Abstract
The incretin hormone glucagon-like peptide-1 (GLP-1) has been implicated in the regulation of appetite by acting as an anorexigenic gut-brain signal. The postprandial release of GLP-1 can be blunted in obese humans and animals. However, it remains unknown whether obesogenic diets with varying fat and carbohydrate content may differentially influence the effectiveness of GLP-1 feedback. To investigate this, male Sprague-Dawley rats were fed a standard (low fat) chow diet, or one of two high-energy diets varying in fat content (45 or 60 kcal%) for 28 weeks. Intake of sucrose and fructose solutions, two commonly added sugars in the Western diet, was then tested in non-deprived rats following administration of the GLP-1 receptor agonist, Exendin-4 (0, 0.5, 1, 2, 3 µg/kg; s.c.). Exendin-4 dose-dependently reduced short (2-hr) sucrose and fructose intake. This effect was significantly attenuated in rats fed more dietary fat despite both diets resulting in obesity. These findings demonstrate that intake of carbohydrates when offered as treats can be regulated by GLP-1 and suggests that dietary fat consumption, rather than extra calories or obesity, may lead to impaired GLP-1 feedback to curb carbohydrate intake. Future studies are warranted to investigate relevance of these observations to human and to elucidate the underlying mechanisms.
Keywords: GLP-1, Exendin-4, sucrose, fructose, dietary obesity, gut-brain feedback
Introduction
With the escalating prevalence of high energy diets, increased attention must be given to the physiological changes that follow chronic consumption of obesogenic diets leading to obesity. Obesogenic diets include those high in fat, carbohydrates or combinations of these macronutrients (1). Such diets are readily consumed by both animals and humans and often lead to weight gain and obesity (1–2). Diet-induced obesity is associated with changes in a number of physiological signals (3–4). One such signal is the gut hormone glucagon-like peptide-1 (GLP-1).
GLP-1 is released by enteroendocrine L cells of the ileum following ingestion of a meal (5–7). While GLP-1 is able to produce feelings of satiety in lean and obese humans following peripheral administration (8–9), the postprandial GLP-1 release has also been shown to be reduced in some obese humans and animals (10–11). Normally, GLP-1 release results in a suppression of food intake (6–7). Reports show that exogenous GLP-1, as well as the synthetic analog Exendin-4, also reduce food intake when administered systemically or directly into the brain in normal weight subjects (12–13). While less studied, others have begun to investigate how GLP-1 influences aspects of sucrose consumption as well (14). The effects of GLP-1 receptor activation in a model of diet-induced obesity, and its role in hedonically-driven consumption (i.e. consumption driven by palatably of the food, rather than hunger), is not as well characterized. Differences due to various obesogenic diets also remain unknown, although the varying plasma GLP-1 levels of obese individuals indicate possible differences (10–11, 15–17). Such ambiguity points strongly to factors other than the presence of obesity alone in producing GLP-1 dysregulation. Based on previous literature and this gap in the knowledge, we investigated how different obesogenic diets may differentially influence the inhibitory effects on carbohydrate intake produced by activation of the GLP-1 receptor, using the long-acting synthetic GLP-1 analog Exendin-4.
Obesogenic diets, such as high-sugar and high-fat diets, often produce hyperphagia, leading to increased body weight, increased fat mass, and eventually can produce hyperinsulemia, hyperleptinemia and insulin resistance (4, 18). Based on the macronutrient content and the similarities between the obesogenic diet-fed rat and the obese human, these diet-induced obesity models are considered applicable to human obesity (4). On this basis, we used two obesogenic diets of varying macronutient content to induce obesity in rats. Obese rats and their lean counterparts were then tested in a one-bottle intake taste to two sweet carbohydrate solutions with and without activation of GLP-1 receptors. We chose two common carbohydrates, sucrose and fructose, which are both preferred by rats and humans but have been shown to differ in their post-ingestive effects, including their rewarding properties. Specifically, whereas sucrose has been shown to produce conditioned effects based on both its taste and post-ingestive properties (19) fructose appears to exert behaviorally relevant stimulation exclusively by its taste and not by reinforcing post-ingestive effects (20). To our knowledge, changes in intake of these common carbohydrate following activation of GLP-1 receptors is yet unreported in obese rats. Furthermore, rats remained sated with ad libitum food available throughout the experiments to discern the possible role of GLP-1 in hedonic (palatability-driven) consumption, instead of consumption due to homeostatic regulation (hunger due to caloric deprivation).
