Abstract
Oxytocin neurons have a physiological role in food intake and energy balance. Central administration of oxytocin is powerfully anorexigenic, reducing food intake and meal duration. The central mechanisms underlying this effect of oxytocin have become better understood in the past few years. Parvocellular neurons of the paraventricular nucleus project to the caudal brainstem to regulate feeding via autonomic functions including the gastrointestinal vago-vagal reflex. In contrast, magnocellular neurons of the supraoptic and paraventricular nuclei release oxytocin from their dendrites to diffuse to distant hypothalamic targets involved in satiety. The ventromedial hypothalamus, for example, expresses a high density of oxytocin receptors but does not contain detectable oxytocin nerve fibers. Magnocellular neurons represent targets for the anorexigenic neuropeptide α-melanocyte stimulating hormone. In addition to homeostatic control, oxytocin may also have a role in reward-related feeding. Evidence suggests that oxytocin can selectively suppress sugar intake and that it may have a role in limiting the intake of palatable food by inhibiting the reward pathway.
Keywords: oxytocin, food, appetite, satiety, reward
Introduction
Unlike Roald Dahl’s “enormously fat” Augustus Gloop – a boy who pursued eating as a hobby – humans usually eat much less than they could. Obesity is typically the result of a modest excess of energy intake over expenditure, but one that is sustained over a prolonged period. In fact, humans, like other animals, are very efficient at balancing energy intake and expenditure. If rats are allowed unlimited access to a high-energy palatable diet, their energy intake diverges quickly from control animals fed a bland diet, but the difference in energy intake stabilizes within days (Archer and Mercer, 2007). Thus rats, which presumably do not feel societal pressure to be slim, will overeat palatable food, but only to a certain extent. When their access to palatable food ends, rats typically undereat, failing to defend the extra body weight they have accumulated.
A variety of peripheral signals convey information to control meal size and, in the longer term, these signals are modified according to physiological state and the size of energy reserves. Several “satiety” peptides are secreted from the gastrointestinal tract, including cholecystokinin (CCK), glucagon-like-peptide-1 (GLP-1), and peptide YY (PYY) (Strader and Woods, 2005); some of these act on the brain at sites that lack a blood-brain barrier, others are transported across the blood-brain barrier, and others act via vagal neuronal afferents (Verbalis et al., 1986). Leptin, which is secreted from adipose tissue in proportion to the size of fat stores, is not itself a satiety signal, but by signaling the size of peripheral fat reserves it has a long term anorectic influence, and in part it may act by moderating acute satiety signals arising from the gastrointestinal tract. Whether these satiety signals and leptin all converge at a discrete “satiety center” in the brain is unclear but several neuronal populations have been identified as likely candidates for mediating satiety. In part, these have been identified because they synthesize peptides that have marked anorexic actions when administered centrally.
One of those potently anorectic neuropeptides is oxytocin – a peptide classically thought to be involved mainly in reproductive functions. However, as we review here, there is now considerable evidence that oxytocin also plays an important role in satiety.
Physiological Roles of Oxytocin
Oxytocin is produced in two hypothalamic regions: the supraoptic nucleus (SON) and paraventricular nucleus (PVN). Magnocellular neuroendocrine neurons in these nuclei project to the posterior pituitary gland, from where oxytocin is secreted into the blood. Classically, oxytocin secreted from the pituitary gland is involved in efficient and timely fetal expulsion, and is indispensable for the milk-ejection reflex and successful lactation (Nishimori et al., 1996); in some species, including rodents but not humans, it also regulates sodium excretion (natriuresis) (Verbalis et al., 1991), both by direct actions at the kidneys and indirectly be regulating the secretion of natriuretic peptides from the heart (McCann et al., 2003); in these species it is secreted in response to raised plasma osmotic pressure (Huang et al., 1996). All of the oxytocin neurons in the SON are magnocellular neuroendocrine neurons, but the PVN also contains parvocellular oxytocin neurons that project within the brain – to the spinal cord, caudal brainstem, amygdala, and substantia nigra (Sofroniew, 1980). These neurons are important in sexual behavior in males (Melis et al., 1986), and, as we discuss below, in the regulation of gastric reflexes.
Recently it has become apparent that oxytocin has an important “pro-social” role. In rodents, it has been implicated in recognition and positive social behavior between rodent mothers and their offspring and between adult members of the same social group (Neumann, 2009). However, behaviors linked to oxytocin are not all positive. In the rat, for example, patterns of central oxytocin release correlate with maternal aggression against unfamiliar intruders (Bosch et al., 2005). Various “social” effects of oxytocin in humans have also been reported, but mainly following the intranasal administration of doses of oxytocin so large that it is hard to be confident of where or how they are acting.
It is unclear to what extent these “behavioral” roles of oxytocin are attributable to the parvocellular oxytocin system or the magnocellular system, because many of the sites of action of oxytocin in this regard lack conspicuous innervation by oxytocin-containing fibers. Accordingly, it seems likely that oxytocin reaches these sites by volume transmission following release from possibly quite distant sites. Large amounts of oxytocin are released in some circumstances from the dendrites of magnocellular neurons (Ludwig and Leng, 2006), and interestingly, such dendritic released is regulated independently of axonal secretion – thus magnocellular neurons can release oxytocin either centrally or peripherally depending upon the stimulus (Sabatier et al., 2003).
Oxytocin and Food Intake
In early studies of hypothalamic function, lesions of oxytocin-containing hypothalamic nuclei were shown to result in an increase in food intake and body weight (Leibowitz et al., 1981; Shor-Posner et al., 1985; Sims and Lorden, 1986; Kirchgessner et al., 1988). Then, in the 1990s, several studies reported anorexigenic effects of central oxytocin: low doses of oxytocin given icv dose-dependently inhibited food intake in rats, increased the latency to begin feeding and reduced meal duration in both hungry and satiated animals, and these actions could be blocked by oxytocin receptor antagonists (Arletti et al., 1990; Olson et al., 1991a). Longer term central infusions of oxytocin were also reported to reduce body weight gain in rats given a high-fat diet, but in contrast to oxytocin’s acute effects, chronic oxytocin infusions did not alter total food intake or meal patterning, but instead appeared to stimulate lipid metabolism in adipose tissue (Deblon et al., 2011). It was noted that, in rats, dehydration or sodium loading potently increased oxytocin secretion and at the same time suppressed appetite (Flanagan et al., 1992a); given that (in rats) oxytocin promotes natriuresis (Verbalis et al., 1991), the suppression of appetite by oxytocin appeared to be part of a general homeostatic role in sodium balance. As oxytocin secretion is not stimulated by hyperosmolarity in humans (Williams et al., 1986) it seemed that this role of oxytocin might be one peculiar to rodents. However, recent findings in humans with rare genetic mutations linked to monogenic obesity indicate that oxytocin may also have a role in appetite regulation in humans.
Male (but not female) oxytocin receptor-deficient mice express an obese phenotype in later adulthood despite no difference in food intake or motor activity (Takayanagi et al., 2008). Male and female oxytocin-knockout mice show an elevation in body weight and fat stores in adulthood but as with oxytocin receptor-deficient mice this is not due to an increase in food intake (Nishimori et al., 1996). There have been no published reports of humans completely lacking either oxytocin or its receptor, probably because the absence of oxytocin or its receptor is incompatible with successful reproduction, but a partial deficiency in central oxytocin production has been associated with the development of obesity in humans in two documented conditions.
