Abstract
Magnesium is one of the most predominant intracellular divalent cations and is requisite to the regulation of a diverse array of cellular functions. Although accumulating data from multiple studies have begun to illuminate the critical role(s) played by Mg2+ transporters in pathways involved in cell signaling, metabolism, growth and proliferation, there is still a lack of understanding of the underlying molecular mechanisms that govern those various functions. In this review, we focus on the recently described SLC41 family of magnesium transporters, two members of which have been shown to mediate Mg2+ uptake and transport, and highlight what is known about their expression, localization, and function, as well as their roles and contributions to cellular Mg2+ transport.
Keywords: Transporter, MgtE, Magnesium, Solute carrier 41 (SLC41), TRPM7-deficient, membrane
1. Overview of Mg2+ uptake and transport
Intracellular Mg2+ exists at total cellular concentrations estimated to be in the range of several to tens of mM depending on the cell type (reviewed in (Romani, 2011; Romani and Scarpa, 2000b; Saris et al., 2000)). Mg2+ is involved in multiple functions including as an enzyme cofactor (comprising of every enzyme and signaling protein which utilizes a nucleotide triphosphate cofactor), maintenance of active conformations of macromolecules, regulation of phosphoinositide-derived second messengers, charge compensation for negatively charged groups (particularly phosphate), and regulation of various transporters and ion channels (Chien and Cambier, 1990; Eskes et al., 1998; Mandel and Goodman, 1999; Morrill et al., 1998; O’Rourke et al., 1992; Wolf and Cittadini, 1999, 2003).
There are two important features of Mg2+ biochemistry, which constrain how Mg2+ may function in biological systems. The first is the existence of a large Mg2+ buffering capacity of intracellular phosphometabolites (particularly ATP), such that only a small fraction of free Mg2+ entering the cytosol or other compartments is able to remain free in solution intracellularly. The second is the total amount of Mg2+ present within cells. Mg2+ exists within a cell’s various intracellular compartments in millimolar total concentrations. Although the majority of this is bound in various forms, free Mg2+ is typically within the range of 0.3-1.0 mM. On account of these two factors, a total flux of Mg2+ into a cell equivalent in magnitude to that occurring acutely for example with a typical Ca2+ signal, will result in only negligible changes in total intracellular free Mg2+ (reviewed in (Romani and Scarpa, 2000a) and (Romani and Maguire, 2002)). However, it remains possible that Mg2+ may function as an intracellular messenger in localized regions near Mg2+ transporters, as has been recently suggested to occur following T-cell receptor activation (Li et al., 2011).
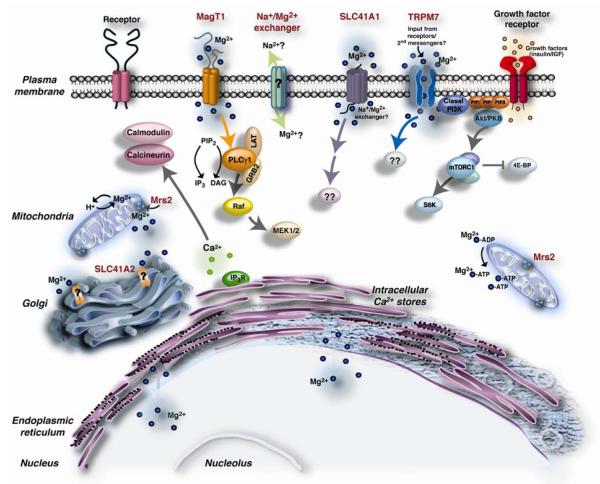
Over the past decade, a number of magnesium transporters and transport pathways have been molecularly identified or proposed to exist in vertebrate cells, and it is the coordinated function of these proteins and pathways that determines vertebrate cellular Mg2+ homeostasis (Figure 1). Members of the SoLute Carrier family 41 (SLC41A1 and A2) are prominent among the molecularly identified transporters, due to their recognized homology to the prokaryotic MgtE Mg2+ transporter family. SLC41A1 is thought to mediate Mg2+ transport across the plasma membrane, whereas SLC41A2 may mediate Mg2+ transport across either the plasma membrane or organellar membranes. In addition to SLC41A1 and SLC41A2, transporters that mediate and/or regulate Mg2+ uptake across the plasma membrane include, Transient Receptor Potential cation channel subfamily Melastatin 7 (TRPM7), Magnesium Transporter protein 1 (MagT1) and Ancient Conserved Domain Protein (ACDP) family proteins ((Deason-Towne et al., 2011; Goytain and Quamme, 2005a; Sahni et al., 2007; Schmitz et al., 2003; Sponder et al., 2010; Wabakken et al., 2003)). The TRPM7 homologue, TRPM6, is also a plasma membrane transporter, and has been linked to Mg2+ uptake