Abstract
Background
Available data are inconsistent regarding factors influencing plasma cholesterol homeostasis marker concentrations and their value in predicting subsequent cardiovascular disease (CVD) events.
Methods and Results
To address this issue, the relationship between markers of cholesterol absorption (campesterol, sitosterol, cholestanol) and synthesis (squalene, desmosterol, lathosterol) and 10‐year CVD incidence was assessed in Framingham Offspring Study participants (cycle 6) who were without CVD at baseline and not taking lipid‐lowering medications (N=2616). The primary end point was “hard” coronary heart disease (HCHD; coronary death and myocardial infarction), and the secondary end point was full CVD (HCHD plus stroke, coronary insufficiency, angina pectoris, peripheral artery disease, and congestive heart failure). In cross‐sectional analysis, significant differences by sex, age, body mass index, blood pressure, and smoking status were observed. In both women and men, lower cholesterol absorption was associated with higher triglyceride and lower high‐density lipoprotein (HDL) cholesterol concentrations, whereas lower cholesterol synthesis was associated with higher low‐density lipoprotein (LDL) cholesterol concentrations (P for trend <0.05). In women only, lower cholesterol synthesis and absorption were associated with higher non–HDL cholesterol concentrations. Using Cox proportional hazards model adjusting for standard CVD risk factors, squalene concentrations were associated with lower HCHD in women (hazard ratio=0.70 [0.5 to 0.9]). In contrast, squalene (hazard ratio=1.40 [1.1 to 1.8]) concentrations were associated with higher HCHD in men (P<0.0001 for interaction). The cholesterol absorption markers were not predictive of HCHD or full CVD in either women or men.
Conclusions
These data suggest significant sex differences in the 10‐year prognostic value of cholesterol synthesis markers and HCHD, specifically coronary death and incidence of myocardial infarction.
Clinical Trial Registration
URL:http://ClinicalTrials.gov. Unique identifier: NCT00074464.
Keywords: cardiovascular disease, lipids, metabolism, mortality, myocardial infarction, risk factors
Introduction
High low‐density lipoprotein cholesterol (LDL‐C) and triglyceride concentrations and low high‐density lipoprotein cholesterol (HDL‐C) concentrations are established risk factors for cardiovascular disease (CVD).1 Cholesterol concentrations within the circulatory pool are products of input from gut absorption and endogenous synthesis relative to clearance through hepatic and extrahepatic tissue pathways.2 A disruption in any of these mechanisms can alter this balance, which is reflected in plasma cholesterol concentrations and subsequent CVD progression.3–4 The importance of cholesterol synthesis as a regulator of plasma LDL‐C concentrations has been demonstrated through studies reporting an inverse relationship between 3‐hydroxy‐3‐methylglutaryl–coenzyme A (HMG‐CoA) reductase inhibitors (statin therapy) and cholesterol synthesis rates,5–10 and dietary cholesterol and de novo biosynthesis rates.11 Similarly, studies have demonstrated that altering the efficiency of cholesterol absorption plays a critical role in determining LDL‐C and HDL‐C concentrations.12–13
Using circulating indicators of cholesterol homeostasis—plasma phytosterol and cholestanol concentrations, which are validated markers of cholesterol absorption, and squalene, desmosterol, and lathosterol concentrations, which are validated markers of cholesterol synthesis13–15—we have previously documented in patients with coronary artery disease that LDL‐C concentrations were positively associated with cholesterol synthesis markers and negatively associated with cholesterol absorption markers.16 In contrast, HDL‐C concentrations were negatively associated with cholesterol synthesis markers and positively associated with absorption markers. Interestingly, a positive association between HDL‐C concentrations and cholesterol absorption efficiency has been reported in obese and hypercholesterolemic individuals without heart disease.17
Given this association between cholesterol homeostasis and plasma lipoprotein concentrations, we and others have started assessing whether cholesterol absorption and synthesis are also associated with prevalent CVD. Results from these cross‐sectional case–control studies have been equivocal with some studies reporting that higher cholesterol absorption and/or lower cholesterol synthesis marker concentrations are associated with increased,18–25 decreased,26–27 or no difference in28–30 CVD risk. Of note, the cohorts used in these studies differed in baseline characteristics, including mean age, body mass index (BMI), and plasma lipoprotein profile, all of which could affect the stage of the disease and thus the magnitude or direction of the associations observed. Also, limits in sample sizes and study duration have potentially precluded assessment of the prognostic value of the cholesterol homeostasis markers on CVD events. The overall objective of the present study was to determine prospectively the validity of cholesterol absorption and synthesis markers as predictors of CVD events during a 10‐year period in Framingham Offspring Study (FOS) participants without prevalent CVD at baseline and not taking lipid‐lowering medication. A secondary objective was to assess cross‐sectionally the relationship between markers of cholesterol absorption/synthesis and established CVD risk factors.
Methods
Study Population
The FOS is a longitudinal community‐based study initiated in 1971 with a sample of 5135 men and women, consisting of the offspring of the original Framingham Heart Study cohort31 and their spouses. Of the 3532 participants who underwent a standardized medical history and physical examination at the sixth examination cycle (1995–1998), we identified 1785 women and 1593 men without CVD (<5% carotid stenosis and no myocardial infarction, coronary insufficiency, angina pectoris, peripheral artery disease, heart failure, or stroke before the sixth examination cycle) who were not taking lipid‐lowering medications (statins, cholestyramine, niacin, or fibrates). Plasma samples for measurement of cholesterol homeostasis markers were available for 1463 women and 1153 men. The baseline characteristics of subjects with and without cholesterol homeostasis data were similar. This study was approved by the institutional review boards for human research at Tufts University–Tufts Medical Center and Boston University.
CVD Outcomes
“Hard” coronary heart disease (HCHD) was defined as a composite of coronary death and myocardial infarction. Full CVD was defined as HCHD plus stroke (atherothrombotic infarction, hemorrhagic stroke, and transient ischemic attack), coronary insufficiency and angina pectoris, peripheral artery disease (intermittent claudication), and congestive heart failure.32 HCHD and full CVD events were confirmed by medical histories, physical examinations at the study clinic, hospitalization records, and communication with personal physicians as previously described.3,31
Anthropometric and Biochemical Measures
Height, weight, and waist circumference were measured with the subject standing. BMI was calculated (kg/m2). Subjects were classified as hypertensive if their diastolic blood pressure was ≥85 mm Hg or systolic blood pressure was ≥130 mm Hg or if use of antihypertensive medications was reported. Current smokers were defined as those who reported smoking ≥1 cigarette per day during the previous year. Estrogen use was classified as current or no use at the time of the examination. Diabetes was defined as fasting glucose ≥126 mg/dL or use of insulin or oral hypoglycemic medications. Metabolic syndrome (MetS) was defined as having ≥3 of the following individual components: abdominal obesity (for men, waist circumference ≥102 cm; for women, waist circumference ≥88 cm), low HDL‐C (for men, <40 mg/dL; for women, <50 mg/dL), elevated blood pressure (≥130/85 mm Hg) or treatment of hypertension, elevated glucose (≥100 mg/dL) or treatment of hyperglycemia, and elevated triglyceride levels (≥150 mg/dL) or treatment for hypertriglyceridemia.
Fasting plasma total cholesterol, HDL‐C, triglyceride, and glucose concentrations were measured using standard enzymatic methods, as previously described.33–34 LDL‐C concentrations were calculated according to the formula of Friedewald et al.35 The assays were standardized through the Lipid Standardization Program of the Centers for Disease Control and Prevention (Atlanta, GA).