The transcription factor Single-minded 1 (Sim1) is one of the few genes associated with human monogenic obesity (Holder et al., 2000; Farooqi and O’Rahilly, 2005). In mice, Sim1 is expressed in the SON and PVN and is essential for the development of these nuclei (Michaud et al., 1998). Homozygous Sim1 knockout mice do not survive gestation; but heterozygous mice are viable and are hyperphagic and become obese early in life (Michaud et al., 2001). These mice have much-reduced levels of oxytocin mRNA and immunoreactive oxytocin in both the SON and PVN (Michaud et al., 2001), and their hyperphagia can be reversed by oxytocin given icv (Kublaoui et al., 2008). Conditional deletion of Sim1 after gestational development of the PVN also results in both a reduction in oxytocin mRNA expression, and in hyperphagia and obesity (Tolson et al., 2010). In contrast, mice overexpressing Sim1 do not increase their food intake when given a high-fat diet, and are resistant to diet-induced obesity (Kublaoui et al., 2006b).
Patients affected by Prader–Willi syndrome (PWS) caused by the lack of a segment in the paternal chromosome 15 suffer from morbid obesity due to extreme hyperphagia. The PVN of these patients contains fewer oxytocin neurons than controls (Swaab et al., 1995), leading to speculation that a deficiency in oxytocin may be instrumental in the development of obesity in this condition. Mice in which specific genes associated with the PWS syndrome have been knocked out similarly develop late-onset obesity due to hyperphagia, and this can be partly explained by a deficient production of oxytocin in the hypothalamus (Dombret et al., 2012) or by a reduction in the number of oxytocin neurons (Muscatelli et al., 2000).
Where Does Appetite-Inhibitory Oxytocin Come From?
In the last 20 years it has become apparent that oxytocin neurons in both the SON and PVN are powerfully regulated by appetite-related signals (Renaud et al., 1987; Olson et al., 1991a,b). The role of central oxytocin in the regulation of energy homeostasis appears to involve both the magnocellular neurons and centrally projecting parvocellular neurons.
Parvocellular neurons of the PVN
The PVN has a major role in the regulation of appetite and metabolism, and is an important direct target of projections from the primary leptin- and ghrelin-receptive neurons of the arcuate nucleus – from orexigenic neurons that co-express neuropeptide Y (NPY) and agouti-related peptide (AgRP) and the inhibitory neurotransmitter GABA, and from pro-opiomelanocortin (POMC)-containing neurons, which express the potent satiety peptides α-melanocyte stimulating hormone (α-MSH) and cocaine-and amphetamine regulating transcript (CART) (Valassi et al., 2008). The PVN regulates metabolism via neuroendocrine neurons that release thyrotropin releasing hormone to regulate the thyroid gland (Alkemade, 2010; Nillni, 2010), it regulates glucocorticoid production via its regulation of pituitary adrenocorticotropin secretion (Herman et al., 2003), and it regulates the sympathetic nervous system via a large population of pre-autonomic neurons (Ferguson et al., 2008; Kc and Dick, 2010). However, the oxytocin neurons in the PVN, like those in the SON, are also conspicuous targets for α-MSH (Kim et al., 2000), and are particularly powerfully influenced by food intake and a variety of nutritionally related signals.
In rats, the expression of oxytocin mRNA in the PVN is markedly reduced by fasting; this reduction can be reversed by leptin administration (Kublaoui et al., 2008) and these effects apparently involve both magnocellular neurons and parvocellular neurons. Oxytocin neurons in the PVN are also contacted by fibers arising from the NPY/AgRP/GABA neurons of the arcuate nucleus. The PVN expresses abundant GABA receptors (Kalsbeek et al., 2004) and NPY Y1 receptors (Yokosuka et al., 1999). Optogenetic activation of the GABAergic axons in the PVN that arise from the arcuate nucleus increases food intake. Similarly, direct optogenetic activation of PVN oxytocin neurons increases c-Fos expression in these neurons and suppresses food intake. In the same study, a pharmacogenetic approach showed that while acute silencing of arcuate POMC neurons has surprisingly little effect, silencing Sim1-expressing PVN neurons markedly increases food intake (Atasoy et al., 2012).
Many parvocellular oxytocin neurons project to the nucleus tractus solitarii (NTS) in the caudal brainstem (Rinaman, 1998) where oxytocin modulates vagal efferent pathways that regulate gastric motility (McCann and Rogers, 1990). These neurons are critically involved in a reflex that is triggered by food intake, and mediated in part by gastric distension and in part by the secretion of CCK from the duodenum. Peripheral administration of CCK leads to activation of gastric vagal afferent neurons and thence to activation of brainstem structures, notably the NTS and ventrolateral medulla (Simpson et al., 2012). These in turn project to, and activate, centrally projecting oxytocin neurons of the PVN. As the CCK-stimulated NTS neurons are densely innervated by oxytocin-containing fibers from the PVN (Blevins et al., 2003), it appears that there is a recurrent circuit involving parvocellular oxytocin neurons that modulates the gastrointestinal vago-vagal reflex.
The gastrointestinal vago-vagal reflex involves main three-components: the gastrointestinal tract, the NTS, and the dorsal motor nucleus of the vagus (DMV). Visceral afferent fibers carrying digestion-related information ascend the vagus nerve and terminate in the medial NTS. NTS neurons integrate the information and send afferent projections to the DMV, and DMV neurons in turn project back down to the intrinsic ganglia in the gastrointestinal tract. In addition to this main loop, catecholaminergic and peptidergic neurons of the NTS also send ascending projections to structures in the forebrain including the PVN and the SON, and parvocellular PVN neurons project back to both the NTS and the DMN (Saper et al., 1976; Swanson and Kuypers, 1980). There is clear evidence that oxytocin acts in the DMN to inhibit gastric motility: the DMN contains oxytocin receptors (Dubois-Dauphin et al., 1992) and injection of oxytocin into the DMN decreases gastric motility, while oxytocin antagonists have the opposite effect (Rogers and Hermann, 1987). Electrical stimulation of the PVN inhibits gastric motility, and this effect can be attenuated by injection of an oxytocin antagonist in the DMN (Rogers and Hermann, 1987). Similar results were observed in conscious, freely moving animals, in which gastric motility was reduced by icv injection of oxytocin and by stimulation of the PVN, and in both cases the inhibition was prevented by icv injection of an oxytocin antagonist (Flanagan et al., 1992b). Finally, icv administration of an oxytocin antagonist alone increased baseline gastric motility, suggesting a tonic inhibitory effect of oxytocin on gastric motility. However, it seems unlikely that the inhibitory action of CCK on gastric motility is mediated by oxytocin, as icv injection of an oxytocin antagonist did not prevent CCK-induced inhibition of gastric motility (Flanagan et al., 1992b).
Thus there is a well-established role of parvocellular oxytocin neurons in the regulation of the gastrointestinal tract. Moreover, the magnocellular oxytocin neurons of the SON and PVN also appear to be involved in regulating appetite, possibly by the actions of dendritically released oxytocin on the ventromedial nucleus of the hypothalamus (VMN). This large nucleus is known to have an important role in both energy balance and sexual behavior, and is a site at which oxytocin receptors are expressed at an exceptionally high density (Tribollet et al., 1988) as well as insulin-regulated aminopeptidase (IRAP), an enzyme involved in the inactivation of oxytocin (Fernando et al., 2005).