from the gut lumen and urine for the purpose of maintaining organism-level Mg2+ homeostasis (Schlingmann et al., 2002; Voets et al., 2004). In conjunction with progress in the characterization of Mg2+ transport systems at the molecular level, physiological studies have accumulated evidence that Mg2+ transport is dynamically regulated, emphasizing the crucial role Mg2+ transport must play in eukaryotic cell function. While total cellular free ionized cytosolic Mg2+ concentrations remain relatively constant under all but extreme conditions of Mg2+ deprivation or supplementation (Romani and Maguire, 2002; Wolf et al., 2003), total Mg2+ contents have been shown to change acutely to a considerable extent in vertebrate cells subjected to various types of stimulation (Grubbs, 1991; Gunther and Hollriegl, 1993). Although significant alterations in Mg2+ transport may also occur for regulatory purposes between one or more cellular subcompartments, the regulation of intercompartmental Mg2+ transport has proven difficult to study due to a lack of compartment-specific probes.
Figure 1. Mg2+ transporters in vertebrate cells.
Pictured are various proposed transporters and their predicted transport mechanisms: SLC41A1/A2 (channel transport mechanism based on structural homology with prokaryotic MgtE transporters); Mrs2, a mitochondrial Mg2+ uptake system that transports Mg2+ with ΔΨ (channel transport mechanism based on similarity with yeast Alr family); TRPM7 channel kinase (channel transport mechanism), and MagT1 (thought to be a plasma membrane Mg2+ transporter with an ion channel transport mechanism). Overexpression of either TRPM7 or SLC41A1 in TRPM7-deficient cells allows them to grow and proliferate due to Mg2+ transport via the respective overexpressed transporters, suggesting they may have some overlap in function. TRPM7 has been implicated to regulate lymphocyte growth via rapid onset of PI3K activation and its downstream signaling events. MagT1 has been proposed to regulate the PLCγ1 signaling pathway in T-lymphocytes, based on studies of human patients with mutations in MagT1. Note that Mg2+ transport into both the endoplasmic reticulum and nucleus are very poorly characterized and hence are pictured simply as passive exchange mechanisms. Although the gene for Na+/Mg2+ exchanger (?) has not been cloned, such an exchange mechanism has been proposed to exist and mediate Mg2+ efflux in vertebrate cells.
2. SLC41 Mg2+ transporters: vertebrate representatives of the MgtE family
The SLC41 family of vertebrate magnesium transporters was first identified and characterized in 2003 (Wabakken et al., 2003) and comprises three members – SLC41A1, SLC41A2 and SLC41A3 (Table 1). Members of the SLC41 transporter family are found in all eukaryotes, and display distant homology to the prokaryotic MgtE family of Mg2+ transporters (Hattori et al., 2009; Hattori et al., 2007; Ishitani et al., 2008). The Transporter Classification DataBase (TCDB; Saier et al., 2009) categorizes SLC41 transporters in the MgtE family - 9.A.19, with 9.A being reserved for ‘transporters of unknown biochemical mechanisms’. Comparison of the SLC41 transporters with protein databases (Pfam and NCBI), identified two domains - D1 and D2, homologous to Pfam10769, a domain found in the prokaryotic MgtE transporter (Wabakken et al., 2003). Two conserved motifs – PX6GN and P(D/A)X4PX6D with functional implications have also been identified in both D1 and D2 domains (Wabakken et al., 2003). Furthermore, SLC41 proteins appear to possess the same basic structural features of prokaryotic MgtE transporters: MgtE proteins create a Mg2+-selective pore via homodimerization (Hattori et al., 2007), and examination of the SLC41 topology suggests the presence of two five TM span MgtE domains, connected by a TM spanning linker. Similarly, the large N-terminal domains of prokaryotic MgtE proteins have been implicated in regulation of their Mg2+ transport, and the shorter N-terminal cytoplasmic domain of SLC41A1 has recently been implicated in regulation of SLC41A1 function (Hattori et al., 2009; Mandt et al., 2011).