Cholesterol Homeostasis Analyses
Plasma concentrations of the cholesterol absorption and synthesis markers were quantified using gas chromatography as previously described.16,36 Peaks of interest were identified by comparison with authentic standards (Supelco, Bellefonte, PA) and expressed relative to the internal standard. Interassay coefficient of variation was on average ≤5.5% for most of the sterols. External quality control samples were routinely interspersed per 20 samples and analyzed with study samples. The cholesterol homeostasis markers measured included the cholesterol synthesis precursors (squalene, desmosterol, and lathosterol) and the absorption markers (cholestanol, campesterol, and sitosterol). Their concentrations have been expressed relative to the concentration of plasma total cholesterol (μmol/mmol of cholesterol) to correct for the different number of lipoprotein acceptor particles.
Statistical Analyses
All variables were summarized using means and SEM. Two‐sample t test was used to compare cholesterol homeostasis markers between women and men. ANOVA was used to determine the equality of mean cholesterol homeostasis markers among different categories. Sex‐specific Cox proportional hazards models were used to relate the cholesterol homeostasis markers to the incidence of a first CVD event during a maximum follow‐up period of 10 years after confirming that the assumption of proportionality of hazards was met. We focused on HCHD as the primary outcome and full CVD as a secondary outcome. Covariates included in the Cox models were age, BMI, blood pressure, antihypertensive medication, LDL‐C, HDL‐C, triglycerides, smoking, and diabetes status. Estrogen use was not included because there was no statistical difference in the cholesterol homeostasis markers between premenopausal and postmenopausal women. Triglyceride concentrations and all cholesterol homeostasis markers were log‐transformed in the models to correct for their skewed distributions and standardized to express the results on a comparable scale. All analyses were performed using SAS version 9.2 or higher, and P values <0.05 were considered statistically significant.
Results
Cholesterol Homeostasis Markers and CVD Outcomes
There was a marked difference in the relationship of cholesterol synthesis markers and HCHD risk between women and men. Using Cox proportional hazards models, after controlling for standard risk factors, diabetes, and antihypertensive medication use, squalene was associated with a lower risk of HCHD (hazard ratio [HR]=0.70 [0.5 to 0.9] in women but with a higher risk of HCHD in men (HR=1.40 [1.1 to 1.8]). Values are HR per 1 SD of log with 95% CIs. The P value for interaction between men and women was significant (P<0.0001). These sex‐specific differences were also observed with desmosterol (HR=0.71 [0.5 to 1.0] and HR=1.19 [0.9 to 1.5] for women and men, respectively) and lathosterol (HR =0.73 [0.5 to 1.1] and HR =1.26 [1.0 to 1.6] for women and men, respectively) concentrations, but the associations did not reach statistical significance (Figure 1A). In contrast to the cholesterol synthesis markers, cholesterol absorption markers were not predictive of HCHD in either women or men (Figure 1B). The cholesterol synthesis:absorption ratios (Figure 1C) tended to be lower in women and associated with higher risk in men, but only the lathosterol:sitosterol ratio reached significance in the women (HR=0.66 [0.4 to 0.9]). These ratios provide an overall assessment of cholesterol homeostasis because they take into account the relative contributions of cholesterol synthesis as well as absorption.37 We also assessed the prognostic value of the cholesterol homeostasis markers and full CVD (a composite of HCHD plus stroke, coronary insufficiency and angina pectoris, peripheral artery disease, and congestive heart failure). No significant associations were observed (Figure 2A through 2C); however, the sex‐specific trends observed for HCHD were similar to those observed with full CVD events, with the cholesterol synthesis markers being associated with lower risk in women and higher risk in men. Of note, the multivariable model adjusted for lipid parameters including LDL‐C. Similar results were obtained when LDL‐C was not included in the model. In addition, to determine if the cholesterol homeostasis markers could be used for risk prediction above and beyond LDL‐C, we calculated the net reclassification improvement after the addition of each marker into the model. Results suggest that there might be some improvement in risk classification for women when squalene was added to the model (0.1298 [−0.0188 to 0.5554]; net reclassification improvement with 95% CIs), but it was widely variable, possibility due to the smaller number of events in our cohort. No improvement was observed with the other cholesterol synthesis markers.
Figure 1.
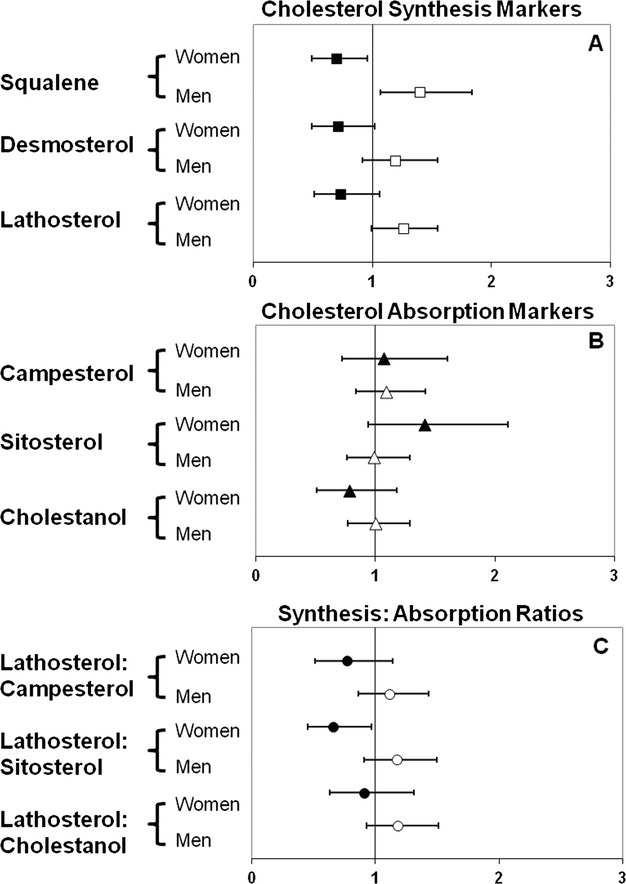
Multivariable‐adjusted hazard ratios for cholesterol synthesis markers (A), cholesterol absorption markers (B), cholesterol synthesis:absorption ratios (C), and “hard” coronary heart disease (coronary death and myocardial infarction) based on Cox proportional hazards model. Error bars represent 95% CIs.
Figure 2.
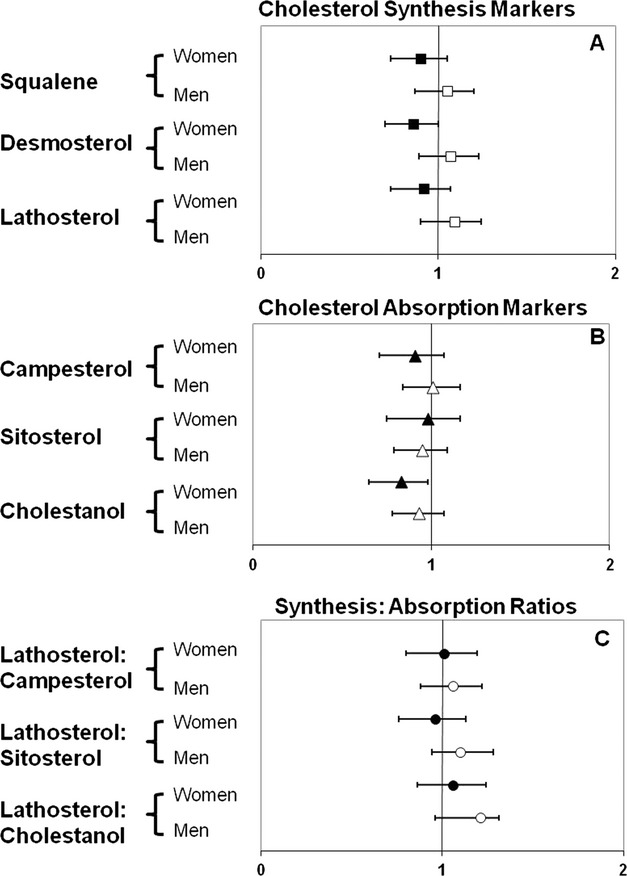
Multivariable‐adjusted hazard ratios for cholesterol synthesis markers (A), cholesterol absorption markers (B), cholesterol synthesis:absorption ratios (C), and full cardiovascular disease (“hard” coronary heart disease plus stroke, coronary insufficiency, angina pectoris, peripheral artery disease, and congestive heart failure) based on Cox proportional hazards model. Error bars represent 95% CIs.