Magnocellular neurons of the SON and PVN
As we discuss further below, there is powerful evidence that magnocellular oxytocin neurons have an important role in the regulation of appetite – but it is important to note that this role is not necessarily mediated by oxytocin alone. In magnocellular neurons of the SON and PVN, oxytocin is co-localized with a number of anorexigenic factors, including the neuropeptides CART (Vrang et al., 1999), pituitary adenylate cyclase activating polypeptide (PACAP) (Koves et al., 1994), corticotropin-releasing factor (CRF) (Sawchenko et al., 1984), CCK (Vanderhaeghen et al., 1981), and nesfatin-1 (Foo et al., 2008). Indeed, it has been suggested that oxytocin actually mediates the inhibitory action of nesfatin-1 on food intake, as nesfatin-1 induces the release of oxytocin in the PVN (Maejima et al., 2009) and as the anorexigenic effect of nesfatin-1 can be blocked by an oxytocin antagonist (Yosten and Samson, 2010). The fat mass and obesity-associated (FTO) gene that has been associated with obesity in humans is also co-localized with oxytocin in both the PVN and SON. This gene encodes a transcription co-factor (Fto) that is believed to regulate the expression of appetite-related genes (Jia et al., 2008), and Fto over-expression increases oxytocin mRNA levels in cell cultures (Olszewski et al., 2011).
Magnocellular oxytocin neurons are activated during feeding: thus, in schedule-fed rats trained to expect to receive food for just 2 h each day, magnocellular oxytocin neurons in both the SON and PVN densely express Fos protein soon after the initiation of food intake (Johnstone et al., 2006). These neurons are also activated by gastric distension, and by systemic application of the satiety peptides CCK (Renaud et al., 1987) and GLP-1 (Bojanowska and Stempniak, 2000). Moreover, the intestinal lipid amide oleoylethanolamide (OEA), which suppresses feeding via activation of the vagus nerve (Lo Verme et al., 2005), stimulates oxytocin mRNA expression in the PVN and SON, this anorexic effect is prevented by blockade of central oxytocin receptors (Gaetani et al., 2010).
The best understood pathway involving feeding-evoked activation of magnocellular oxytocin neurons is that where peripheral injections of CCK leads to secretion of oxytocin from the posterior pituitary gland in rats. In brief, CCK is released from the duodenum in response to food intake and peripheral administration of CCK can inhibit food intake via stimulation of vagal afferent neurons and activation of brainstem structures, notably the NTS and ventrolateral medulla. CCK given intraperitoneally or intravenously increases Fos expression in oxytocin neurons in the PVN and SON (Caquineau et al., 2010), increases the electrical activity of oxytocin neurons in the SON (Renaud et al., 1987; Leng et al., 1991) and increases plasma oxytocin secretion (Kutlu et al., 2010). These actions are mediated by a direct projection from noradrenergic neurons of the A2 cell group, which co-express the potent appetite-inhibiting peptide prolactin-releasing peptide (PrRP). The activation of oxytocin neurons can be blocked by selective lesioning of the noradrenergic afferents or by blocking the actions at the SON of noradrenaline itself or those of PrRP (Onaka et al., 2012). Interestingly, central pretreatment with an oxytocin receptor antagonist reduces the anorexigenic effect of CCK (Olson et al., 1991b; Blevins et al., 2003) suggesting that CCK-evoked satiety may be mediated in part by oxytocin.
Interactions between magnocellular oxytocin neurons and the melanocortins
Like parvocellular oxytocin neurons, magnocellular oxytocin neurons abundantly express leptin receptors and are a target for this important hormone (Hakansson et al., 1998; Yarnell et al., 1998; Brogan et al., 2000). Leptin activates Fos expression in the PVN (Yokosuka et al., 1998; Caquineau et al., 2010; Qi et al., 2010), particularly in parvocellular PVN neurons projecting to CCK-sensitive neurons in the NTS. In the SON, however, central administration of leptin does not induce Fos expression (Caquineau et al., 2010) or nuclear translocation of STAT3 (Hakansson and Meister, 1998), but it does increase nuclear STAT5 expression (Mutze et al., 2007).
In addition to this direct modulation by leptin, magnocellular oxytocin neurons are regulated indirectly via the effects of leptin on POMC neurons of the arcuate nucleus. Like the PVN, the SON receives a strong projection from POMC neurons. Oxytocin neurons in the SON, like those in the PVN, densely express α-MSH receptors (MC4) (Garza et al., 2008). α-MSH is a powerful anorexigenic peptide: centrally administered α-MSH reduces food intake and body weight, and mice lacking MC4 receptors are hyperphagic and obese (Adan et al., 2006). As mentioned above, magnocellular oxytocin neurons can secrete a large amount of peptide from their dendrites in response to certain stimuli, and notably they do so in response to α-MSH. In magnocellular oxytocin neurons of the SON, α-MSH acts at MC4 receptors to increase the intracellular calcium concentration; this increase directly evokes oxytocin release from the large dendrites of these neurons and also results in the production of endocannabinoids by the oxytocin neurons. Endocannabinoids produced in response to α-MSH act presynaptically to suppress glutamatergic afferent inputs to the oxytocin neurons. Thus, remarkably, the response of magnocellular oxytocin neurons to α-MSH is an increase in central release of oxytocin but a simultaneous suppression of electrical activity and hence a suppression of secretion into the systemic circulation (Sabatier and Leng, 2006).
Further evidence of an interaction between oxytocin and α-MSH is illustrated in the model of Sim1 heterozygous mice. In wild type mice, an agonist of α-MSH MC4 receptor (MC4R) reduced food intake, and induced Fos expression in PVN neurons, of which some co-express oxytocin and MC4R (Liu et al., 2003), and oxytocin and Sim1 (Kublaoui et al., 2008). However in Sim1 heterozygous mice, MC4R agonist had a much attenuated effect on both food intake and Fos expression in the PVN (Kublaoui et al., 2006a). This suggests that the α-MSH agonist actions on the PVN of Sim1± mice were impaired by the lack of oxytocin production in these mice (Michaud et al., 2001).
The ventromedial nucleus of the hypothalamus – a site of action of oxytocin
It seems possible that oxytocin released from the dendrites of magnocellular neurons is involved in the regulation of appetite, presumably reaching its targets by volume transmission. We have estimated that a release rate of just one vesicle per oxytocin cell every 10 s would be enough to achieve a mean basal concentration of ∼260 pg/ml throughout the anterior hypothalamus within a minute (Leng and Ludwig, 2008). The half life of oxytocin in CSF is ∼20 min – it is likely to be less than this in the extracellular fluid, but there are no clear data on this point. We have argued elsewhere that the effects of oxytocin depends less on its sites of release but rather on the location of its receptors (Sabatier et al., 2007). Thus it is likely that food intake is inhibited in various physiological conditions in which oxytocin is released from the dendrites of magnocellular neurons. Indeed, appetite is inhibited in rats subjected to dehydration or sodium loading (Flanagan et al., 1992a), two stimuli that result in a hyperosmolar environment, which is known to stimulate the dendritic release of oxytocin from supraoptic neurons (Neumann et al., 1993). The main physiological circumstances in which there is extensive central secretion of oxytocin is in lactation, when suckling-induced dendritic oxytocin release is an essential part of the milk-ejection reflex (Rossoni et al., 2008). Lactation is associated with a marked increase of food intake, rather than a reduction, but despite this, lactation is a time of negative energy balance, because the increased food intake does not adequately compensate for the energy demands of the suckling young. Accordingly, it seems that appetite during lactation is not increased to the degree needed to maintain energy homeostasis – and it may be that suppression of appetite during suckling is necessary to ensure that the mother nurses the young rather than searches for food.
One particularly intriguing potential target for dendritic oxytocin is the VMN. This large hypothalamic nucleus contains a very high density of oxytocin receptors, as shown by intense labeling both for oxytocin receptor binding sites (Tribollet et al., 1988) and oxytocin receptor mRNA expression (Yoshimura et al., 1993), particularly in the ventrolateral region. Oxytocin receptors in the VMN have an established role in sexual behavior in female rats (McCarthy et al., 1994), and we have previously suggested that they are involved in the reciprocal regulation of appetite and sexual behavior (Leng and Ludwig, 2008). Although the VMN has a high density of oxytocin receptors, it contains very few oxytocin fibers, and is therefore a likely target for oxytocin released from the dendrites of magnocellular oxytocin neurons (Leng and Ludwig, 2008).