Table 1.
SLC41-MgtE-like magnesium transporter family
| Human gene name |
Protein name |
Aliases | Predominant substrates |
Transport type*)/ coupling ions |
Tissue distribution & cellular/subcellular expression |
Link to disease |
Human gene Iocus |
Sequence accession ID |
Splice variants |
|---|---|---|---|---|---|---|---|---|---|
| SLC41A1 | MgtE | Mg2+(Sr2+,Zn2+,Cu2+, Fe2+,Co2+,Ba2+,Cd2+) |
Ch | Kidney,heart, testis, skeletal muscle, prostate, adrenal gland and thyrold |
Mutations in PARK 16 locus |
1q32.1 | NM_173854 | ||
| SLC41A2 | SLC1A1-L1 SLC41A1-like 1 |
Mg2+(Ba2+,Ni2+,Co2+ Fe2+,Mn2+) |
Ch | Highest expression in cerebellum, lymph nodes, stomach, lungs, testis and skin |
12q23.3 | NM_032148.3 | 2 potential splice variants |
||
| SLC41A3 | SLC41A1-L2 SLC41A1-like 2 FLJ20473 |
Ch? (predicted based on homology with the other 2 members/MgtE transporter) |
3q21.2 |
NM_001008485.1 NM_017836.3 NM_001008486.1 NM_001008487.1 NM_001164475.1 |
5 splice variants |
*)C:Cotransporter; E:Exchanger; F:Facilitated transporter; O:Orphan transporter; Ch: channel
3. SLC41A1
3.1 Expression and localization
SLC41A1 was the first member of the family to be described, and was identified using the modified Signal Sequence Trap (SST) method (Wabakken et al., 2003). High expression levels of SLC41A1 have been detected in the human heart and testis, whereas prostate, adrenal gland, skeletal muscle and thyroid have lower expression levels. Hematopoietic tissues, brain, lungs and colon displayed the weakest expression in surveys of primary tissue, although lymphoid cell lines including Tom-1 and Jurkat cells were observed to express SLC41A1 as a distinct band (Wabakken et al., 2003). SLC41A1 was initially implicated in vertebrate Mg2+ transport when mice that were kept on low magnesium versus normal magnesium diet for 5 days were shown to have increased Slc41a1 mRNA expression in the kidney, colon and heart, suggesting its involvement in Mg2+ homeostasis (Goytain and Quamme, 2005a).
SLC41A1 protein appears to exist primarily in intracellular compartments and on the plasma membrane. An N-terminal FLAG-tagged version of the protein can be detected at the plasma membrane of HEK293 cells by confocal microscopy, an observation that has been further confirmed biochemically (Kolisek et al., 2008). Flow cytometric analysis of SLC41A1 tagged at both N and C-termini with epitope tags also demonstrates plasma membrane expression (Mandt et al., 2011).
3.2 Characterization and topology
The protein encoded by human SLC41A1 has a predicted molecular weight of ~56kDa, and this has been well established by a number of studies (Kolisek et al., 2008; Mandt et al., 2011; Wabakken et al., 2003). Although initial studies proposed that the protein had 10 transmembrane (TM) spans, epitope tagging studies indicate that the transporter has an odd number of TM spans, most likely 11, with an N-terminus in/C-terminus out topology. In addition, our lab has shown that intracellular transport serves as a regulatory mechanism for expression of SLC41A1 on the cell surface (Mandt et al., 2011). Protein-protein interaction resulting in formation of large Multiprotein functional complexes (MPCs) have been shown to play a role in cellular signaling processes, and biochemical analysis of SLC41A1 indicates that it might be part of such a MPC in HEK293 cells (Kolisek et al., 2008). The nature of the MPC’s which may contain SLC41A1 remain obscure, as although increasing concentrations of detergent are able to dissociate SLC41A1 from its associated MPC, it has yet to be determined what proteins are interacting with SLC41A1 and constitute the MPC (Kolisek et al., 2008).