Cholesterol Homeostasis Markers and CVD Risk Factors
In cross‐sectional analysis, marked differences on the basis of sex were observed in cholesterol homeostasis marker concentrations that were not explained by differences in BMI (Table 1). On average, women had significantly lower concentrations of all cholesterol absorption markers assessed compared with men (P<0.01). Among the cholesterol synthesis markers, squalene was significantly higher (P<0.01) in women than in men, whereas desmosterol (P<0.0001), but not lathosterol, was significantly lower in women. Regardless of these differences, the ratios of lathosterol to campesterol, sitosterol, and cholestanol were not significantly different between women and men.
Table 1.
Plasma Noncholesterol Sterol Concentrations by Sex
| Variable | Women (n=1463) | Men (n=1153) | P Value* | BMI Adjusted P Value* |
|---|---|---|---|---|
| Cholesterol synthesis markers,* 102 mmol/mol of cholesterol | ||||
| Squalene | 39.8±0.4 | 38.4±0.5 | 0.006 | 0.026 |
| Desmosterol | 53.8±0.6 | 59.4±0.8 | <0.0001 | <0.0001 |
| Lathosterol | 110.3±1.2 | 114.2±1.5 | 0.141 | 0.239 |
| Cholesterol absorption markers,* 102 mmol/mol of cholesterol | ||||
| Campesterol | 214.8±2.5 | 225.0±2.9 | 0.002 | <0.0001 |
| Sitosterol | 160.2±1.8 | 167.7±2.2 | 0.008 | <0.0001 |
| Cholestanol | 120.7±1.2 | 126.2±1.4 | 0.002 | 0.004 |
| Synthesis:absorption ratio | ||||
| Lathosterol:campesterol | 0.64±0.01 | 0.61±0.01 | 0.177 | 0.011 |
| Lathosterol:sitosterol | 0.85±0.02 | 0.83±0.02 | 0.344 | 0.021 |
| Lathosterol:cholestanol | 1.03±0.01 | 1.03±0.02 | 0.290 | 0.239 |
Values are mean±SE.
P value based on 2‐sample t test.
P value based on regression coefficient for sex from the model with body mass index (BMI).
In both women and men, older age was associated with higher desmosterol and lathosterol concentrations and lower squalene, campesterol, and sitosterol concentrations (Tables 2 and 3; correlations provided in Table 4). Women with higher BMI values (≥30 kg/m2) and waist circumferences (≥88 cm) had higher desmosterol and lathosterol concentrations and lower campesterol and sitosterol concentrations. Men with higher BMI values (≥30 kg/m2) and waist circumferences (≥102 cm) also had lower campesterol and sitosterol concentrations but, in contrast to women, without an apparent compensatory change in cholesterol synthesis marker concentrations.
Table 2.
Plasma Cholesterol Homeostasis Marker Concentrations for Women by CVD Risk Factors
| Variable | N | Cholesterol Synthesis Markers* | Cholesterol Absorption Markers* | ||||
|---|---|---|---|---|---|---|---|
| Squalene | Desmosterol | Lathosterol | Campesterol | Sitosterol | Cholestanol | ||
| Age, y | |||||||
| 30 to 49 | 318 | 42.0±0.8 | 50.8±1.0 | 106.3±2.3 | 239.0±5.6 | 179.7±4.1 | 122.8±2.7 |
| 50 to 59 | 552 | 39.8±0.6 | 52.0±0.9 | 108.5±1.8 | 207.7±4.0 | 152.5±2.7 | 119.1±1.7 |
| 60 to 69 | 404 | 39.6±0.7 | 54.8±1.4 | 112.0±2.2 | 208.0±4.6 | 154.6±3.3 | 119.1±2.1 |
| >70 | 189 | 36.1±1.1 | 62.4±2.3 | 118.4±3.9 | 209.5±7.9 | 162.2±5.8 | 125.1±3.8 |
| P value* | <0.0001 | <0.0001 | 0.022 | <0.0001 | <0.0001 | 0.682 | |
| Body mass index, kg/m2 | |||||||
| <25 | 603 | 40.5±0.6 | 51.2±0.8 | 106.0±1.6 | 240.4±4.3 | 181.5±3.1 | 119.2±1.9 |
| ≥25, <30 | 504 | 39.1±0.6 | 54.6±1.1 | 109.9±2.0 | 203.8±3.8 | 152.9±2.7 | 120.9±1.9 |
| ≥30 | 352 | 39.2±0.8 | 57.4±1.5 | 118.3±2.7 | 187.0±4.8 | 134.5±3.1 | 122.7±2.4 |
| P value* | 0.164 | 0.022 | 0.003 | <0.0001 | <0.0001 | 0.298 | |
| Waist circumference, cm | |||||||
| ≤88 | 592 | 40.4±0.6 | 50.8±0.8 | 104.4±1.5 | 240.2±4.2 | 181.9±3.1 | 120.8±1.9 |
| >88 | 848 | 39.3±0.5 | 55.8±0.9 | 114.1±1.6 | 197.9±3.1 | 145.7±2.1 | 120.5±1.5 |
| P value* | 0.106 | 0.004 | 0.001 | <0.0001 | <0.0001 | 0.875 | |
| Smoking | |||||||
| Nonsmoker | 1243 | 39.9±0.4 | 53.9±0.7 | 110.2±1.2 | 213.3±2.8 | 160.5±2.0 | 120.9±1.3 |
| Current smoker | 220 | 39.0±1.0 | 53.5±1.7 | 110.9±3.1 | 223.7±5.9 | 159.0±4.4 | 119.5±3.2 |
| P value* | 0.362 | 0.914 | 0.931 | 0.037 | 0.919 | 0.425 | |
| Blood pressure (SBP/DBP), mm Hg | |||||||
| <130/<85 | 797 | 41.4±0.5 | 52.6±0.8 | 109.3±1.5 | 224.1±3.5 | 167.8±2.4 | 121.6±1.6 |
| ≥130/≥85 | 666 | 37.7±0.5 | 55.2±1.0 | 111.5±1.8 | 203.7±3.7 | 151.2±2.7 | 119.5±1.7 |
| P value* | <0.0001 | 0.257 | 0.495 | <0.0001 | <0.0001 | 0.272 | |
| Glucose, mg/dL | |||||||
| <100 | 1017 | 40.1±0.4 | 52.1±0.7 | 108.8±1.3 | 225.5±3.1 | 169.8±2.2 | 120.1±1.4 |
| ≥100 | 426 | 38.8±0.7 | 58.3±1.5 | 114.3±2.4 | 188.9±4.2 | 137.0±2.9 | 122.7±2.1 |