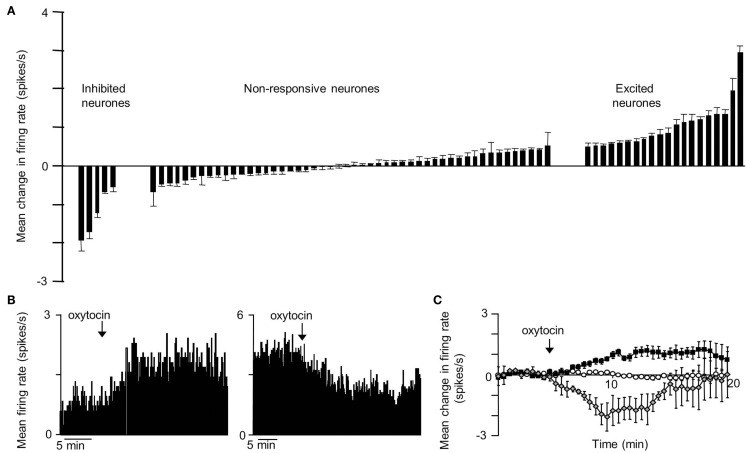
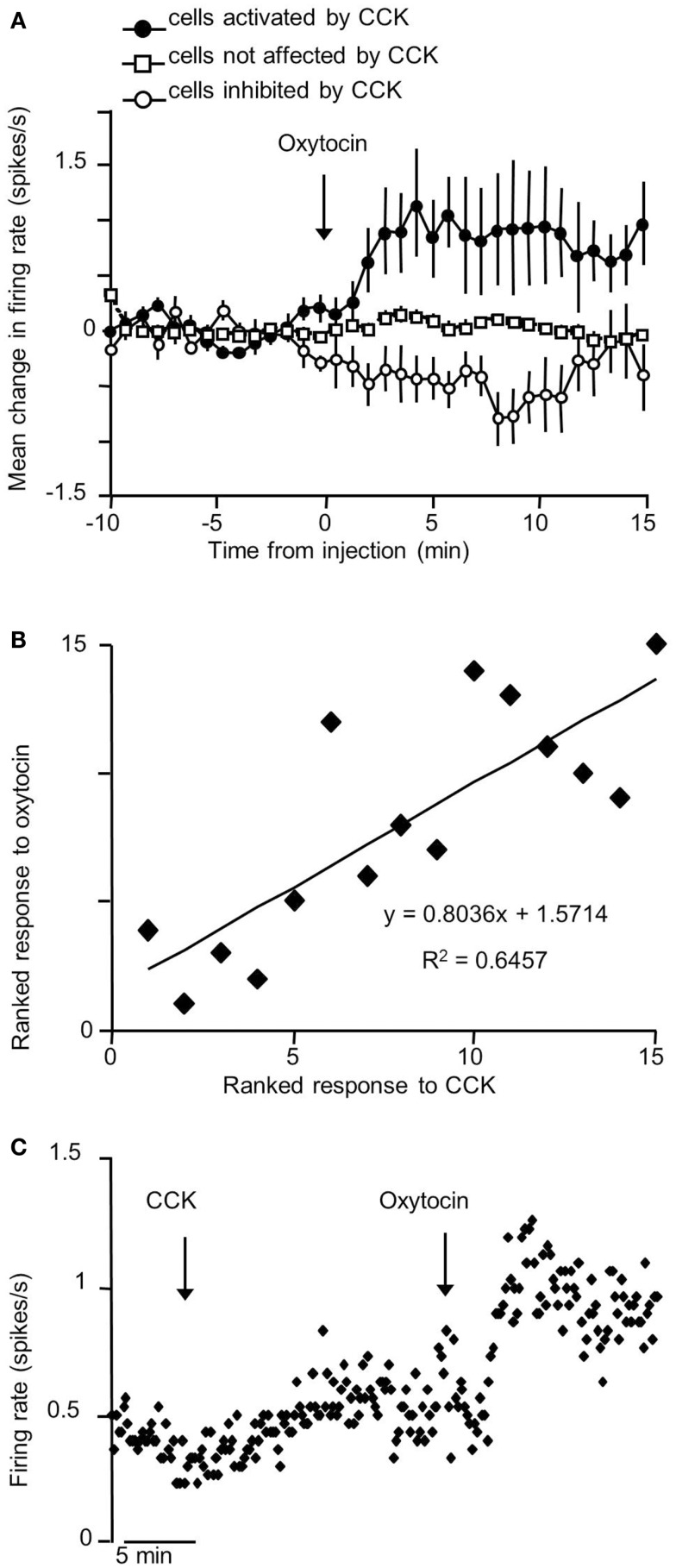
The VMN is not a functionally homogeneous nucleus so it is not surprising that systemic injections of CCK have diverse effects on VMN neurons, but the most common effect of CCKon the electrical activity of VMN neurons is inhibitory (Sabatier and Leng, 2010); these responses varied particularly between different subpopulations of VMN that displayed contrasting electrophysiological features. To test whether the appetite-inhibiting effects of oxytocin and those of CCK converge at the level of the VMN, we have studied the effects of central icv injection of oxytocin on the electrical activity of VMN neurons in vivo, and compared these responses with those of the same neurons to CCK. As with CCK, the responses to oxytocin were diverse. About 30% of neurons responded to oxytocin, 78% with a significant increase in their mean firing rate (Figure 1), and again there were differences in the responses of electrophysiologically distinct subpopulations. However, when we compared the responses of the same neurons to CCK and oxytocin, we noted particularly that, while one subpopulation of neurons had clearly divergent responses to CCK and oxytocin, in all other subpopulations the responses were remarkably convergent – for most neuronal types in the VMN, the response to icv oxytocin was a very strong predictor of the response to systemic CCK (Figure 2), supporting the hypothesis that VMN neurons are part of a common pathway mediating satiety.
Figure 1.
Responses to icv oxytocin in VMN neurons in vivo. Single VMN neurons were recorded extracellularly from the VMN of urethane-anesthetized rats (Sabatier and Leng, 2008). (A) Each bar represent the mean change in firing rate (±SD) averaged over the first 10 min after icv injection of oxytocin (1–10 ng) in each VMN cell tested. The cells are ranked by response magnitude and are classed as inhibited, non-responsive, and excited according to whether the responses were significant or not. (B) Representative example of an inhibition (left panel) and activation (right panel) of the mean firing rate in response to icv oxytocin in a single VMN cell recorded extracellularly. (C) Mean change in firing rate (±SE) in the VMN cells that were significantly activated  ; n = 14), significantly inhibited
; n = 14), significantly inhibited  ; n = 4), and not significantly affected
; n = 4), and not significantly affected  ; n = 43) by icv oxytocin.
; n = 43) by icv oxytocin.
Figure 2.
Correlation of responses to icv oxytocin and iv CCK in VMN neurons in vivo. (A) Mean change in firing rate (±SE) in response to icv oxytocin (1–10 ng) in single VMN cells that were activated  ; n = 5), inhibited
; n = 5), inhibited  ; n = 4), and not affected
; n = 4), and not affected  ; n = 6) by iv CCK injection. (B) Rank correlation of responses to CCK and oxytocin (high ranks excited; low ranks inhibited). The solid line is the linear fit to the ranks (r2 = 0.65). (C) Example of convergent responses to icv oxytocin (1–10 ng) and iv CCK (20 μg/kg) in a single VMN neuron.