3.3 Functional studies
An initial study suggested that SLC41A1 functions as a nonspecific divalent cation channel, since expression of the mouse Slc41a1 in Xenopus oocytes led to the generation of Mg2+ specific currents as well as mediated transport of Fe2+, Zn2+, Cu2+, Co2+ and Cd2+(Goytain and Quamme, 2005a). In contrast, whole cell patch clamp analysis by our group following expression of SLC41A1 in TRPM7-deficient DT40 cells was not able to detect any currents associated with SLC41A1 expression (J. Sahni and A. M. Scharenberg, unpublished observations), and Kolisek and colleagues observed that overexpression of the human SLC41A1 in HEK293 cells resulted in development of endogenous Cl− currents, which were repressed by DIDS (4,4′ Diisothiocyanatostilbene-2, 2′-disulfonic acid), a broad-spectrum inhibitor of chloride transport (Kolisek et al., 2008). These observations raise the question as to why development of prominent Mg2+ specific currents was observed in the first study, especially as the mouse and human sequences display a high degree of homology (98% identity). One explanation could be that SLC41A1’s interaction with a larger multi-protein complex in the HEK-293 system results in activation or regulation of associated Cl− channels, whereas the absence of its partner proteins in the DT40 or Xenopus oocytes contexts results in alternative modes of function.
Using the TRPM7-deficient DT40 B-cell model system previously characterized in our lab (Schmitz et al., 2003), we have used functional complementation of cell growth to infer that SLC41A1 is capable of trans-plasma membrane Mg2+ transport. Furthermore, we identified the N-terminus of SLC41A1 as a defined protein domain of SLC41A1 that is required for regulation of its intracellular transport, suggesting that it was involved in the sensing or receipt by SLC41A1 of information regarding the status of intracellular Mg2+ homeostasis (Mandt et al., 2011). Our findings suggest a model wherein under Mg2+-replete conditions, SLC41A1 is internalized and predominantly shuttled to the lysosomes for degradation. However, under low or Mg2+-deficient conditions, the transporter either avoids internalization or is internalized, but does not undergo lysosomal degradation and is instead recycled back to the cell surface. Such a model is similar to that proposed for the regulation of the Alr family of Mg2+ transporters in yeast by Graschopf and colleagues (Graschopf et al., 2001).
Kolisek and colleagues recently found that the expression of human SLC41A1 in HEK293 cells leads to Mg2+ efflux, on the basis of which they proposed that SLC41A1 may function as a Na+/ Mg2+ exchanger (Kolisek et al., 2008; Kolisek et al., 2012). The disparate observation of enhanced Mg2+ uptake in TRPM7-deficient cells vs. enhanced Mg2+ export in HEK293 cells likely reflects differences in the presence of protein interaction partners, or regulation of Mg2+ transport in the two systems used in the studies, and suggests the presence of a deeper level of coordination of Mg2+ transport among transporters of the various families. These results emphasize that understanding the coordination of Mg2+ transport among the various families of Mg2+ transporters, and defining specific functions or roles for Mg2+ transport mediated by each family member, are an important priority for the field.
3.4 Implications in disease
The PARK16 (Parkinson disease 16) locus has been shown to carry mutations in patients of both European and Asian ancestries (Pankratz et al., 2009; Satake et al., 2009). Sequencing analysis of this locus recently revealed novel mutations in the SLC41A1 gene - A350V (Tucci et al., 2010), A436G and A1440G (Yan et al., 2011). However, it’s still unclear how SLC41A1 impacts this neurodegenerative disease at a biochemical level.
4. SLC41A2
4.1 Expression and localization
The second member of the SLC41 transporter family, SLC41A2, is expressed in normal human tissues with lymph nodes, stomach, lungs, testis and skin with the highest expression followed by spleen, intestine, heart, breast and kidneys exhibiting moderate expression. Respiratory epithelia, liver, pancreas, thyroid, uterine glands and glial cells show weak or negative expression (expression data obtained from - http://www.proteinatlas.org/ENSG00000136052/normal). Real-time RT-PCR analysis of Mouse Distal Convoluted Tubule (MDCT) cells cultured in low Mg2+ media (1mM) or Mg2+ free media for 16h indicated no change in Slc41a2 expression and the same was found to be the case with kidney cortical tissue harvested from mice kept on normal or low Mg2+ diet for 5 days (Goytain and Quamme, 2005b).