| P value* | 0.011 | 0.002 | 0.086 | <0.0001 | <0.0001 | 0.237 | |
| LDL cholesterol,* mg/dL | |||||||
| <100 | 331 | 42.8±0.8 | 55.2±1.3 | 114.9±2.4 | 218.3±5.5 | 164.7±4.1 | 138.0±2.7 |
| ≥100, <130 | 481 | 40.1±0.7 | 55.0±1.2 | 111.6±2.1 | 216.9±4.6 | 162.0±3.3 | 124.1±2.0 |
| ≥130, <160 | 399 | 39.0±0.7 | 53.2±1.3 | 109.5±2.3 | 210.5±4.8 | 157.7±3.3 | 115.6±2.1 |
| ≥160, <190 | 168 | 35.8±1.0 | 51.1±1.7 | 106.6±3.1 | 212.1±7.0 | 154.3±4.8 | 101.7±2.5 |
| ≥190 | 69 | 36.7±1.4 | 49.3±2.2 | 96.1±3.2 | 220.2±12.6 | 162.9±8.8 | 92.5±3.5 |
| P value* | <0.0001 | 0.042 | 0.011 | 0.838 | 0.663 | <0.0001 | |
| HDL cholesterol,* mg/dL | |||||||
| ≤40 | 168 | 40.5±1.2 | 54.6±1.9 | 108.4±3.7 | 191.0±7.0 | 140.0±4.8 | 127.3±3.5 |
| 40 to 60 | 648 | 39.3±0.5 | 54.3±1.0 | 109.7±1.7 | 208.8±3.8 | 157.0±2.7 | 120.1±1.8 |
| ≥60 | 644 | 40.0±0.6 | 53.2±0.9 | 111.4±1.7 | 227.1±3.9 | 168.9±2.8 | 119.6±1.7 |
| P value* | 0.713 | 0.818 | 0.245 | <0.0001 | <0.0001 | 0.086 | |
| Non–HDL cholesterol,* mg/dL | |||||||
| <130 | 428 | 42.0±0.7 | 55.6±1.1 | 112.8±2.0 | 227.0±4.8 | 173.0±3.6 | 135.7±2.4 |
| ≥130, <160 | 459 | 39.4±0.6 | 53.8±1.2 | 111.9±2.2 | 212.6±4.4 | 158.0±3.1 | 120.6±2.0 |
| ≥160, <190 | 321 | 39.4±0.8 | 53.7±1.5 | 110.4±2.6 | 204.2±5.5 | 151.4±4.0 | 116.9±2.4 |
| ≥190 | 252 | 37.0±0.8 | 51.1±1.4 | 103.0±2.6 | 211.6±6.0 | 154.0±4.0 | 100.3±2.0 |
| P value* | 0.001 | 0.019 | 0.006 | 0.002 | <0.0001 | <0.0001 | |
| Triglycerides,* mg/dL | |||||||
| <150 | 1059 | 40.0±0.4 | 54.1±0.7 | 110.6±1.3 | 225.8±3.1 | 169.8±2.2 | 122.2±1.4 |
| ≥150, <200 | 213 | 38.6±0.9 | 53.5±1.8 | 111.8±3.5 | 192.9±5.7 | 139.6±3.7 | 118.9±2.9 |
| ≥200 | 191 | 39.7±1.1 | 53.0±1.8 | 106.7±3.7 | 178.2±5.5 | 130.5±4.0 | 114.0±2.9 |
| P value* | 0.640 | 0.266 | 0.059 | <0.0001 | <0.0001 | 0.057 | |
| Metabolic syndrome | |||||||
| Absence | 40.3±0.4 | 52.2±0.7 | 108.8±1.3 | 228.9±3.2 | 172.7±2.3 | 120.6±1.4 | |
| Presence | 38.5±0.7 | 57.2±1.3 | 113.7±2.3 | 186.8±3.7 | 135.6±2.6 | 120.8±2.0 | |
| P value* | 0.02 | 0.0002 | 0.05 | <0.0001 | <0.0001 | 0.94 | |
| Diabetes mellitus | |||||||
| Absence | 1369 | 39.8±0.4 | 53.1±0.6 | 110.0±1.2 | 216.6±2.6 | 161.9±1.9 | 120.3±1.2 |
| Presence | 94 | 38.4±1.4 | 64.4±4.0 | 114.3±5.2 | 189.1±9.4 | 136.7±6.3 | 125.9±4.9 |
| P value* | 0.366 | 0.006 | 0.656 | 0.002 | 0.0002 | 0.261 | |
CVD indicates cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein.
Values are mean±SE.
P value based on ANOVA.
To convert values for cholesterol and triglycerides to millimoles per liter, divide by 38.67 and 88.54, respectively.
Table 3.
Plasma Cholesterol Homeostasis Marker Concentrations for Men by CVD Risk Factors
| Variable | N | Cholesterol Synthesis Markers* | Cholesterol Absorption Markers* | ||||
|---|---|---|---|---|---|---|---|
| Squalene | Desmosterol | Lathosterol | Campesterol | Sitosterol | Cholestanol | ||
| Age, y | |||||||
| 30 to 49 | 245 | 40.2±0.8 | 55.0±1.3 | 109.5±2.9 | 258.2±7.6 | 187.4±5.4 | 123.0±2.7 |
| 50 to 59 | 450 | 38.8±0.6 | 55.5±1.2 | 108.8±2.1 | 225.3±4.6 | 165.7±3.3 | 125.6±2.1 |
| 60 to 69 | 322 | 38.1±1.0 | 61.4±1.6 | 119.6±3.0 | 215.1±5.0 | 163.4±3.8 | 130.0±2.6 |
| >70 | 136 | 35.0±1.7 | 75.6±2.8 | 127.6±4.8 | 187.8±6.0 | 148.9±5.5 | 124.7±4.5 |
| P value* | <0.0001 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | 0.299 | |
| Body mass index, kg/m2 | |||||||
| <25 | 251 | 38.2±0.8 | 61.7±1.7 | 113.9±2.9 | 239.0±6.1 | 182.6±4.5 | 126.8±2.9 |
| ≥25, <30 | 567 | 38.6±0.6 | 58.8±1.1 | 113.7±2.1 | 234.3±4.5 | 174.4±3.3 | 124.1±1.9 |
| ≥30 | 331 | 38.5±1.0 | 58.7±1.6 | 115.4±2.9 | 199.4±4.5 | 145.4±3.3 | 129.5±2.6 |
| P value* | 0.825 | 0.078 | 0.928 | <0.0001 | <0.0001 | 0.221 | |
| Waist circumference, cm | |||||||
| ≤102 | 668 | 38.2±0.5 | 59.6±1.0 | 112.6±1.8 | 240.1±4.1 | 179.6±3.0 | 125.6±1.8 |
| >102 | 480 | 38.7±0.8 | 59.0±1.4 | 116.6±2.3 | 204.3±3.9 | 151.6±2.9 | 126.7±2.1 |
| P value* | 0.713 | 0.212 | 0.200 | <0.0001 | <0.0001 | 0.835 | |
| Smoking | |||||||
| Nonsmoker | 985 | 38.6±0.5 | 59.6±0.9 | 114.7±1.6 | 225.2±3.2 | 169.3±2.3 | 124.9±1.5 |
| Current smoker | 168 | 37.7±1.0 | 58.3±2.1 | 111.1±4.0 | 224.2±8.0 | 158.4±5.5 | 133.5±3.5 |
| P value* | 0.801 | 0.571 | 0.199 | 0.498 | 0.017 | 0.017 | |
| Blood pressure (SBP/DBP), mm Hg | |||||||
| <130/<85 | 503 | 39.0±0.6 | 56.7±1.0 | 114.0±2.1 | 239.8±4.5 | 179.4±3.3 | 128.1±2.1 |
| ≥130/≥85 | 649 | 38.0±0.6 | 61.5±1.2 | 114.3±2.0 | 213.2±3.8 | 158.4±2.8 | 124.7±1.8 |
| P value* | 0.086 | 0.054 | 0.728 | <0.0001 | <0.0001 | 0.183 | |
| Glucose, mg/dL | |||||||