Methods and Procedures
Animals
Naïve adult male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 250–275 g at the beginning of the study were housed in individual cages in a temperature-controlled vivarium and maintained on a 12:12-hr light-dark cycle (lights on at 0700). Animals were maintained on ad libitum standard laboratory chow (Chow; n=8), a high energy diet of high-fat content (HFHE; n=11) or a high energy diet consisting of both fat and carbohydrate content (FCHE; n=11). Animals were maintained on the diet for 28 weeks prior to experiments and throughout the testing period. During this time, rats received equal but limited exposure to sucrose and fructose in behavior-only test paradigms. Food and water was available ad libitum throughout testing.
Diets, Test Solutions, and Drugs
Standard laboratory chow (Teklad #2018, Somerville, NJ) was used as the control diet (3.4 kcal/g, 17% kcal from fat, 60% kcal from carbohydrates and 23% kcal from protein). High energy diets were obtained from Research Diets (New Brunswick, NJ). The high-fat high energy diet (HFHE; Research Diets #D12492) consisted of 5.24 kcal/gram (60% kcal from fat, 20% kcal from carbohydrates and 20% kcal from protein). The fat-carbohydrate high energy diet (FCHE; Research Diets #D12451) consisted of 4.73 kcal/gram (45% kcal from fat, 35% kcal from carbohydrates and 20% kcal from protein). Sucrose and fructose (Fisher-Scientific, Fair Lawn, NJ) were dissolved in filtered tap water (source identical to water available in home cages) and prepared no more than 24 hours prior to testing. Exendin-4 was obtained from Tocris Biosciences (Ellisville, MO) and dissolved in sterile saline containing 1% albumin. Vehicle, i.e. saline containing 1% albumin, was used as the control solution.
Behavioral tests
To test the effects of GLP-1 on carbohydrate consumption, we used the synthetic analog Exendin-4 (0, 0.5, 1, 2, and 3 µg/kg; s.c.). Previous research has shown that doses within this range reduce chow intake in rats (21–22). Whereas many studies have been carried out in food deprived rats, in our design the rats were fed ad libitum throughout the whole experiment, including the test phase. The rationale for that is the fact that an increasing number of calories consumed by the average U.S. adult comes from high-sugar content beverages and snacks which are consumed independent of main meals, i.e. as treats (23–24). All tests were conducted in the middle of the light phase and in the home cage. Test solutions were administered via a single 100 ml bottle attached to the front of the home cage, with the spout extending into the home cage. Rats were trained to lick sucrose and fructose in this manner prior to Exendin-4 testing. Animals were treated with Exendin-4 ten minutes before 2-hr access to 0.3M sucrose solution. Animals were given 4 days to recover, and then Exendin-4 was administered immediately prior to 2 hour access to 0.4M fructose. These concentrations were chosen as they are readily consumed by rats and have been used extensively in the literature (25–26). Amount consumed (in ml) was measured. A minimum of two days was given between injections.
Statistical Analyses
Body weight data were analyzed using a two-way analysis of variance (ANOVA) with diet and dose as the independent variables, with separate ANOVAs conducted for body weight during sucrose and fructose testing periods.
Carbohydrate intake was measured as ml consumed. To evaluate differences in baseline intake, a two-way ANOVA was conducted on intake after vehicle (0 µg/kg) with diet group and carbohydrate as independent variables. The ANOVA revealed no effect of diet (F(2,54)=2.8802, p=0.06) or diet × carbohydrate (F(2,54)=0.7096, p=0.50), but did reveal a significant difference between in baseline intake of the two carbohydrates (F(2,54)=8.1669, p<0.01). Therefore, intake was converted to percent reduction from baseline (intake following dose X of Exendin-4/intake following 0 µg/kg Exendin-4, (27)) and is presented as mean ± SEM. Percent reduction was analyzed using a two-way ANOVA with Diet and Treatment (0, 0.5, 1, 2, or 3 µg/kg Exendin-4) as independent variables. Data was further analyzed for median inhibitory dose (ID50), calculated as previously described (28) and compared as a function of diet and carbohydrate using Student’s t-tests. For all experiments, significant findings were further analyzed using Fischer’s least significant difference (LSD) post-hoc tests when appropriate. For all statistical analyses the software Statistica (version 6.0, StatSoft® Inc., Tulsa OK) was used.
Results
Body weight
ANOVA revealed a consistent difference in body weight between Chow and obesogenic diet groups throughout the experiment. For the sucrose test period, ANOVA revealed a significant effect of Diet (F(2,162)=68.16, p<0.001) but not Dose (F(5,162)=0.06, p=0.998) or Diet × Dose interaction (F(10,162)=0.01, p=1.00) on body weight. Post-hoc analysis showed HFHE and FCHE rats weighed significantly more than Chow rats at each time point, while no significant differences were observed between obese groups (versus Chow, HFHE: p<0.001; FCHE: p<0.001, all time points, Figure 1a).