The subcellular localization of SLC41A2 remains unclear - although heterologously expressed epitope tagged protein showed detectable plasma membrane localization in TRPM7-deficient DT40 cells by flow cytometry, its orientation appeared to be the opposite of that predicted by the structure of prokaryotic MgtE proteins or SLC41A1 (see discussion below), raising the possibility that the protein detected on the plasma membrane localization may have reflected aberrant cell surface transport due to overexpression (Sahni et al., 2007). The N-terminal out orientation observed in these studies would be consistent with a role for SLC41A2 in Mg2+ transport across organellar membranes, where the N-terminal domain of SLC41A2 would be involved in regulation of the internal Mg2+ content of the organelle
4.2 Characterization and topology
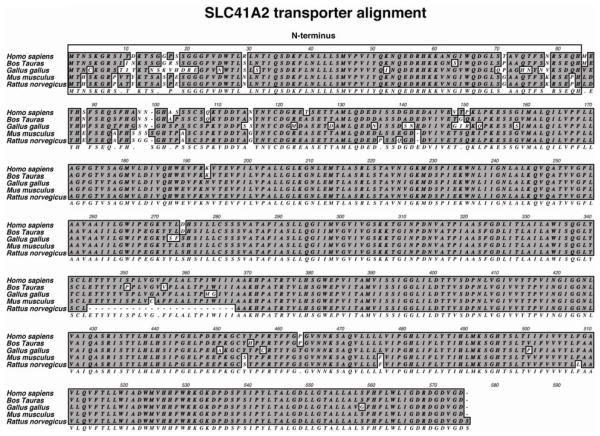
SLC41A2 has now been identified in a large number of vertebrate species. The initial characterization of the human SLC41A2 in the NCBI database (accession# AAI06873) indicated that it comprised of 490 amino acid residues with a molecular weight of ~53.2kDa and was shorter than the reported mouse SLC41A2 protein (573 aa residues; MW=62.1kDa) (Goytain and Quamme, 2005b). Interestingly, upon ORF analysis of the human SLC41A2, we found presence of 83 extra amino acid residues in-frame at 5′ of the reported N-terminus (J. Sahni and A.M.Scharenberg, unpublished observation), which displayed close homology to the mouse SLC41A2 present in the database. Subsequently, the originally reported shorter sequence (referred to as SS in this review) was replaced by an updated full-length (FL) SLC41A2 sequence in the NCBI database (accession# NP_115524.3). The predicted molecular weight of the full-length protein is ~62.3kDa (573 aa residues) and sequence analysis of its long N-terminus indicated absence of any signal peptide and/or conserved domains. Although existing data unambiguously shows that the extended N-terminal region of SLC41A2 is highly conserved amongst vertebrates (Figure 2), it’s exact role remains undefined.
Figure 2. ClustalW alignment of the full-length human SLC41A2 with other identified homologues in the NCBI database.
Sequence comparison of the full-length human SLC41A2 showed that the long N-terminus (outlined box) is highly conserved amongst most of its homologous sequences, although its precise function is unclear.
Goytain and Quamme characterized the mouse SLC41A2 and their hydophobicity analysis suggested that it consisted of 12 TM spans (Goytain and Quamme, 2005b). However, heterologous expression of the epitope-tagged SS human SLC41A2 in DT40 TRPM7-deficient cells revealed the presence of N and C-terminal epitopes on opposite sides of the plasma membrane, most consistent with SLC41A2 possessing 11 TM spans in an unexpected N-terminus-outside/C-terminus inside topology (Sahni et al., 2007). As noted above, because this orientation is the reverse of that observed for the B. subtilis MgtE homologue, SLC41A1, one possibility is that SLC41A2 is normally involved in organellar Mg2+ transport (where its topology would place its N-terminus inside an intracellular organelle), and that SLC41A2 transporters may have aberrantly accumulated on the plasma membrane in our initial study due to overexpression.