| <100 | 565 | 38.6±0.6 | 60.3±1.2 | 115.0±2.0 | 235.4±4.3 | 176.4±3.2 | 128.0±1.9 |
| ≥100 | 555 | 38.2±0.7 | 58.6±1.2 | 114.1±2.2 | 214.3±4.1 | 159.0±2.9 | 124.4±2.0 |
| P value* | 0.338 | 0.280 | 0.485 | <0.0001 | <0.0001 | 0.086 | |
| LDL cholesterol,* mg/dL | |||||||
| <100 | 186 | 41.1±1.4 | 68.6±2.1 | 121.7±3.9 | 223.3±7.1 | 166.1±5.4 | 146.5±4.0 |
| ≥100, <130 | 412 | 38.6±0.7 | 60.1±1.4 | 114.5±2.5 | 220.4±4.1 | 163.9±3.2 | 131.8±2.2 |
| ≥130, <160 | 341 | 38.2±0.7 | 56.8±1.4 | 115.0±2.6 | 231.7±5.9 | 173.9±4.2 | 118.9±2.3 |
| ≥160, <190 | 159 | 34.4±1.1 | 54.5±1.9 | 104.7±3.3 | 231.8±9.5 | 169.8±6.5 | 111.2±2.5 |
| ≥190 | 35 | 36.0±2.1 | 52.3±4.1 | 101.5±5.9 | 217.0±16.3 | 165.6±12.0 | 94.2±6.9 |
| P value* | 0.001 | <0.0001 | 0.016 | 0.929 | 0.727 | <0.0001 | |
| HDL cholesterol,* mg/dL | |||||||
| ≤40 | 476 | 40.1±0.8 | 60.0±1.4 | 117.9±2.5 | 210.5±3.8 | 156.8±2.9 | 129.2±2.2 |
| 40 to 60 | 541 | 37.5±0.6 | 58.6±1.1 | 111.4±2.0 | 235.0±4.7 | 173.8±3.3 | 125.2±1.9 |
| ≥60 | 136 | 36.3±1.1 | 60.6±2.0 | 112.0±3.6 | 236.2±8.8 | 181.5±7.0 | 119.7±3.9 |
| P value* | 0.007 | 0.448 | 0.392 | 0.001 | 0.0002 | 0.108 | |
| Non–HDL cholesterol,* mg/dL | |||||||
| <130 | 263 | 39.3±1.0 | 64.5±1.7 | 115.8±2.9 | 231.0±5.8 | 172.8±4.5 | 143.2±3.3 |
| ≥130, <160 | 359 | 38.3±0.7 | 60.9±1.5 | 116.7±2.8 | 220.0±4.9 | 163.7±3.5 | 127.6±2.3 |
| ≥160, <190 | 335 | 38.7±0.9 | 57.9±1.6 | 115.0±2.8 | 229.5±5.9 | 170.2±4.4 | 123.3±2.3 |
| ≥190 | 196 | 37.0±1.3 | 52.4±1.8 | 105.9±3.0 | 218.6±7.3 | 163.8±5.2 | 105.6±2.6 |
| P value* | 0.284 | <0.0001 | 0.112 | 0.227 | 0.377 | <0.0001 | |
| Triglycerides,* mg/dL | |||||||
| <150 | 797 | 37.6±0.5 | 59.5±0.9 | 110.0±1.5 | 235.0±3.7 | 174.3±2.7 | 127.2±1.6 |
| ≥150, <200 | 168 | 38.4±1.0 | 58.8±2.3 | 118.9±4.3 | 206.9±6.4 | 159.0±5.1 | 123.4±3.6 |
| ≥200 | 188 | 41.9±1.6 | 59.6±2.4 | 127.3±4.7 | 199.0±6.0 | 147.6±4.5 | 124.3±3.4 |
| P value* | 0.053 | 0.2306 | 0.003 | <0.0001 | <0.0001 | 0.337 | |
| Metabolic syndrome | |||||||
| Absence | 699 | 37.7±0.5 | 59.3±1.0 | 110.7±1.7 | 239.2±4.0 | 179.6±2.9 | 126.4±1.7 |
| Presence | 444 | 39.6±0.8 | 59.5±1.5 | 120.1±2.7 | 202.3±4.0 | 148.5±2.9 | 125.9±2.3 |
| P value* | 0.04 | 0.90 | 0.002 | <0.0001 | <0.0001 | 0.85 | |
| Diabetes mellitus | |||||||
| Absence | 1058 | 38.7±0.5 | 58.8±0.8 | 113.7±1.5 | 227.8±3.1 | 169.6±2.3 | 125.7±1.4 |
| Presence | 93 | 35.9±1.5 | 66.0±3.5 | 119.7±6.1 | 194.3±8.0 | 147.3±6.3 | 133.1±5.7 |
| P value* | 0.039 | 0.073 | 0.571 | 0.001 | 0.005 | 0.302 | |
CVD indicates cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein.
Values are mean±SE.
P value based on ANOVA.
To convert values for cholesterol and triglycerides to millimoles per liter, divide by 38.67 and 88.54, respectively.
Table 4.
Correlation Between Cholesterol Homeostasis Markers and CVD Risk Factors*
| Variable | Women | Men | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BMI | LDL‐C | HDL‐C | Non–HDL‐C | TG* | Age | BMI | LDL‐C | HDL‐C | Non–HDL‐C | TG | |
| Cholesterol synthesis markers* | ||||||||||||
| Squalene | −0.14* | −0.06* | −0.13* | 0.02 | −0.12* | −0.04 | −0.16* | 0.03 | −0.10* | −0.09* | −0.04 | 0.07* |
| Desmosterol | 0.11* | 0.06* | −0.07* | −0.03 | −0.07* | −0.03 | 0.19* | 0.06 | −0.17* | 0.02 | −0.18* | −0.08* |
| Lathosterol | 0.07* | 0.08* | −0.08* | 0.02 | −0.08* | −0.04 | 0.11* | 0.02 | −0.10* | −0.05 | −0.06* | 0.07* |
| Cholesterol absorption markers* | ||||||||||||
| Campesterol | −0.12* | −0.23* | −0.01 | 0.14* | −0.09* | −0.26* | −0.21* | −0.15* | 0.02 | 0.10* | −0.04 | −0.19* |
| Sitosterol | −0.12* | −0.30* | −0.03 | 0.14* | −0.12* | −0.31* | −0.17* | −0.20* | 0.03 | 0.11* | −0.04 | −0.19* |
| Cholestanol | −0.02 | 0.03 | −0.31* | −0.04 | −0.30* | −0.10* | −0.01 | 0.01 | −0.27* | −0.07* | −0.28* | −0.08* |
| Cholesterol synthesis:absorption ratio* | ||||||||||||
| Lathosterol:campesterol | 0.13* | 0.22* | −0.04 | −0.09* | 0.01 | 0.16* | 0.22* | 0.12* | −0.08* | −0.10* | −0.01 | 0.18* |
| Lathosterol:sitosterol | 0.13* | 0.26* | −0.02 | −0.08* | 0.04 | 0.19* | 0.19* | 0.15* | −0.09* | −0.11* | −0.01 | 0.18* |
| Lathosterol:cholestanol | 0.07* | 0.04 | 0.17* | 0.04 | 0.15* | 0.03 | 0.09* | 0.003 | 0.11* | 0.01 | 0.14* | 0.11* |
CVD indicates cardiovascular disease; BMI, body mass index; LDL‐C, LDL cholesterol; HDL‐C, HDL cholesterol; TG, triglycerides.
Pearson product moment correlation coefficient.
Triglycerides and cholesterol synthesis and absorption markers and ratios were log‐transformed during analysis to correct for their skewed distributions.
‡P<0.05; §P<0.01; ¶P<0.001.