; n = 6) by iv CCK injection. (B) Rank correlation of responses to CCK and oxytocin (high ranks excited; low ranks inhibited). The solid line is the linear fit to the ranks (r2 = 0.65). (C) Example of convergent responses to icv oxytocin (1–10 ng) and iv CCK (20 μg/kg) in a single VMN neuron.
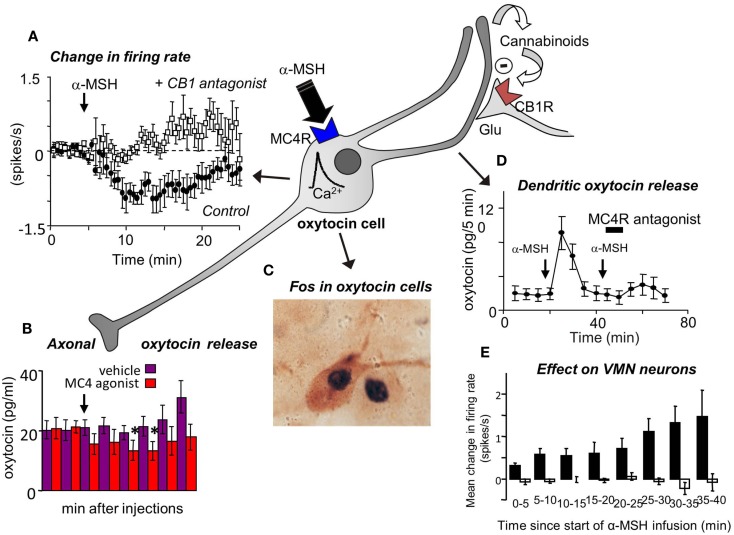
Finally, to test the feasibility of the hypothesis that oxytocin affects VMN neurons via dendritic release of oxytocin from magnocellular neurons, we studied the electrical activity of single VMN neurons while applying α-MSH directly to the ipsilateral SON by microdialysis to evoke dendritic oxytocin release. Like the effects of icv oxytocin, this too had long-lasting, mainly excitatory effects upon a subset of VMN neurons (Figure 3).
Figure 3.
(A) Magnocellular oxytocin neurons are inhibited by icv injections of α-MSH and this effect can be blocked by pre-administration of a CB1 cannabinoid antagonist (Sabatier et al., 2003). (B) Oxytocin secretion from the posterior pituitary gland is reduced by icv injection of MC4 agonists. (C) However, α-MSH and MC4 agonists induce Fos expression in magnocellular oxytocin neurons and (D) induce dendritic oxytocin release. (E) Application of α-MSH retrodialysis to the SON affects the electrical activity of VMN neurons in vivo. Mean change in firing rate (±SE) in VMN neurons that were not affected (open bars; n = 13), and activated (solid bars; n = 8; P < 0.005, 0–30 min vs. control) in the 30 min period from the start of α-MSH infusion (100 μM) on the SON.
Oxytocin and Reward
In addition to the drive to eat for metabolizable energy and nutritional factors (homeostatic feeding), humans and many other animals attend selectively to palatable foods and are motivated to eat them for pleasure (hedonic feeding). In addition to its effects on homeostatic food intake, oxytocin may have a role in the modulation of hedonic food intake.
Activation of dopaminergic mesolimbic neurons of the ventral tegmental area (VTA) is currently thought to be closely associated with reward and motivation. During palatable food intake, dopamine is released in the VTA’s target regions such as the nucleus accumbens (NAcc) and dopamine release in these structures can also be evoked by stimuli associated with or predictive of palatable food. It is likely that activation of this system equates with the motivation to attend to, pursue and consume rewarding stimuli like palatable food. Oxytocin-containing axons from the PVN contact mesolimbic neurons (Sofroniew, 1980; Succu et al., 2008) and likely have an inhibitory action as exogenous oxytocin activates NO synthase in mesolimbic dopaminergic neurons in vivo (Succu et al., 2008. Exogenous oxytocin also reduces amphetamine-evoked dopamine turnover in the NAcc (Qi et al., 2008) and inhibits the formation of a place-preference to methamphetamine when given into the NAcc core (Baracz et al., 2012). However, administration of oxytocin does not always lead to a reduction in dopamine release, administration into the ventral subiculum for example, a hippocampal region innervated by the PVN and potentially involved in restraining the stress response and reinstating non-food reward responding behavior, results in increased dopamine release in the NAcc (Melis et al., 2009). Inhibition of the PVN by optical stimulation of GABAergic fibers from arcuate NPY/AgRP/GABA neurons increases motivation to obtain sugar in a progressive ratio lever pressing task (Atasoy et al., 2012) suggesting that signals arising from the PVN may suppress the motivation for rewarding foods.
Consumption of readily catabolizable sugars seems to be innate in animals. Without training, rodents given a choice between water and sucrose will consume large volumes of a 10% sucrose solution [over 200 ml a day in adult male Sprague-Dawley rats (unpublished observations)] and voluntarily cease water drinking. Even transgenic mice lacking functional sweet taste receptors prefer sucrose over water (de Araujo et al., 2008) indicating that a post-ingestive reward is provided by sucrose. Transgenic mice lacking oxytocin show an enhanced preference for and consumption of sweet solutions over water in a two-bottle choice paradigm (Amico et al., 2005; Billings et al., 2006). The increase in intake is driven by a greater number of feeding bouts rather than by an increase in their duration (Sclafani et al., 2007). However, no such effect of oxytocin deficiency is seen on consumption of a palatable high-fat liquid formulation (Miedlar et al., 2007) or of a sucrose-containing solid food (Amico et al., 2005), though scheduled feeding of a high-sugar diet to rats increases oxytocin gene expression (Olszewski et al., 2009). Progressive ratio operant conditioning paradigms are often used as a measure of motivation to work for a reward. In contrast to an effect of oxytocin deficiency on freely available sweet solutions, there is less evidence that oxytocin-deficient mice have enhancements (or deficiencies) in motivation to work for food as they are not different to wild type mice in this task (Sclafani et al., 2007). In addition to Fos expression observed at the termination of bland food intake (Johnstone et al., 2006), Fos expression is also increased in PVN oxytocin neurons at the termination of a bout of feeding where only either sucrose or a high-fat food was available (Olszewski et al., 2010). In this study, hypothalamic oxytocin mRNA levels were higher in mice allowed access to a high-sugar diet compared to a high-fat diet despite both being palatable and readily consumed. Furthermore, oxytocin receptor antagonism led to an increase in sucrose consumption but has no effect on fat consumption when presented separately. Regular exposure in rats to a diet high in sugar reduces Fos expression in PVN and SON oxytocin neurons after a high- or low-sugar meal compared to animals regularly receiving a low-sugar diet (Mitra et al., 2010). This suggests that regular sugar consumption might blunt the activity of oxytocin neurons in response to any meal, whether high in sugar or not. Nonetheless, it may be that oxytocin has a satiating effect related to certain components of diet rather than a general effect. Thus, the balance of evidence suggests that oxytocin might have a role in limiting the intake of palatable food by suppressing the activation of the reward pathway.
Conclusion
It now seems clear that oxytocin has a physiological role in energy balance through its actions in the caudal brainstem, but probably also through actions within the hypothalamus, including at the VMN, and possibly at other sites in the brain. While the actions in the brainstem appear to be part of a suite of autonomic regulatory functions exercised by parvocellular oxytocin neurons of the PVN, the hypothalamic actions appear to be more associated with motivational drive to eat, and are probably attributable to the magnocellular oxytocin system rather than the parvocellular system. It is possible that the inhibitory effects of oxytocin on appetite are closely linked to the stimulatory effects of oxytocin in sexual behavior and that this link reflects an evolutionarily conserved reciprocal regulation of these two key motivational drives (Caquineau et al., 2012). Whether it is possible to find a way of dissociating the appetite-inhibiting effects of oxytocin from the sexual arousal effects is unclear at present, but this may be the key to success in using the oxytocin system as a target for appetite-reducing drug therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by the EU Seventh Framework program under grant agreements FP7-KBBE-2009-245009 (NeuroFAST) and FP7-KBBE-2010-266408 (Full4Health). This research was supported in part by a grant award to Dr. Nancy Sabatier from Medical Research Scotland (Grant award 316-FRG-L-0901).