Figure 1. Body weight during pharmacological tests.
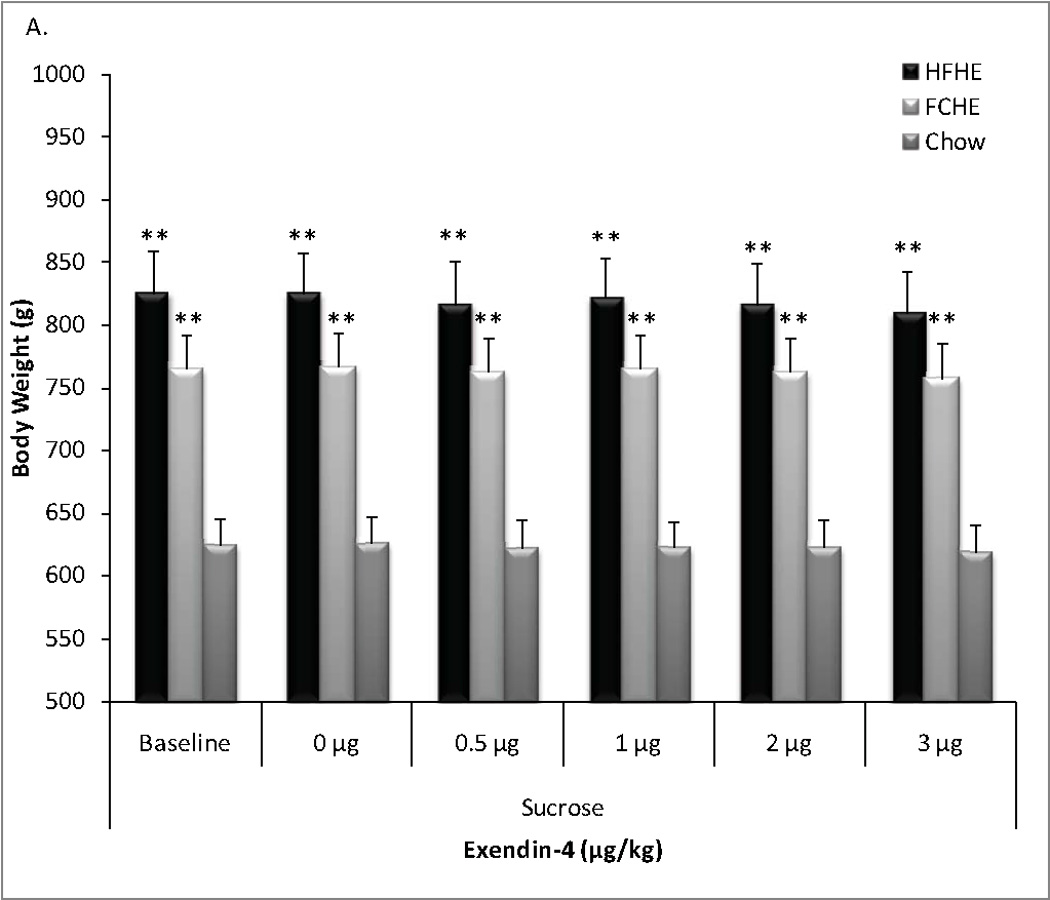
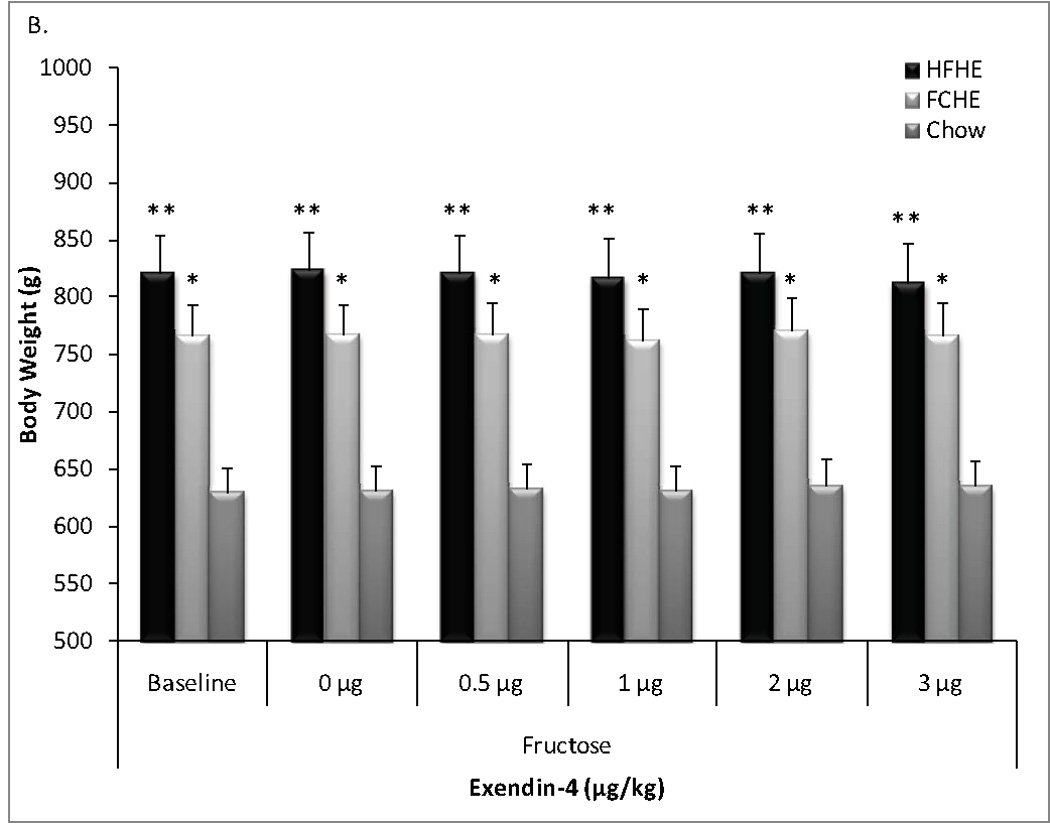
Rats maintained on obesogenic diets for 28 weeks and throughout testing had significantly higher body weight than Chow-fed rats. There was no difference in body weight between HFHE or FCHE groups. A. Average body weight by groups throughout the sucrose tests administered 24 hours after no treatment (baseline weight), vehicle (0 µg/kg) or Exendin-4 treatment. B. Body weight by group throughout the fructose test measured 24 hours after no treatment, vehicle, or Exendin-4 treatment. * p<0.01, ** p<0.001 compared to Chow at the same time point.
ANOVA also revealed a significant effect of Diet (F(2,162)=56.62, p<0.001) but not Dose (F(5,162)=0.02, p=0.999) or Diet × Dose interaction (F(10,162)=0.01, p=1.00) on body weight throughout the fructose test period. Post-hoc analysis again showed Chow rats had significantly lower body weight that HFHE or FCHE at each time point, but no significant differences were observed between obesogenic diet groups (versus Chow, HFHE: p<0.001; FCHE: p<0.01, all time points, Figure 1b).
Effects of GLP-1 receptor agonist on sucrose intake
A two-way ANOVA of normalized sucrose intake revealed significant effects of Diet (F(2,135)=9.468, p<0.001) and Dose (F(4,135)=66.636, p<0.001) but not a significant Diet × Dose interaction (F(8,135)=0.990, p=0.45) on percent reduction of baseline (Figure 2). Whereas all groups showed a dose-dependent reduction in sucrose intake, the effect was dependent upon diet conditions, with post-hoc analysis showing overall sucrose intake was suppressed in HFHE rats to a lesser degree than FCHE (p<0.01) and Chow (p<0.001). Analysis of dose effects revealed that Exendin-4 significantly reduced sucrose intake in HFHE rats at 1 µg/kg and higher doses (1, 2, and 3 µg/kg; all p<0.001) while all doses suppressed sucrose intake in FCHE rats (0.5 µg/kg: p<0.01; 1, 2, and 3 µg/kg: p<0.001). Chow rats showed reductions at 1 µg/kg and above (1, 2, and 3 µg/kg; all p<0.001).
Figure 2. Changes in sucrose intake following Exendin-4.