There was some indication that smoking status was associated with altered cholesterol absorption but not synthesis marker concentrations. Among smokers, women had higher campesterol concentrations, whereas men had lower sitosterol and higher cholestanol concentrations. Regardless of sex, participants with high blood pressure (≥130/≥85 mm Hg) and high glucose (≥100 mg/dL) concentrations had lower campesterol and sitosterol concentrations. However, women with high blood pressure and high glucose concentrations also had differences in the cholesterol synthesis marker concentrations characterized by lower squalene and higher desmosterol and a trend toward higher lathosterol concentrations. Such relationships or trends in relationships were not observed in the men.
With regard to plasma lipid profiles (Tables 2 and 3), lower cholesterol absorption (campesterol and sitosterol concentrations) was associated with higher triglyceride and lower HDL‐C concentrations, whereas lower cholesterol synthesis (squalene, desmosterol, and lathosterol concentrations) was associated with higher LDL‐C concentrations in both women and men. Sex differences were observed in non–HDL‐C and triglyceride concentrations. In women, lower cholesterol synthesis and absorption marker concentrations were associated with higher non–HDL‐C concentrations, whereas in men, higher lathosterol concentrations were associated with higher triglyceride concentrations.
Given the association between the cholesterol absorption/synthesis markers and several CVD risk factors that comprise or contribute to MetS and type 2 diabetes, we further assessed whether the presence or absence of these metabolic conditions was related to differences in cholesterol homeostasis (Tables 2 and 3). Women and men classified as having MetS or diabetes had lower campesterol and sitosterol concentrations (both P<0.05) and higher desmosterol and lathosterol concentrations (P<0.05 for desmosterol and lathosterol in women; P<0.05 for lathosterol in men). Squalene concentrations were lower in women with MetS (−4.5%, P=0.02) but higher in men with MetS (5%, P=0.04) compared with their counterparts not classified as having MetS. Similarly, diabetic women had higher desmosterol concentrations (21%, P<0.01), whereas diabetic men had lower squalene concentrations (−7%, P=0.04) with no difference in desmosterol or lathosterol concentrations.
Discussion
In cross‐sectional analyses from this community‐based observational study, significant differences in cholesterol absorption and synthesis markers by sex, age, BMI, blood pressure, lipoprotein concentrations, smoking, and metabolic status were observed. When studied prospectively during a 10‐year period, after controlling for standard CVD risk factors, the cholesterol synthesis markers predicted HCHD; however, sex‐specific differences were observed. In women, squalene was associated with lower risk of coronary death and incidence of myocardial infarction, whereas in men, it was associated with higher risk of coronary death and incidence of myocardial infarction.
To our knowledge, this is the largest and most comprehensive study to date to examine the relationship between cholesterol homeostasis marker concentrations and CVD risk factors and events. To adequately address the primary and secondary aims of this work, we measured one cholesterol synthesis marker, squalene, formed as an early intermediate in the biosynthetic pathway, as well as 2 cholesterol synthesis markers formed from squalene later in the biosynthetic pathway, desmosterol and lathosterol, representing 2 alternate cholesterol biosynthetic pathways (Figure 3). In the Kandutsch–Russell pathway, lathosterol is a precursor of cholesterol, and in the Bloch pathway, desmosterol is a precursor of cholesterol. It is thought that these pathways may be independently regulated.38 Among the cholesterol absorption markers, we measured campesterol and sitosterol, which are derived from the diet, as well as cholestanol, a 5α saturated derivative of cholesterol, which is not influenced by dietary intake.
Figure 3.
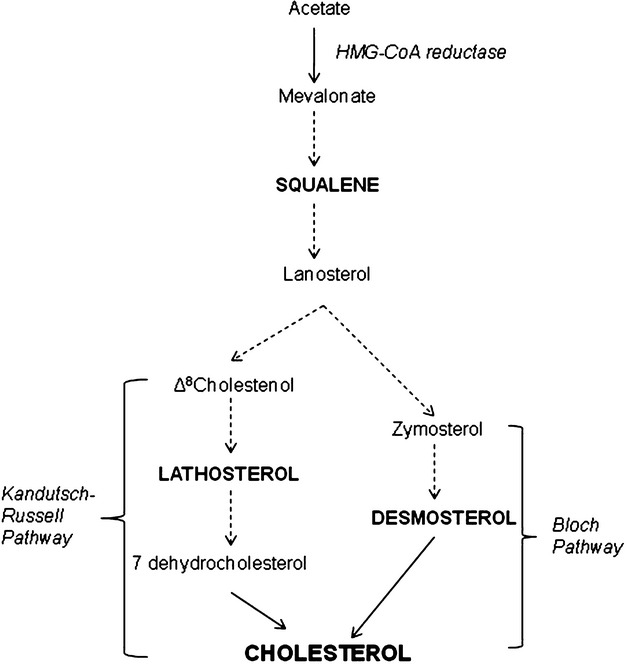
Cholesterol biosynthetic pathway highlighting the formation of squalene, an early intermediate, as well as desmosterol (Bloch pathway) and lathosterol (Kandutsch–Russell pathway) formed from squalene later in the biosynthetic pathway. HMG‐CoA indicates 3‐hydroxy‐3‐methylglutaryl–coenzyme A.
Several case–control studies have assessed the relationship between cholesterol homeostasis marker concentrations and prevalent CVD risk, but the results have been inconsistent.18–30 Data are more limited regarding the association between cholesterol homeostasis markers and CVD events. Among the 4 studies identified, the Drugs and Evidence BAsed medicine in The Elderly (DEBATE) study39 included 247 women and 149 men aged >75 years with documented CVD and abnormalities in glucose metabolism and reported that individuals in the highest quartile of cholestanol concentrations had the greatest increase in mortality (HR=3.53 [1.52 to 8.19]) and recurrence of CVD events relative to the lowest quartile. In addition, in this cohort, cholestanol and sitosterol, but not lathosterol, concentrations were higher in individuals who died after 3.4 years of follow‐up. However, given the age of the cohort, survivor bias cannot be ruled out. The LUdwigshafen RIsk and Cardiovascular health (LURIC)20 and the Helsinki Aging40 studies also focused on an elderly population. In the LURIC study, 1257 participants with CVD and not taking statins were followed for ≈7 years. All‐cause and CVD mortality rates were lower in individuals in the highest versus the lowest lathosterol tertile (HR=0.61 [0.5 to 0.8] and HR=0.60 [0.4 to 0.9) for all‐cause and CVD mortality, respectively), whereas the third cholestanol tertile was associated with increased all‐cause (HR=1.71 [1.3 to 2.3]) and CVD (HR=1.60 [1.1 to 2.3]) mortality. No significant association was observed across sitosterol tertiles. Similar results were observed in the Helsinki Businessmen Study 41, which followed 232 middle‐aged men with CVD and not using statin therapy for a period of 22 years. In multivariate analysis, men in the highest sitosterol tertile had a 42% to 47% lower mortality rate (HR=0.51 [0.3 to 0.9]) than those in the highest desmosterol to sitosterol tertile and had a 67% higher mortality rate (HR=1.67 [1.1 to 2.5]) than those men in the lowest tertiles. Taken together, these results suggest that higher cholesterol synthesis and lower absorption predict long‐term mortality. In contrast, low cholesterol synthesis and low cholesterol absorption were associated with higher mortality in 1 of the 2 studies.
Expanding the previous data sets available on cholesterol homeostasis markers and CVD risk factors and outcomes, our study identified notable sex‐specific associations between some of the cholesterol synthesis marker concentrations and HCHD. In women, squalene was associated with a 30% lower incidence of myocardial infarction and coronary death. Desmosterol (29%) and lathosterol (28%) showed similar but nonsignificant protective trends. In men, squalene was associated with 40% higher risk of coronary death and myocardial infarction. Desmosterol (19%) and lathosterol (26%) showed similar risk trends. Sex‐specific differences were not observed in the LURIC study; however, given the age of the cohort, it might have been difficult to tease out these differences. The other 2 studies were not powered to determine sex‐specific associations. We did not find an association between cholesterol absorption markers and HCHD or full CVD.