References
- Adan R. A., Tiesjema B., Hillebrand J. J., La Fleur S. E., Kas M. J., de Krom M. (2006). The MC4 receptor and control of appetite. Br. J. Pharmacol. 149, 815–827 10.1038/sj.bjp.0706929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkemade A. (2010). Central and peripheral effects of thyroid hormone signalling in the control of energy metabolism. J. Neuroendocrinol. 22, 56–63 10.1111/j.1365-2826.2009.01932.x [DOI] [PubMed] [Google Scholar]
- Amico J. A., Vollmer R. R., Cai H. M., Miedlar J. A., Rinaman L. (2005). Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1798–R1806 10.1152/ajpregu.00558.2005 [DOI] [PubMed] [Google Scholar]
- Archer Z. A., Mercer J. G. (2007). Brain responses to obesogenic diets and diet-induced obesity. Proc. Nutr. Soc. 66, 124–130 10.1017/S0029665107005356 [DOI] [PubMed] [Google Scholar]
- Arletti R., Benelli A., Bertolini A. (1990). Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830 10.1016/0031-9384(90)90234-U [DOI] [PubMed] [Google Scholar]
- Atasoy D., Betley J. N., Su H. H., Sternson S. M. (2012). Deconstruction of a neural circuit for hunger. Nature 488, 172–177 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz S. J., Rourke P. I., Pardey M. C., Hunt G. E., McGregor I. S., Cornish J. L. (2012). Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav. Brain Res. 228, 185–193 10.1016/j.bbr.2011.11.038 [DOI] [PubMed] [Google Scholar]
- Billings L. B., Spero J. A., Vollmer R. R., Amico J. A. (2006). Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav. Brain Res. 171, 134–141 10.1016/j.bbr.2006.03.028 [DOI] [PubMed] [Google Scholar]
- Blevins J. E., Eakin T. J., Murphy J. A., Schwartz M. W., Baskin D. G. (2003). Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 993, 30–41 10.1016/j.brainres.2003.08.036 [DOI] [PubMed] [Google Scholar]
- Bojanowska E., Stempniak B. (2000). Effects of centrally or systemically injected glucagon-like peptide-1 (7-36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul. Pept. 91, 75–81 10.1016/S0167-0115(00)00119-1 [DOI] [PubMed] [Google Scholar]
- Bosch O. J., Meddle S. L., Beiderbeck D. I., Douglas A. J., Neumann I. D. (2005). Brain oxytocin correlates with maternal aggression: link to anxiety. J. Neurosci. 25, 6807–6815 10.1523/JNEUROSCI.1342-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan R. S., Grove K. L., Smith M. S. (2000). Differential regulation of leptin receptor but not orexin in the hypothalamus of the lactating rat. J. Neuroendocrinol. 12, 1077–1086 10.1046/j.1365-2826.2000.00559.x [DOI] [PubMed] [Google Scholar]
- Caquineau C., Douglas A. J., Leng G. (2010). Effects of cholecystokinin in the supraoptic nucleus and paraventricular nucleus are negatively modulated by leptin in 24-h fasted lean male rats. J. Neuroendocrinol. 22, 446–452 10.1111/j.1365-2826.2010.01982.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caquineau C., Leng G., Douglas A. J. (2012). Sexual behaviour and neuronal activation in the vomeronasal pathway and hypothalamus of food-deprived male rats. J. Neuroendocrinol. 24, 712–723 10.1111/j.1365-2826.2012.02290.x [DOI] [PubMed] [Google Scholar]
- de Araujo I. E., Oliveira-Maia A. J., Sotnikova T. D., Gainetdinov R. R., Caron M. G., Nicolelis M. A., et al. (2008). Food reward in the absence of taste receptor signaling. Neuron 57, 930–941 10.1016/j.neuron.2008.01.032 [DOI] [PubMed] [Google Scholar]
- Deblon N., Veyrat-Durebex C., Bourgoin L., Caillon A., Bussier A. L., Petrosino S., et al. (2011). Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 6:e25565. 10.1371/journal.pone.0025565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombret C., Nguyen T., Schakman O., Michaud J. L., Hardin-Pouzet H., Bertrand M. J., et al. (2012). Loss of Maged1 results in obesity, deficits of social interactions, impaired sexual behavior and severe alteration of mature oxytocin production in the hypothalamus. Hum. Mol. Genet. 21, 4703–4717 10.1093/hmg/dds310 [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M., Raggenbass M., Widmer H., Tribollet E., Dreifuss J. J. (1992). Morphological and electrophysiological evidence for postsynaptic localization of functional oxytocin receptors in the rat dorsal motor nucleus of the vagus nerve. Brain Res. 575, 124–131 10.1016/0006-8993(92)90431-8 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., O’Rahilly S. (2005). Monogenic obesity in humans. Annu. Rev. Med. 56, 443–458 10.1146/annurev.med.56.062904.144924 [DOI] [PubMed] [Google Scholar]
- Ferguson A. V., Latchford K. J., Samson W. K. (2008). The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 12, 717–727 10.1517/14728222.12.6.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando R. N., Larm J., Albiston A. L., Chai S. Y. (2005). Distribution and cellular localization of insulin-regulated aminopeptidase in the rat central nervous system. J. Comp. Neurol. 487, 372–390 10.1002/cne.20585 [DOI] [PubMed] [Google Scholar]
- Flanagan L. M., Blackburn R. E., Verbalis J. G., Stricker E. M. (1992a). Hypertonic NaCl inhibits gastric motility and food intake in rats with lesions in the rostral AV3V region. Am. J. Physiol. 263, R9–R14 [DOI] [PubMed] [Google Scholar]
- Flanagan L. M., Olson B. R., Sved A. F., Verbalis J. G., Stricker E. M. (1992b). Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 578, 256–260 10.1016/0006-8993(92)90255-8 [DOI] [PubMed] [Google Scholar]
- Foo K. S., Brismar H., Broberger C. (2008). Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156, 563–579 10.1016/j.neuroscience.2008.07.054 [DOI] [PubMed] [Google Scholar]
- Gaetani S., Fu J., Cassano T., Dipasquale P., Romano A., Righetti L., et al. (2010). The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J. Neurosci. 30, 8096–8101 10.1523/JNEUROSCI.0036-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza J. C., Kim C. S., Liu J., Zhang W., Lu X. Y. (2008). Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J. Endocrinol. 197, 471–482 10.1677/JOE-08-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M. L., Brown H., Ghilardi N., Skoda R. C., Meister B. (1998). Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18, 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M. L., Meister B. (1998). Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology 68, 420–427 10.1159/000054392 [DOI] [PubMed] [Google Scholar]
- Herman J. P., Figueiredo H., Mueller N. K., Ulrich-Lai Y., Ostrander M. M., Choi D. C., et al. (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 24, 151–180 10.1016/j.yfrne.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Holder J. L., Jr., Butte N. F., Zinn A. R. (2000). Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 9, 101–108 10.1093/hmg/9.1.101 [DOI] [PubMed] [Google Scholar]
- Huang W., Lee S. L., Arnason S. S., Sjoquist M. (1996). Dehydration natriuresis in male rats is mediated by oxytocin. Am. J. Physiol. 270, R427–R433 [DOI] [PubMed] [Google Scholar]
- Jia G., Yang C. G., Yang S., Jian X., Yi C., Zhou Z., et al. (2008). Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 582, 3313–3319 10.1016/j.febslet.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone L. E., Fong T. M., Leng G. (2006). Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 4, 313–321 10.1016/j.cmet.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., La Fleur S., Van Heijningen C., Buijs R. M. (2004). Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 24, 7604–7613 10.1523/JNEUROSCI.5328-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc P., Dick T. E. (2010). Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir. Physiol. Neurobiol. 174, 55–64 10.1016/j.resp.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Rossi M., Abusnana S., Sunter D., Morgan D. G., Small C. J., et al. (2000). Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes 49, 177–182 10.2337/diabetes.49.5.847 [DOI] [PubMed] [Google Scholar]