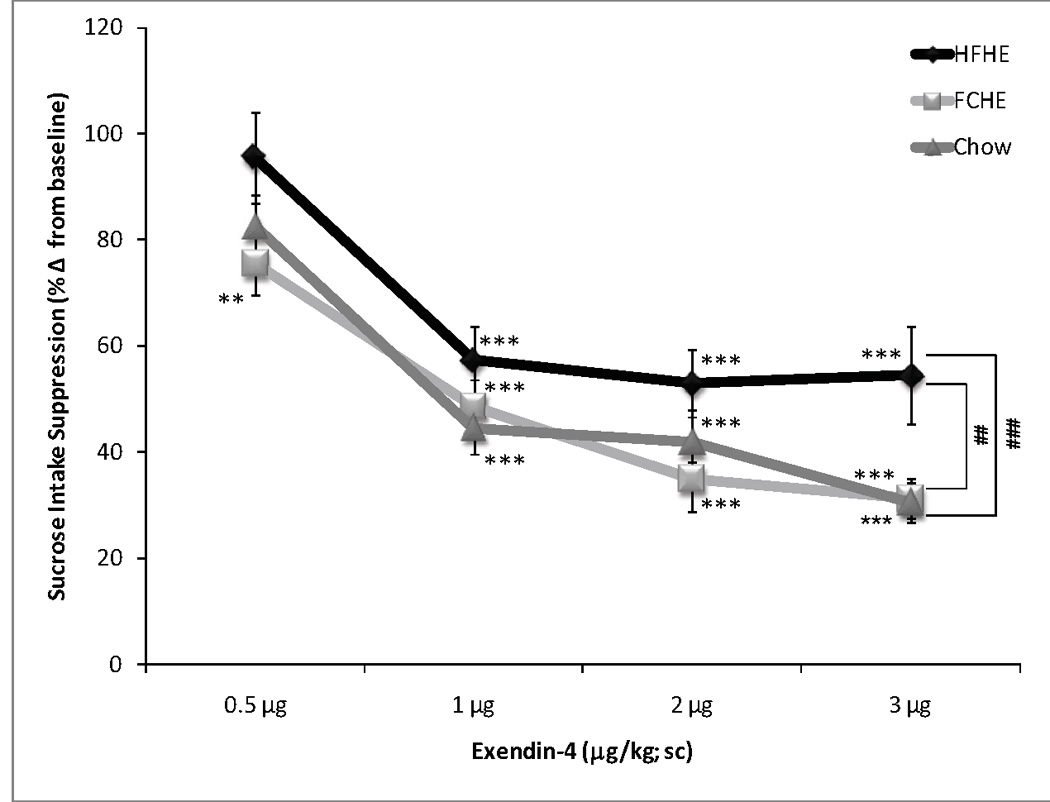
Data is depicted as a reduction from baseline intake (i.e. intake following vehicle injection; set at 1.0 or 100% on y-axis). Sucrose intake was significantly reduced from baseline in all groups at 1, 2, and 3 µg/kg. However, Exendin-4 was less effective at suppressing sucrose intake in HFHE rats. ** p <0.01; *** p<0.001 compared to 0 µg/kg Exendin-4. ## p <0.01, ### p<0.001 indicates diet group differences.
Effects of GLP-1 receptor agonist on fructose intake
A two-way ANOVA of normalized fructose intake revealed significant effects of Diet (F(2,135)=14.329, p<0.001) and Dose (F(4,135)=25.549, p<0.001) but not a significant Diet×Dose interaction (F(8,135)=1.097, p=0.369; Figure 3). All groups showed a dose-dependent reduction in fructose intake, with differential sensitivity by different diet conditions. Suppression of fructose intake in HFHE was blunted compared to FCHE (p<0.001) or Chow (p<0.001). Post-hoc analyses of percent reduction from baseline revealed that only the highest doses (2 µg/kg: p<0.01; 1 µg/kg: p<0.001) reduced intake in HFHE rats. All doses significantly reduced intake in FCHE rats in a dose-dependent manner (0.5 µg/kg: p<0.01; 1, 2, and 3 µg/kg: p<0.001). Chow rats also showed a dose-dependent reduction in fructose intake following all Exendin-4 doses (0.5 µg/kg: p<0.01; 1 µg/kg: p<0.01; 2 and 3 µg/kg: p<0.001).
Figure 3. Changes in fructose intake following Exendin-4.
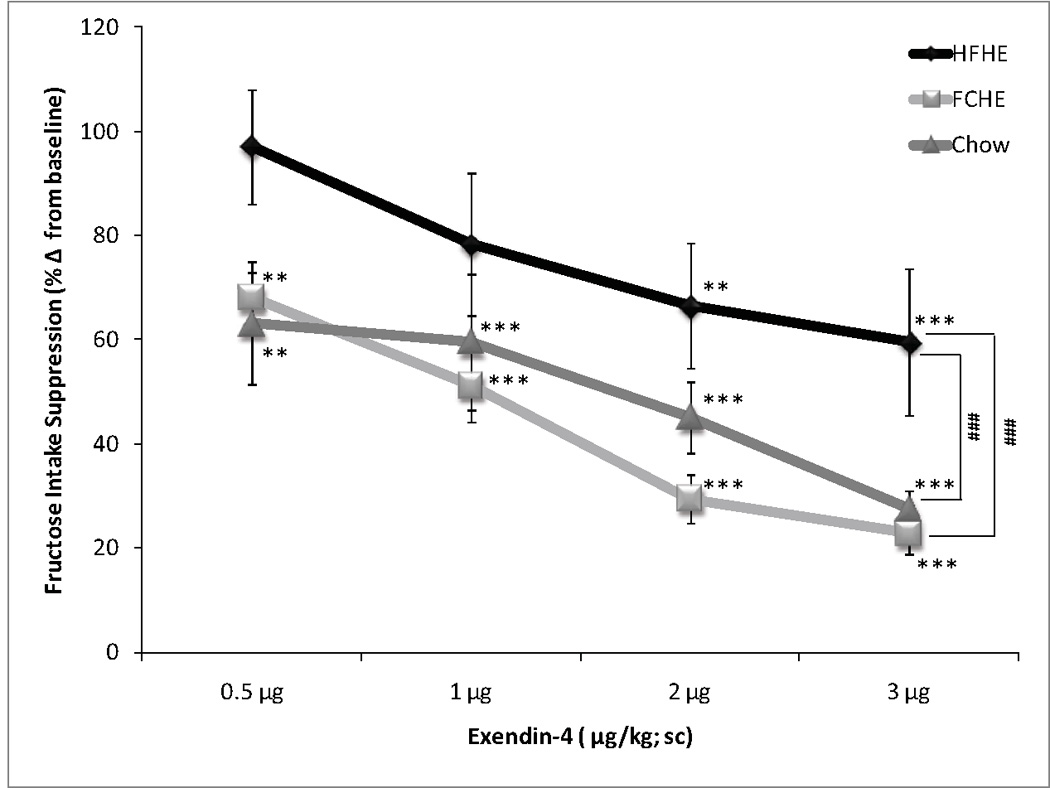
Data is depicted as a reduction from baseline intake (i.e. intake following vehicle injection; set at 1.0 or 100% on y-axis). Fructose intake was reduced in FCHE and Chow by all doses. Only the highest doses, 2 and 3 µg/kg Exendin-4, significantly reduced intake in HFHE rats, which were overall less sensitive to Exendin-4 than FCHE and Chow groups. ** p <0.01; *** p<0.001 compared to 0µg/kg Exendin-4. ### p<0.001 indicates diet group differences.
Median inhibitory dose (ID50)
Calculation and analyses of the ID50 revealed significant differences in the sensitivity to Exendin-4 based on diet (Table 1). Calculation of the ID50 for both tests further supported the findings of the previous analysis and demonstrated that HFHE rats were the least sensitive of all groups to Exendin-4 treatment. For sucrose, independent t-tests revealed significant differences between HFHE and FCHE groups (t(86)=2.99, p<0.01) and HFHE and Chow groups (t(74)=2.34, p<0.05), but not between FCHE and Chow groups (t(74)=0.061, p=0.952). Analysis of the ID50 for fructose again revealed significant differences between HFHE and FCHE groups (t(86)=2.71, p<0.01) and HFHE and Chow groups (t(74)=2.62, p<0.01) but not between FCHE and Chow groups (t(74)=0.061, p=0.952).