In our cross‐sectional analysis, women had lower concentrations of the absorption and synthesis markers compared with men, and this was not explained by differences in BMI. This is consistent with some,26–27,42 but not all, of the prior data available.25,20,43 As indicated previously, limited sample size in several of the prior studies may have precluded analysis on the basis of sex. A novel finding of the present work is that squalene concentrations were markedly higher in women relative to men. Squalene can be obtained in small amounts from foods (eg, olive oil), but because this was not a predominant component of the diet, the higher concentrations in women most likely reflect lower conversion to cholesterol via the sterol pathways than in men. Were this hypothesis correct, it could also account for the lower desmosterol and lathosterol concentrations observed in men compared with women in our cohort.
Increasing age was associated with lower squalene and higher desmosterol and lathosterol concentrations, a finding observed in both sexes. Consistent with prior reports, we also observed that higher cholesterol synthesis marker concentrations were associated with lower absorption marker concentrations.19–20,40,42 Changes in body composition (loss of lean protein mass and increase in fat mass) could partially account for these age‐related changes.44 This hypothesis is supported by our findings of lower cholesterol absorption marker concentrations in men and women and of higher synthesis marker concentrations in women with higher BMI values and waist circumferences, as well prior observations in obese subjects17,45–47 and those with MetS48–49 and diabetes.50–53
Lower cholesterol absorption marker concentrations were associated with higher triglyceride and lower HDL‐C concentrations, whereas lower cholesterol synthesis marker concentrations were associated with higher LDL‐C concentrations, in both women and men. These results are consistent with previous reports that have had the power to address sex differences.22,26,20 The inverse association between cholesterol synthesis marker concentrations and plasma LDL‐C concentrations has been attributed to upregulation of the LDL receptor, which, in turn, increases internalization of LDL from the circulation leading to release of cholesterol into the cell, thereby downregulating HMG‐CoA reductase activity, the rate‐limiting enzyme in cholesterol biosynthesis.54
The association between non–HDL‐C concentrations and cholesterol homeostasis marker concentrations was also assessed in the present study given the strong association between non–HDL‐C and the pathophysiology of atherosclerosis55 and the documented sex‐specific difference in non–HDL‐C concentrations between women and men.56 Our results indicate that the lower non–HDL‐C concentrations in women are associated with lower cholesterol synthesis and absorption.
Although the regulatory mechanisms of cholesterol homeostasis have been established, little data are available to speculate on the mechanism(s) responsible for the sex‐specific differences observed. HMG‐CoA reductase activity, the rate‐limiting enzyme in cholesterol biosynthesis, has been reported to be is lower in women than in men.57 It has been suggested that this factor contributes, in part, to a lower lifetime burden of cholesterol in women41 and, thus, lower event rates. Consistent with these data, in our cohort, squalene and, to a lesser extent, desmosterol and lathosterol were associated with a lower risk of HCHD in women but not in men. These markers are intermediates of cholesterol biosynthesis that have been correlated with HMG‐CoA reductase activity.58
A limitation of this study is that the associations observed could have been influenced by factors that we were not able to control for, such as genotype. Second, the phytosterol content of the diets could not be assessed. However, plasma samples were collected before the introduction of phytosterol‐enriched products into the marketplace, which would eliminated this potential source of confounding. Third, only a single determination of cholesterol absorption and synthesis markers was made during cycle 6, which could have been responsible for some of the variability in the data. To minimize this variation, only fasted samples collected according to a strict protocol within a specified timeframe were used. Finally, the FOS population studied was predominantly white, so care must be taken in extrapolating the results to other populations.
In conclusion, our results suggest significant sex differences in the 10‐year prognostic value of cholesterol synthesis markers, specifically squalene, on coronary death and incidence of myocardial infarction (HCHD). This could have implications in the management of CVD risk and choice of lipid‐lowering therapy, including newer agents such as the squalene synthase inhibitors.
Sources of Funding
This work was supported by grants HL 074388 (N.R.M., A.H.L., E.J.S.) and N01‐HC‐25195 (L.Z., M.P., R.B.D.) from the National Institutes of Health and the United States Department of Agriculture, under agreement No. 58‐1950‐4‐401. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of authors and do not necessarily reflect the view of the U. S. Department of Agriculture.
Disclosures
None.
Acknowledgments
We thank FOS participants and staff and Jane LaRocque and Kara Fedors, for performing the gas chromatographic analysis of the cholesterol homeostasis markers.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143-3421 [PubMed] [Google Scholar]
- 2.Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 2004; 174:197-205 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979; 110:281-290 [DOI] [PubMed] [Google Scholar]
- 4.Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. 1991; 338:985-992 [DOI] [PubMed] [Google Scholar]
- 5.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002; 360:23-3312114037 [Google Scholar]
- 6.Long‐term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998; 339:1349-1357 [DOI] [PubMed] [Google Scholar]
- 7.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. J Am Med Assoc. 1998; 279:1615-1622 [DOI] [PubMed] [Google Scholar]
- 8.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998; 67:25-30 [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995; 333:1301-1307 [DOI] [PubMed] [Google Scholar]
- 10. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383-1389 [PubMed] [Google Scholar]
- 11.Cuchel M, Schaefer EJ, Millar JS, Jones PJ, Dolnikowski GG, Vergani C, Lichtenstein AH. Lovastatin decreases de novo cholesterol synthesis and LDL Apo B‐100 production rates in combined‐hyperlipidemic males. Arterioscler Thromb Vasc Biol. 1997; 17:1910-1917 [DOI] [PubMed] [Google Scholar]
- 12.Miettinen TA. Cholesterol absorption inhibition: a strategy for cholesterol‐lowering therapy. Int J Clin Pract. 2001; 55:710-716 [PubMed] [Google Scholar]
- 13.Kesaniemi YA, Ehnholm C, Miettinen TA. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest. 1987; 80:578-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990; 131:20-31 [DOI] [PubMed] [Google Scholar]
- 15.Kuksis A. Plasma non‐cholesterol sterols. J Chromatogr A. 2001; 935:203-236 [DOI] [PubMed] [Google Scholar]
- 16.Matthan NR, Giovanni A, Schaefer EJ, Brown BG, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res. 2003; 44:800-806 [DOI] [PubMed] [Google Scholar]
- 17.Miettinen TA, Gylling H. Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis. 2000; 153:241-248 [DOI] [PubMed] [Google Scholar]