4.3 Functional studies
Expression of the mouse Slc41a2 in Xenopus oocytes was shown to mediate Mg2+ currents as well as the transport of a variety of divalent cations including Ba2+, Ni2+, Co2+ and Fe2+, suggesting that it might be associated with cellular Mg2+ transport (Goytain and Quamme, 2005b). Confirmation that SLC41A2 (SS) could indeed function as a plasma membrane Mg2+ transporter upon overexpression in TRPM7-deficient DT40 cells was provided when these cells were able to grow and proliferate in regular cell culture media without supplemental Mg2+ upon induction of the protein. However, similar to our results with SLC41A1, investigation of SLC41A2 expressing cells by whole cell patch clamp did not reveal any novel currents associated with its expression (Goytain and Quamme, 2005b; Sahni et al., 2007). Our inability to detect any apparent currents, despite the capacity of SLC41A2 to complement the Mg2+ uptake and growth defects of TRPM7-deficient cells, suggests that the regulation or function of SLC41A2 in the DT40 context differs from that in the Xenopus oocyte system. It will be interesting to resolve the differences in the behavior of SLC41A2 in order to generate a clearer understanding of both its regulatory and Mg2+-transport functions.
Consistent with our observations, a recent study carried out by Liu et al in which the authors analyzed the role of TRPM7 in vertebrate embryogenesis, found that SLC41A2 (SS) was able to rescue the phenotype caused by depletion of TRPM7 (Liu et al., 2011). Additional findings by the same group further reported that expression of SLC41A2 in fibroblasts with knocked down TRPM7 could restore both cell morphology as well as cell motility (Su et al., 2011). Taken together, the above studies provide further support for the concept that SLC41A2 is involved in maintenance of cellular Mg2+ homeostasis in vertebrates, and that Mg2+ transport mediated by SLC41 transporters may have some functional overlap with that mediated by TRPM7. Moreover, in-depth functional analysis of both the SS and full-length SLC41A2 in TRPM7-deficient cells showed that inducible expression of only the SS version was able to restore their cell growth and proliferative defects (J. Sahni and A.M.Scharenberg, unpublished observations). This alludes to a likelihood of the shorter form of the protein functioning as a potential splice variant of the full-length protein. However, it’s also conceivable the epitope-tagged full-length SLC41A2 does not fold properly or has intracellular trafficking issues due to the presence of epitope tags, which might hinder its transport and localization at the plasma membrane (e.g. see (Yewdell et al., 2011) , (Snapp, 2009)).
Despite the abundant evidence that links SLC41A2 to Mg2+ transport, a plethora of questions regarding SLC41A2 remain unanswered: the functional role, if any, for its N-terminus that is analogous to what has been observed for SLC41A1; identification of signals or metabolic cues that regulate its expression and activity; and determination of its specific role in maintenance of Mg2+ homeostasis in intracellular or organellar milieus.
4.4 Implications in disease
There is no current evidence to indicate association of SLC41A2 or its mutated forms with any specific disease, although SLC41A2 is reported as either over or under expressed in 75 disease states including a number of carcinomas and lymphomas according to the database on European Bioinformatics Institute (EBI) website (ref-http://www.ebi.ac.uk/gxa/gene/ENST00000258538).
5. SLC41A3
As both SLC41A1 and SLC41A2 have been shown to function as magnesium transporters, it’s quite likely that SLC41A3 plays a similar functional role in vertebrates. However, in contrast to SLC41A1 and SLC41A2, no studies have yet been published regarding the third member of this family, to date. Unpublished data from our lab using recombinant SLC41A3 constructs have not been able to consistently detect protein expression or capacity to complement TRPM7-deficient DT40 cell lines, suggesting that SLC41A3 may be more dependent on partner proteins, or possess specialized functions. This may in part be because five splice isoforms of SLC41A3 have been reported, and their explicit biochemical role(s) may need to be individually characterized in the specific cell or tissue context where they are normally expressed. As bioinformatics data on EBI suggests that SLC41A3 is differentially expressed in a number of human organs including cerebellum, testis, prostate, etc. as well as in some cell lines - SW480, MOLT4, MCF-7, etc, it seems likely that role(s) for the various SLC41A3 isoforms in Mg2+ transport will eventually be defined.