Table 1. Effectiveness of GLP-1 receptor stimulation by Exendin-4 as calculated by median inhibitory dose (ID50).
The calculated doses at which intake is reduced by 50% of vehicle baseline (0 µg/kg) are depicted. For both carbohydrates, HFHE rats had a significantly higher ID50 than either HFHE or Chow rats suggesting decreased sensitivity.
| Diet effect on ID50 (µg/kg) | |||
|---|---|---|---|
| HFHE | FCHE | Chow | |
| Sucrose | 3.3 ± 1.1**## | 2.2 ± 0.3 | 2.5 ± 0.1 |
| Fructose | 3.7 ± 1.9**## | 1.6 ± 0.3 | 1.5 ±0.9 |
p > 0.01; compared to Chow,
p<0.01 compared to HFHE.
Discussion
The present study assessed the sensitivity to GLP-1 receptor activation in two animal models of dietary obesity on suppressing intake of palatable carbohydrates. Extended exposure to the obesogenic diets resulted in significantly greater body weight in HFHE and FCHE groups compared to Chow controls, while no differences were observed between obesogenic diets groups (Figure 1). In contrast, we observed marked differences between diet groups following Exendin-4 treatment. HFHE rats were less sensitive overall to GLP-1 receptor activation, particularly when fructose was the test stimulus. Exendin-4 treatments did not significantly alter body weight measured 24 hours after the injection, indicating GLP-1’s effects on carbohydrate intake were not likely due to a dramatic change in homeostatic, energy-regulatory systems. These data support the notion that palatable carbohydrate intake above and beyond homeostatic needs can be regulated by GLP-1 receptor activation. Furthermore, this data demonstrates for the first time that GLP-1 signaling may be differentially altered in different models of dietary obesity, possibly explaining some of the incongruent findings on GLP-1 release in obese humans (10, 15–17). We found that rats fed a high energy diet from which the kilocalories came predominantly from high dietary fat content were markedly less sensitive to the inhibitory effects of Exendin-4 (Figures 2 and 3). On the other hand, rats on a high energy diet consisting of moderately high dietary fat and carbohydrate content did not differ from lean controls, despite their significantly greater body weight (Figure 1). These findings were further supported by analyzing the inhibitory dose at which intake was reduced to 50% of baseline (ID50, Table 1). For both sucrose and fructose, HFHE rats required a higher ID50 than FCHE or Chow fed rats. These findings collectively suggest that a history of dietary fat intake, not obesity alone, may diminish GLP-1 signaling to curb carbohydrate intake.