- Kirchgessner A. L., Sclafani A., Nilaver G. (1988). Histochemical identification of a PVN-hindbrain feeding pathway. Physiol. Behav. 42, 529–543 10.1016/0031-9384(88)90153-9 [DOI] [PubMed] [Google Scholar]
- Koves K., Gorcs T. J., Kausz M., Arimura A. (1994). Present status of knowledge about the distribution and colocalization of PACAP in the forebrain. Acta Biol. Hung. 45, 297–321 [PubMed] [Google Scholar]
- Kublaoui B. M., Gemelli T., Tolson K. P., Wang Y., Zinn A. R. (2008). Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 22, 1723–1734 10.1210/me.2008-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublaoui B. M., Holder J. L., Jr., Gemelli T., Zinn A. R. (2006a). Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol. Endocrinol. 20, 2483–2492 10.1210/me.2005-0483 [DOI] [PubMed] [Google Scholar]
- Kublaoui B. M., Holder J. L., Jr., Tolson K. P., Gemelli T., Zinn A. R. (2006b). SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology 147, 4542–4549 10.1210/en.2006-0453 [DOI] [PubMed] [Google Scholar]
- Kutlu S., Aydin M., Alcin E., Ozcan M., Bakos J., Jezova D., et al. (2010). Leptin modulates noradrenaline release in the paraventricular nucleus and plasma oxytocin levels in female rats: a microdialysis study. Brain Res. 1317, 87–91 10.1016/j.brainres.2009.12.044 [DOI] [PubMed] [Google Scholar]
- Leibowitz S. F., Hammer N. J., Chang K. (1981). Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27, 1031–1040 10.1016/0031-9384(81)90366-8 [DOI] [PubMed] [Google Scholar]
- Leng G., Ludwig M. (2008). Neurotransmitters and peptides: whispered secrets and public announcements. J. Physiol. (Lond.) 586, 5625–5632 10.1113/jphysiol.2008.159103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Way S., Dyball R. E. (1991). Identification of oxytoxin cells in the rat supraoptic nucleus by their response to cholecystokinin injection. Neurosci. Lett. 122, 159–162 10.1016/0304-3940(91)90847-M [DOI] [PubMed] [Google Scholar]
- Liu H., Kishi T., Roseberry A. G., Cai X., Lee C. E., Montez J. M., et al. (2003). Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J. Neurosci. 23, 7143–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J., Gaetani S., Fu J., Oveisi F., Burton K., Piomelli D. (2005). Regulation of food intake by oleoylethanolamide. Cell. Mol. Life Sci. 62, 708–716 10.1007/s00018-004-4494-0 [DOI] [PubMed] [Google Scholar]
- Ludwig M., Leng G. (2006). Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- Maejima Y., Sedbazar U., Suyama S., Kohno D., Onaka T., Takano E., et al. (2009). Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 10, 355–365 10.1016/j.cmet.2009.09.002 [DOI] [PubMed] [Google Scholar]
- McCann M. J., Rogers R. C. (1990). Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J. Physiol. (Lond.) 428, 95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann S. M., Gutkowska J., Antunes-Rodrigues J. (2003). Neuroendocrine control of body fluid homeostasis. Braz. J. Med. Biol. Res. 36, 165–181 10.1590/S0100-879X2003000200003 [DOI] [PubMed] [Google Scholar]
- McCarthy M. M., Kleopoulos S. P., Mobbs C. V., Pfaff D. W. (1994). Infusion of antisense oligodeoxynucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat. Neuroendocrinology 59, 432–440 10.1159/000126689 [DOI] [PubMed] [Google Scholar]
- Melis M. R., Argiolas A., Gessa G. L. (1986). Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Res. 398, 259–265 10.1016/0006-8993(86)91485-X [DOI] [PubMed] [Google Scholar]
- Melis M. R., Succu S., Sanna F., Boi A., Argiolas A. (2009). Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur. J. Neurosci. 30, 1349–1357 10.1111/j.1460-9568.2009.06912.x [DOI] [PubMed] [Google Scholar]
- Michaud J. L., Boucher F., Melnyk A., Gauthier F., Goshu E., Levy E., et al. (2001). Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10, 1465–1473 10.1093/hmg/10.14.1465 [DOI] [PubMed] [Google Scholar]
- Michaud J. L., Rosenquist T., May N. R., Fan C. M. (1998). Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275 10.1101/gad.12.20.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlar J. A., Rinaman L., Vollmer R. R., Amico J. A. (2007). Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1063–R1068 10.1152/ajpregu.00228.2007 [DOI] [PubMed] [Google Scholar]
- Mitra A., Gosnell B. A., Schioth H. B., Grace M. K., Klockars A., Olszewski P. K., et al. (2010). Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides 31, 1346–1352 10.1016/j.peptides.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatelli F., Abrous D. N., Massacrier A., Boccaccio I., Le Moal M., Cau P., et al. (2000). Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum. Mol. Genet. 9, 3101–3110 10.1093/hmg/9.20.3101 [DOI] [PubMed] [Google Scholar]
- Mutze J., Roth J., Gerstberger R., Hubschle T. (2007). Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci. Lett. 417, 286–291 10.1016/j.neulet.2007.02.074 [DOI] [PubMed] [Google Scholar]
- Neumann I. D. (2009). The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front. Neuroendocrinol. 30, 483–496 10.1016/j.yfrne.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Neumann I., Ludwig M., Engelmann M., Pittman Q. J., Landgraf R. (1993). Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology 58, 637–645 10.1159/000126604 [DOI] [PubMed] [Google Scholar]
- Nillni E. A. (2010). Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front. Neuroendocrinol. 31, 134–156 10.1016/j.yfrne.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K., Young L. J., Guo Q., Wang Z., Insel T. R., Matzuk M. M. (1996). Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699–11704 10.1073/pnas.93.21.11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson B. R., Drutarosky M. D., Chow M. S., Hruby V. J., Stricker E. M., Verbalis J. G. (1991a). Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12, 113–118 10.1016/0196-9781(91)90227-G [DOI] [PubMed] [Google Scholar]
- Olson B. R., Drutarosky M. D., Stricker E. M., Verbalis J. G. (1991b). Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129, 785–791 10.1210/endo-129-2-785 [DOI] [PubMed] [Google Scholar]
- Olszewski P. K., Fredriksson R., Eriksson J. D., Mitra A., Radomska K. J., Gosnell B. A., et al. (2011). Fto colocalizes with a satiety mediator oxytocin in the brain and upregulates oxytocin gene expression. Biochem. Biophys. Res. Commun. 408, 422–426 10.1016/j.bbrc.2011.04.037 [DOI] [PubMed] [Google Scholar]
- Olszewski P. K., Klockars A., Olszewska A. M., Fredriksson R., Schioth H. B., Levine A. S. (2010). Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology 151, 4736–4744 10.1210/en.2010-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski P. K., Shaw T. J., Grace M. K., Hoglund C. E., Fredriksson R., Schioth H. B., et al. (2009). Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides 30, 226–233 10.1016/j.peptides.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka T., Takayanagi Y., Yoshida M. (2012). Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J. Neuroendocrinol. 24, 587–598 10.1111/j.1365-2826.2012.02300.x [DOI] [PubMed] [Google Scholar]