6. Pharmaceutical aspects
Currently, no small molecule drugs have been reported that specifically target the SLC41 family of transporters.
7. Coordination with other vertebrate Mg2+ transporters
Given emerging evidence that multiple vertebrate Mg2+ transporters are expressed in many vertebrate cell types and collectively contribute to Mg2+ homeostasis, the existence of a dynamic interplay between the various individual transporters seems likely. One example of such functional overlap or linkage is Mg2+ transport mediated by SLC41 Mg2+ transporters and TRPM7, as suggested by the observation that SLC41A1 is upregulated in a Mg2+ concentration-dependent manner in TRPM7-deficient DT40 cells (Mandt et al., 2011). As the expression pattern and functions of each family become more clearly defined, it is likely that similar interactions will be identified, both with respect to Mg2+ transport, as well as the regulatory mechanisms that govern the function of each type of protein.
8. Summary
That SLC41 family members play an important role in the maintenance of cellular Mg2+ homeostasis is now well supported, but the mechanisms through which Mg2+ transport mediated by these proteins is regulated, and how their functions are coordinated with Mg2+ transport mediated by other transporters, remain largely obscure. Future studies focused on a mechanistic understanding of these questions promise to provide important insights into how Mg2+ transport mediated by SLC41 family proteins, as well as that mediated by members of other Mg2+ transporter families, is coordinated with myriad aspects of cell biology.
Acknowledgements
We would like to acknowledge past and present members of the Ion Channel group in the Scharenberg lab for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chien MM, Cambier JC. Divalent cation regulation of phosphoinositide metabolism. Naturally occurring B lymphoblasts contain a Mg2(+)-regulated phosphatidylinositol-specific phospholipase C. J Biol Chem. 1990;265(16):9201–9207. [PubMed] [Google Scholar]
- Deason-Towne F, Perraud AL, Schmitz C. The Mg(2+) transporter MagT1 partially rescues cell growth and Mg(2+) uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011;585(14):2275–2278. doi: 10.1016/j.febslet.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143(1):217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goytain A, Quamme GA. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol Genomics. 2005a;21(3):337–342. doi: 10.1152/physiolgenomics.00261.2004. [DOI] [PubMed] [Google Scholar]
- Goytain A, Quamme GA. Functional characterization of the mouse [corrected] solute carrier, SLC41A2. Biochem Biophys Res Commun. 2005b;330(3):701–705. doi: 10.1016/j.bbrc.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Graschopf A, Stadler JA, Hoellerer MK, Eder S, Sieghardt M, Kohlwein SD, Schweyen RJ. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem. 2001;276(19):16216–16222. doi: 10.1074/jbc.M101504200. [DOI] [PubMed] [Google Scholar]
- Grubbs RD. Effect of epidermal growth factor on magnesium homeostasis in BC3H1 myocytes. Am J Physiol. 1991;260(6 Pt 1):C1158–1164. doi: 10.1152/ajpcell.1991.260.6.C1158. [DOI] [PubMed] [Google Scholar]
- Gunther T, Hollriegl V. Na(+)- and anion-dependent Mg2+ influx in isolated hepatocytes. Biochim Biophys Acta. 1993;1149(1):49–54. doi: 10.1016/0005-2736(93)90023-s. [DOI] [PubMed] [Google Scholar]
- Hattori M, Iwase N, Furuya N, Tanaka Y, Tsukazaki T, Ishitani R, Maguire ME, Ito K, Maturana A, Nureki O. Mg(2+)-dependent gating of bacterial MgtE channel underlies Mg(2+) homeostasis. EMBO J. 2009;28(22):3602–3612. doi: 10.1038/emboj.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature. 2007;448(7157):1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- Ishitani R, Sugita Y, Dohmae N, Furuya N, Hattori M, Nureki O. Mg2+-sensing mechanism of Mg2+ transporter MgtE probed by molecular dynamics study. Proc Natl Acad Sci U S A. 2008;105(40):15393–15398. doi: 10.1073/pnas.0802991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M. SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem. 2008;283(23):16235–16247. doi: 10.1074/jbc.M707276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisek M, Nestler A, Vormann J, Schweigel-Rontgen M. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am J Physiol Cell Physiol. 2012;302(1):C318–326. doi: 10.1152/ajpcell.00289.2011. [DOI] [PubMed] [Google Scholar]