Previous research has shown that Exendin-4 administered peripherally reduces chow intake in hungry lean and obese rats (13). Studies in lean and obese humans have also shown suppression of appetite and the intake of a balanced meal following GLP-1 administration (8–9). Our data shows that a GLP-1 receptor agonist can also potently reduce the intake of palatable carbohydrates in lean and obese rats when tested in a sated state. This is a novel observation and is relevant to understanding GLP-1’s role in hedonic-driven eating, i.e. intake that is independent of the drive due to actual energy deficits. Relevant to this are the reports from both human and animal studies demonstrating increased postprandial GLP-1 release following Roux-en-Y gastric bypass (16, 29) and ileal interposition surgeries (30–31), both of which are also known to reduce appetite despite restricted caloric intake. Furthermore, gastric bypass but not food restriction appears to reduce behavioral and neural taste functions for sweet taste in obese rats (32). One potential explanation of these findings is that GLP-1 may directly engage the food reward system in addition to energy regulatory circuits. In fact, current research in our laboratory strongly suggests that GLP-1 released endogenously may modulate activity of dopamine neurons in the ventral tegmental area in vivo (33). Initial behavioral data support this notion by demonstrating modulatory effects of intra-ventral tegmental area infusions of Exendin-4 on sucrose preference (34).
An additional riddle is the relationship between GLP-1 signaling, palatable carbohydrates, and obesity. We observed a blunted response in obese HFHE rats compared to chow controls. Such effects could be due to a reduction in endogenous GLP-1 release in obese HFHE rats. Recent research by Williams and colleagues (35) has shown that rats maintained for 4 weeks on a high-fat, high energy diet identical to that used in the present study had lower fasting levels of active GLP-1 compared to rats fed a high carbohydrate (70%), low fat (10%) diet. In this study, similar to our study, the high-fat diet fed rats also exhibited a blunted response to Exendin-4 treatment in 24-hr food intake tests, compared to the low-fat, high carbohydrate diet-fed group. Unfortunately, chow-fed controls were not available for comparison in that study. Previous research has also shown that GLP-1 secretion may be induced by both sucrose and fructose (36–37). Though not measured directly, this and the study by Williams and colleagues collectively suggests that a reduction in endogenous GLP-1 secretion may result from extended exposure to high-fat diets, and this in turn could lead to impaired processing of taste information (38).
In addition to a plausible change in the regulation of peripheral taste information (38–39), GLP-1 may also alter central taste processing. In fact, central GLP-1 administration has been shown to reduce sham feeding of sucrose (14). Changes to the behavioral sequence of feeding of sucrose following GLP-1 administration indicates that central GLP-1 may indeed regulate positive-feedback from a meal, and in turn, reduce intake of palatable foods by attenuating the perceived orosensory reward of the palatable food (14, 34, 40). Taken together, one potential implication of these findings is that excessive consumption of high dietary fat may render anorexigenic gut-brain feedback less effective and in turn, increased stimulation by sweet taste on intake may remain unchecked. Thus, successful treatment of overweight and obesity must include a reduction of the desire to overconsume palatable and obesogenic foods as well as restoration of diminished anorexigenic signals. A similar effect has been achieved by gastric bypass surgery, which increases postprandial GLP-1 response and also reduces appetite and sweet cravings (16–17, 32). Improved understanding of the underlying mechanisms could help with developing an effective non-invasive treatment of obesity by reducing the drive to overconsume palatable foods. Furthermore, these findings caution that in the quest for identifying novel drug targets, conflicting responses may occur due to differences in the source of obesity, including macronutrient content of the diet.
Acknowledgements
This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grants DK080899 (A.H.).
Footnotes
Disclosures: CE Pritchett has no conflicts of interest and no disclosures to make. A Hajnal has no conflicts of interest and no disclosures to make.