- Qi J., Yang J. Y., Song M., Li Y., Wang F., Wu C. F. (2008). Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch. Pharmacol. 376, 441–448 10.1007/s00210-007-0245-8 [DOI] [PubMed] [Google Scholar]
- Qi Y., Henry B. A., Oldfield B. J., Clarke I. J. (2010). The action of leptin on appetite-regulating cells in the ovine hypothalamus: demonstration of direct action in the absence of the arcuate nucleus. Endocrinology 151, 2106–2116 10.1210/en.2009-1283 [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Tang M., McCann M. J., Stricker E. M., Verbalis J. G. (1987). Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am. J. Physiol. 253, R661–R665 [DOI] [PubMed] [Google Scholar]
- Rinaman L. (1998). Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J. Comp. Neurol. 399, 101–109 [DOI] [PubMed] [Google Scholar]
- Rogers R. C., Hermann G. E. (1987). Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides 8, 505–513 10.1016/0196-9781(87)90017-9 [DOI] [PubMed] [Google Scholar]
- Rossoni E., Feng J., Tirozzi B., Brown D., Leng G., Moos F. (2008). Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput. Biol. 4:e1000123. 10.1371/journal.pcbi.1000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N., Caquineau C., Dayanithi G., Bull P., Douglas A. J., Guan X. M., et al. (2003). Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 23, 10351–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N., Leng G. (2006). Presynaptic actions of endocannabinoids mediate alpha-MSH-induced inhibition of oxytocin cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R577–R584 10.1152/ajpregu.00667.2005 [DOI] [PubMed] [Google Scholar]
- Sabatier N., Leng G. (2008). Spontaneous discharge characteristic of neurons in the ventromedial nucleus of the rat hypothalamus in vivo. Eur. J. Neurosci. 28, 693–706 10.1111/j.1460-9568.2008.06389.x [DOI] [PubMed] [Google Scholar]
- Sabatier N., Leng G. (2010). Responses to cholecystokinin in the ventromedial nucleus of the rat hypothalamus in vivo. Eur. J. Neurosci. 31, 1127–1135 10.1111/j.1460-9568.2010.07144.x [DOI] [PubMed] [Google Scholar]
- Sabatier N., Rowe I., Leng G. (2007). Central release of oxytocin and the ventromedial hypothalamus. Biochem. Soc. Trans. 35, 1247–1251 10.1042/BST0351247 [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. (1976). Direct hypothalamo-autonomic connections. Brain Res. 117, 305–312 10.1016/0006-8993(76)90738-1 [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. (1984). Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J. Neurosci. 4, 1118–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A., Rinaman L., Vollmer R. R., Amico J. A. (2007). Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1828–R1833 10.1152/ajpregu.00826.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor-Posner G., Azar A. P., Insinga S., Leibowitz S. F. (1985). Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol. Behav. 35, 883–890 10.1016/0031-9384(85)90255-0 [DOI] [PubMed] [Google Scholar]
- Simpson K., Parker J., Plumer J., Bloom S. (2012). CCK, PYY and PP: the control of energy balance. Handb. Exp. Pharmacol. 209, 209–230 10.1007/978-3-642-24716-3_9 [DOI] [PubMed] [Google Scholar]
- Sims J. S., Lorden J. F. (1986). Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav. Brain Res. 22, 265–281 10.1016/0166-4328(86)90071-9 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. (1980). Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J. Histochem. Cytochem. 28, 475–478 10.1177/28.5.7381192 [DOI] [PubMed] [Google Scholar]
- Strader A. D., Woods S. C. (2005). Gastrointestinal hormones and food intake. Gastroenterology 128, 175–191 10.1053/j.gastro.2004.10.043 [DOI] [PubMed] [Google Scholar]
- Succu S., Sanna F., Cocco C., Melis T., Boi A., Ferri G. L., et al. (2008). Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur. J. Neurosci. 28, 813–821 10.1111/j.1460-9568.2008.06385.x [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Purba J. S., Hofman M. A. (1995). Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab. 80, 573–579 10.1210/jc.80.2.573 [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. (1980). The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 194, 555–570 10.1002/cne.901940306 [DOI] [PubMed] [Google Scholar]
- Takayanagi Y., Kasahara Y., Onaka T., Takahashi N., Kawada T., Nishimori K. (2008). Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19, 951–955 10.1097/WNR.0b013e3283021ca9 [DOI] [PubMed] [Google Scholar]
- Tolson K. P., Gemelli T., Gautron L., Elmquist J. K., Zinn A. R., Kublaoui B. M. (2010). Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J. Neurosci. 30, 3803–3812 10.1523/JNEUROSCI.5444-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Barberis C., Jard S., Dubois-Dauphin M., Dreifuss J. J. (1988). Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 442, 105–118 10.1016/0006-8993(88)91437-0 [DOI] [PubMed] [Google Scholar]
- Valassi E., Scacchi M., Cavagnini F. (2008). Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 18, 158–168 10.1016/j.numecd.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Lotstra F., Vandesande F., Dierickx K. (1981). Coexistence of cholecystokinin and oxytocin-neurophysin in some magnocellular hypothalamo-hypophyseal neurons. Cell Tissue Res. 221, 227–231 10.1007/BF00216585 [DOI] [PubMed] [Google Scholar]
- Verbalis J. G., Mangione M. P., Stricker E. M. (1991). Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology 128, 1317–1322 10.1210/endo-128-3-1317 [DOI] [PubMed] [Google Scholar]
- Verbalis J. G., McCann M. J., Mchale C. M., Stricker E. M. (1986). Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science 232, 1417–1419 10.1126/science.3715453 [DOI] [PubMed] [Google Scholar]
- Vrang N., Larsen P. J., Clausen J. T., Kristensen P. (1999). Neurochemical characterization of hypothalamic cocaine- and amphetamine-regulated transcript neurons. J. Neurosci. 19, RC5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. D., Abel D. C., King C. M., Jelley R. Y., Lightman S. L. (1986). Vasopressin and oxytocin responses to acute and chronic osmotic stimuli in man. J. Endocrinol. 108, 163–168 10.1677/joe.0.1080163 [DOI] [PubMed] [Google Scholar]
- Yarnell D. O., Knight D. S., Hamilton K., Tulp O., Tso P. (1998). Localization of leptin receptor immunoreactivity in the lean and obese Zucker rat brain. Brain Res. 785, 80–90 10.1016/S0006-8993(97)01388-7 [DOI] [PubMed] [Google Scholar]
- Yokosuka M., Kalra P. S., Kalra S. P. (1999). Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology 140, 4494–4500 10.1210/en.140.10.4494 [DOI] [PubMed] [Google Scholar]
- Yokosuka M., Xu B., Pu S., Kalra P. S., Kalra S. P. (1998). Neural substrates for leptin and neuropeptide Y (NPY) interaction: hypothalamic sites associated with inhibition of NPY-induced food intake. Physiol. Behav. 64, 331–338 10.1016/S0031-9384(98)00065-1 [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Kiyama H., Kimura T., Araki T., Maeno H., Tanizawa O., et al. (1993). Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology 133, 1239–1246 10.1210/en.133.3.1239 [DOI] [PubMed] [Google Scholar]
- Yosten G. L., Samson W. K. (2010). The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1642–R1647 10.1152/ajpregu.00804.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]