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Su LT, Khadka DK, Mezzacappa C, Komiya Y, Sato A, Habas R, Runnels LW. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev Biol. 2011;350(2):348–357. doi: 10.1016/j.ydbio.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel G, Goodman RH. Cell signalling. DREAM on without calcium. Nature. 1999;398(6722):29–30. doi: 10.1038/17933. [DOI] [PubMed] [Google Scholar]
- Mandt T, Song Y, Scharenberg AM, Sahni J. SLC41A1 Mg(2+) transport is regulated via Mg(2+)-dependent endosomal recycling through its N-terminal cytoplasmic domain. Biochem J. 2011;439(1):129–139. doi: 10.1042/BJ20110807. [DOI] [PubMed] [Google Scholar]
- Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipid and lipid second messenger levels in vascular smooth muscle cells. FEBS Lett. 1998;440(1-2):167–171. doi: 10.1016/s0014-5793(98)01446-x. [DOI] [PubMed] [Google Scholar]
- O’Rourke B, Backx PH, Marban E. Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes. Science. 1992;257(5067):245–248. doi: 10.1126/science.1321495. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124(6):593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011;512(1):1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani AM, Maguire ME. Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals. 2002;15(3):271–283. doi: 10.1023/a:1016082900838. [DOI] [PubMed] [Google Scholar]
- Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000a;5:D720–734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000b;5:D720–734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401(2):505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr., Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: recent advances. Nucleic Acids Res. 2009;37(Database issue):D274–278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294(1-2):1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41(12):1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31(2):166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114(2):191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Snapp EL. Fluorescent proteins: a cell biologist’s user guide. Trends Cell Biol. 2009;19(11):649–655. doi: 10.1016/j.tcb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponder G, Svidova S, Schweigel M, Vormann J, Kolisek M. Splice-variant 1 of the ancient domain protein 2 (ACDP2) complements the magnesium-deficient growth phenotype of Salmonella enterica sv. typhimurium strain MM281. Magnes Res. 2010;23(2):105–114. doi: 10.1684/mrh.2010.0206. [DOI] [PubMed] [Google Scholar]
- Su LT, Liu W, Chen HC, Gonzalez-Pagan O, Habas R, Runnels LW. TRPM7 regulates polarized cell movements. Biochem J. 2011;434(3):513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci A, Nalls MA, Houlden H, Revesz T, Singleton AB, Wood NW, Hardy J, Paisan-Ruiz C. Genetic variability at the PARK16 locus. Eur J Hum Genet. 2010;18(12):1356–1359. doi: 10.1038/ejhg.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279(1):19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Wabakken T, Rian E, Kveine M, Aasheim HC. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem Biophys Res Commun. 2003;306(3):718–724. doi: 10.1016/s0006-291x(03)01030-1. [DOI] [PubMed] [Google Scholar]
- Wolf FI, Cittadini A. Magnesium in cell proliferation and differentiation. Front Biosci. 1999;4:D607–617. doi: 10.2741/wolf. [DOI] [PubMed] [Google Scholar]
- Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med. 2003;24(1-3):3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- Wolf FI, Torsello A, Fasanella S, Cittadini A. Cell physiology of magnesium. Mol Aspects Med. 2003;24(1-3):11–26. doi: 10.1016/s0098-2997(02)00088-2. [DOI] [PubMed] [Google Scholar]
- Yan Y, Tian J, Mo X, Zhao G, Yin X, Pu J, Zhang B. Genetic variants in the RAB7L1 and SLC41A1 genes of the PARK16 locus in Chinese Parkinson’s disease patients. Int J Neurosci. 2011;121(11):632–636. doi: 10.3109/00207454.2011.598983. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Lacsina JR, Rechsteiner MC, Nicchitta CV. Out with the old, in with the new? Comparing methods for measuring protein degradation. Cell Biol Int. 2011;35(5):457–462. doi: 10.1042/CBI20110055. [DOI] [PMC free article] [PubMed] [Google Scholar]