an advisory board (Genzyme). Dr Califf has received consulting fees and research funding from Johnson & Johnson; all other industry interactions are listed at www.dcri.org. Dr Fox has received grants and honoraria from Bayer, Lilly, Boehringer Ingelheim, Sanofi‐Aventis, and GlaxoSmithKline.
References
- 1.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation? the ATRIA study. Circ Cardiovasc Qual Outcomes. 2009; 2:297-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck‐Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Agladze V, Aliot E, Balabanski T, Blomstrom‐Lundqvist C, Capucci A, Crijns H, Dahlof B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJ, Kose S, McMurray J. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010; 12:1360-1420 [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e257-e354 [DOI] [PubMed] [Google Scholar]
- 4.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, Lip GY, Manning WJ. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:546S-592S [DOI] [PubMed] [Google Scholar]
- 5.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk‐adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). Circ Cardiovasc Qual Outcomes. 2011; 4:22-29 [DOI] [PubMed] [Google Scholar]
- 6.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236-239 [PubMed] [Google Scholar]
- 7.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 141Suppl 2:e44S-e88S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008; 118:2029-2037 [DOI] [PubMed] [Google Scholar]
- 9.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006; 129:1155-1166 [DOI] [PubMed] [Google Scholar]
- 10.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Patient characteristics associated with oral anticoagulation control: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). J Thromb Haemost. 2010; 8:2182-2191 [DOI] [PubMed] [Google Scholar]
- 11. Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010; 159:340-347.e1 [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883-891 [DOI] [PubMed] [Google Scholar]
- 13.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870 [DOI] [PubMed] [Google Scholar]
- 14.Estes NA, III, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG, Bonow RO, DeLong E, Goff DC, Jr, Grady K, Green LA, Hiniker A, Linderbaum JA, Masoudi FA, Pina IL, Pressler S, Radford MJ, Rumsfeld JS. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation) developed in collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2008; 51:865-884 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247-254 [DOI] [PubMed] [Google Scholar]
- 16.Miller RG., Jr Jackknifing variances. Ann Math Statist. 1968; 39:567-582 [Google Scholar]
- 17.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006; 367:1903-1912 [DOI] [PubMed] [Google Scholar]
- 18.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-1151 [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992 [DOI] [PubMed] [Google Scholar]
- 20.Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010; 376:975-983 [DOI] [PubMed] [Google Scholar]
- 21.You JHS, Chan FWH, Wong RSM, Cheng G. Is INR between 2.0 and 3.0 the optimal level for Chinese patients on warfarin therapy for moderate‐intensity anticoagulation? Br J Clin Pharmacol. 2005; 59:582-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose AJ, Hylek EM, Berlowitz DR, Ash AS, Reisman JI, Ozonoff A. Prompt repeat testing after out‐of‐range INR values: a quality indicator for anticoagulation care. Circ Cardiovasc Qual Outcomes. 2011; 4:276-282 [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003; 290:2685-2692 [DOI] [PubMed] [Google Scholar]
- 24.Wieloch M, Sjalander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo‐embolic complications from the national quality registry AURICULA. Eur Heart J. 2011; 32:2282-2289 [DOI] [PubMed] [Google Scholar]
- 25.McPherson K, Wennberg JE, Hovind OB, Clifford P. Small‐area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982; 307:1310-1314 [DOI] [PubMed] [Google Scholar]
- 26.http://www.wennbergcollaborative.org/ Wennberg International Collaborative. 2012