- 18.Matthan NR, Pencina M, LaRocque JM, Jacques PF, D'Agostino RB, Schaefer EJ, Lichtenstein AH. Alterations in cholesterol absorption/synthesis markers characterize Framingham Offspring Study participants with CHD. J Lipid Res. 2009; 50:1927-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weingartner O, Pinsdorf T, Rogacev KS, Blomer L, Grenner Y, Graber S, Ulrich C, Girndt M, Fliser D, Laufs U, Lutjohann D, Heine GH. The relationships of markers of cholesterol homeostasis with carotid intima‐media thickness. PLoS ONE. 2010; 5:e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibernagel G, Fauler G, Hoffmann MM, Lutjohann D, Winkelmann BR, Marz W. The associations of cholesterol metabolism and plasma plant sterols with all‐cause and cardiovascular mortality. J Lipid Res. 2010; 51:2384-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gylling H, Hallikainen M, Rajaratnam RA, Simonen P, Pihlajamaki J, Laakso M, Miettinen TA. The metabolism of plant sterols is disturbed in postmenopausal women with coronary artery disease. Metabolism. 2009; 58:401-407 [DOI] [PubMed] [Google Scholar]
- 22.Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case–control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr Metab Cardiovasc Dis. 2005; 16:13-21 [DOI] [PubMed] [Google Scholar]
- 23.Rajaratnam RA, Gylling H, Miettinen TA. Serum squalene in postmenopausal women without and with coronary artery disease. Atherosclerosis. 1999; 146:61-64 [DOI] [PubMed] [Google Scholar]
- 24.Rajaratnam RA, Gylling H, Miettinen TA. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol. 2000; 35:1185-1191 [DOI] [PubMed] [Google Scholar]
- 25.Sudhop T, Gottwald BM, von Bergmann K. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism. 2002; 51:1519-1521 [DOI] [PubMed] [Google Scholar]
- 26.Escurriol V, Cofan M, Moreno‐Iribas C, Larranaga N, Martinez C, Navarro C, Rodriguez L, Gonzalez CA, Corella D, Ros E. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J Lipid Res. 2010; 51:618-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassbender K, Lutjohann D, Dik MG, Bremmer M, Konig J, Walter S, Liu Y, Letiembre M, von Bergmann K, Jonker C. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk—the LASA study. Atherosclerosis. 2008; 196:283-288 [DOI] [PubMed] [Google Scholar]
- 28.Pinedo S, Vissers MN, von Bergmann K, Elharchaoui K, Lutjohann D, Luben R, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC‐Norfolk Population Study. J Lipid Res. 2007; 48:139-144 [DOI] [PubMed] [Google Scholar]
- 29.Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol. 2004; 24:2326-2332 [DOI] [PubMed] [Google Scholar]
- 30.Windler E, Zyriax BC, Kuipers F, Linseisen J, Boeing H. Association of phytosterol concentrations with incident coronary heart diease. Data from the CORA study, a case–control study of coronary artery disease in women. Atherosclerosis. 2009; 203:284-290 [DOI] [PubMed] [Google Scholar]
- 31.Dawber TR, Meadors GF, Moore F. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951; 41:279-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting 30‐year risk of cardiovascular disease. The Framingham Heart Study. Circulation. 2009; 119:3078-3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987; 166:1-8 [DOI] [PubMed] [Google Scholar]
- 34.Warnick GR, Benderson J, Albers JJ. Dextran sulfate‐Mg2+ precipitation procedure for quantitation of high‐density‐lipoprotein cholesterol. Clin Chem. 1982; 28:1379-1388 [PubMed] [Google Scholar]
- 35.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502 [PubMed] [Google Scholar]
- 36.Matthan NR, Raeini‐Sarjaz M, Lichtenstein AH, Ausman LM, Jones PJ. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 2000; 35:1037-1044 [DOI] [PubMed] [Google Scholar]
- 37.Nissinen MJ, Gylling H, Miettinen TA. Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br J Nutr. 2008; 99:370-378 [DOI] [PubMed] [Google Scholar]
- 38.Clayton PT. Disorders of cholesterol biosynthesis. Arch Dis Child. 1998; 78:185-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strandberg TE, Tilvis RS, Pitkala KH, Miettinen TA. Cholesterol and glucose metabolism and recurrent cardiovascular events among the elderly. J Am Coll Cardiol. 2006; 48:708-714 [DOI] [PubMed] [Google Scholar]
- 40.Tilvis RS, Valvanne JN, Stranberg TE, Miettinen TA. Prognostic significance of serum cholesterol, lathosterol, and sitosterol in old age; a 17 year population study. Ann Med. 2011; 43:292-301 [DOI] [PubMed] [Google Scholar]
- 41.Strandberg TE, Gylling H, Tilvis RS, Miettinen H. Serum plant and other noncholesterol sterols, cholesterol metabolism and 22 year mortality among middle‐aged men. Atherosclerosis. 2010; 210:282-287 [DOI] [PubMed] [Google Scholar]
- 42.Silbernagel G, Fauler G, Renner W, Landl EM, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. J Lipid Res. 2009; 50:334-341 [DOI] [PubMed] [Google Scholar]
- 43.Kempen HJ, de Knijff P, Boomsma DI, van der Voort HA, Gevers Leuven JA, Havekes L. Plasma levels of lathosterol and phytosterols in relation to age, sex, anthropometric parameters, plasma lipids, and apolipoprotein E phenotype, in 160 Dutch families. Metabolism. 1991; 40:604-611 [DOI] [PubMed] [Google Scholar]
- 44.Buffa R, Floris GU, Putzu PF, Marini E. Body composition variations in ageing. Coll Antropol. 2011; 35:259-265 [PubMed] [Google Scholar]
- 45.Paramsothy P, Knoop RH, Kahn SE, Tretzlaff BM, Fish B, Ma L, Ostlund RE., Jr Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am J Clin Nutr. 2011; 94:1182-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peltola P, Pihlajamaki J, Koutnikova H, Ruotsalainen E, Salmenniemi U, Vauhkonen I, Kainulainen S, Gylling H, Miettinen TA, Auwerx J, Laakso M. Visceral obesity is associated with high levels of serum squalene. Obesity. 2006; 14:1155-1163 [DOI] [PubMed] [Google Scholar]
- 47.Simonen P, Gylling H, Howard AN, Miettinen T. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am J Clin Nutr. 2000; 72:82-88 [DOI] [PubMed] [Google Scholar]
- 48.Chan DC, Watts GF, Barrett HR, O'Neill FH, Thompson GR. Plasma markers of cholesterol homeostasis and apolipoprotein B‐100 kinetics in the metabolic syndrome. Obes Res. 2003; 11:591-596 [DOI] [PubMed] [Google Scholar]
- 49.Assmann G, Cullen P, Kannenberg F, Schulte H. Relationship between phytosterol levels and components of the metabolic syndrome in the PROCAM study. Eur J Cardiovasc Prev Rehabil. 2007; 2:208-214 [DOI] [PubMed] [Google Scholar]
- 50.Briones ER, Steiger DL, Palumbo PJ, O'Fallon WM, Langworthy AL, Zimmerman BR, Kottke BA. Sterol excretion and cholesterol absorption in diabetics and nondiabetics with and without hyperlipidemia. Am J Clin Nutr. 1986; 44:353-361 [DOI] [PubMed] [Google Scholar]
- 51.Gylling H, Miettinen TA. Cholesterol absorption and lipoprotein metabolism in type II diabetes mellitus with and without coronary artery disease. Atherosclerosis. 1996; 126:325-332 [DOI] [PubMed] [Google Scholar]
- 52.Pihlajamaki J, Gylling H, Miettinen T, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid Res. 2004; 45:507-512 [DOI] [PubMed] [Google Scholar]
- 53.Simonen PP, Gylling HK, Miettinen TA. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care. 2002; 25:1511-1515 [DOI] [PubMed] [Google Scholar]
- 54.Lagor WR, Millar JS. Overview of the LDL receptor. Relevance to cholesterol metabolism and future approaches for the treatment of coronary heart disease. J Receptor Ligand Channel Res. 2010; 3:1-14 [Google Scholar]
- 55.Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TANational Lipid Association Taskforce on Non‐HDL Cholesterol The importance of non‐HDL cholesterol reporting in lipid management. J Clin Lipidol. 2008; 4:267-273 [DOI] [PubMed] [Google Scholar]
- 56.Gardner CD, Winkelby M, Fortman SP. Population frequency distribution of non‐high‐density lipoprotein cholesterol (Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994). Am J Cardiol. 2000; 86:299-304 [DOI] [PubMed] [Google Scholar]
- 57.De Marinis E, Martini C, Trentalance A, Pallottini V. Sex differences in hepatic regulation of cholesterol homeostasis. J Endocrinol. 2008; 198:635-643 [DOI] [PubMed] [Google Scholar]
- 58.Bjorkhem I, Miettinen T, Reihner E, Ewerth S, Angelin B, Einarsson K. Correlation between serum levels of some cholesterol precursors and activity of HMG‐CoA reductase in human liver. J Lipid Res. 1987; 28:1137-1143 [PubMed] [Google Scholar]