REFERENCES
- 1.Gibson SA. Are high-fat, high-sugar foods and diets conducive to obesity? Int J Food Sci Nutr. 1996;47:405–415. doi: 10.3109/09637489609006954. [DOI] [PubMed] [Google Scholar]
- 2.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;282:R46–R54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- 3.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutrition Research Reviews. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 4.Madsen AN, Hansen G, Paulsen SJ, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 5.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiology & Behavior. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 7.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Gutzwiller J-P, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naslund E, Barkeling B, King N, et al. Energy intake and appetite is suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes. 1999;3:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 10.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojanowska E. Physiology and pathophysiology of glucagon-like peptide-1 (GLP-1): The role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Med Sci Monit. 2005;11:RA271–RA278. [PubMed] [Google Scholar]
- 12.Turton MD, O'Shea D, Gunn I, et al. A Role for Glucagon-like Peptide-1 in the Central Regulation of Feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 13.Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 14.Asarian L, Corp ES, Hrupka B, Geary N. Intracerebroventricular glucagon-like peptide-1 (7–36) amide inhibits sham feeding in rats without eliciting satiety. Physiology & Behavior. 1998;64:367–372. doi: 10.1016/s0031-9384(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 15.Adam TCM, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. British Journal of Nutrition. 2005;93:845–851. doi: 10.1079/bjn20041335. [DOI] [PubMed] [Google Scholar]
- 16.Morinigo R, Moize V, Musri M, et al. Glucagon-Like Peptide-1, Peptide YY, Hunger, and Satiety after Gastric Bypass Surgery in Morbidly Obese Subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 17.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes. 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 18.Woods SC, D'Alessio DA, Tso P, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiology & Behavior. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Ackroff K. Learned flavor preferences. The variable potency of post-oral nutrient reinforcers. Appetite. 2008;51:743–746. doi: 10.1016/j.appet.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiology & Behavior. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 21.Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. J Physiol Pharmacol. 2007;58:349–360. [PubMed] [Google Scholar]
- 22.Barrera JG, D'Alessio DD, Drucker DJ, Woods SC, Seeley RJ. Differences in the Central Anorectic Effects of Glucagon-Like Peptide-1 and Exendin-4 in Rats. Diabetes. 2009;58:2820–2927. doi: 10.2337/db09-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archer ZA, Corneloup J, Rayner DV, Barrett P, Moar KM, Mercer JG. Solid and Liquid Obesogenic Diets Induce Obesity and Counter-Regulatory Changes in Hypothalamic Gene Expression in Juvenile Sprague-Dawley Rats. J. Nutr. 2007;137:1483–1490. doi: 10.1093/jn/137.6.1483. [DOI] [PubMed] [Google Scholar]
- 24.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Ann Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 25.Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacology Biochemistry and Behavior. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 26.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 27.Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 28.Hajnal A, De Jonghe BC, Covasa M. Dopamine D2 receptors contribute to increased avidity for sucrose in obese rats lacking CCK-1 receptors. Neuroscience. 2007;148:584–592. doi: 10.1016/j.neuroscience.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckman LM, Beckman TR, Earthman CP. Changes in Gastrointestinal Hormones and Leptin after Roux-en-Y Gastric Bypass Procedure: A Review. Journal of the American Dietetic Association. 110:571–584. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Culnan DM, Albaugh V, Sun M, Lynch CJ, Lang CH, Cooney RN. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010;299:G751–G760. doi: 10.1152/ajpgi.00525.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. American Journal of Physiology - Endocrinology And Metabolism. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 32.Hajnal A, Kovacs P, Ahmed TA, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth K, Abraham H, Hajnal A. Glucagon-like peptide-1 (GLP-1) receptors in the ventral tegmental area of the rat: Neuronal distribution and in vivo electrophysiological effects. Society for Neuroscience Annual Meeting Abstract. 2011 [Google Scholar]
- 34.Alhadeff AL, Rupprecht LE, Hayes MR. Society for the Study of Ingestive Behaviors Meeting Abstract. Clearwater, FL: 2011. NTS GLP-1 projections to the mesolimbic dopaminergic reward system reduce food intake. [Google Scholar]
- 35.Williams DL, Hyvarinen N, Lilly N, et al. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiology & Behavior. 2011 doi: 10.1016/j.physbeh.2011.04.005. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gribble FM, Williams L, Simpson AK, Reimann F. A Novel Glucose-Sensing Mechanism Contributing to Glucagon-Like Peptide-1 Secretion From the GLUTag Cell Line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 37.Fukase N, Takahashi H, Manaka H, et al. Differences in glucagon-like peptide-1 and GIP responses following sucrose ingestion. Diabetes Research and Clinical Practice. 1992;15:187–195. doi: 10.1016/0168-8227(92)90024-l. [DOI] [PubMed] [Google Scholar]
- 38.Martin B, Dotson CD, Shin Y-K, et al. Modulation of Taste Sensitivity by GLP-1 Signaling in Taste Buds. Ann Acad N Y Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann Acad N Y Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 40.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R-1695–R-1706. doi: 10.1152/ajpregu.00870.2004. [DOI] [PubMed] [Google Scholar]


