Abstract
Zooarcheological evidence suggests that pigs were domesticated in Southwest Asia ∼8,500 BC. They then spread across the Middle and Near East and westward into Europe alongside early agriculturalists. European pigs were either domesticated independently or more likely appeared so as a result of admixture between introduced pigs and European wild boar. As a result, European wild boar mtDNA lineages replaced Near Eastern/Anatolian mtDNA signatures in Europe and subsequently replaced indigenous domestic pig lineages in Anatolia. The specific details of these processes, however, remain unknown. To address questions related to early pig domestication, dispersal, and turnover in the Near East, we analyzed ancient mitochondrial DNA and dental geometric morphometric variation in 393 ancient pig specimens representing 48 archeological sites (from the Pre-Pottery Neolithic to the Medieval period) from Armenia, Cyprus, Georgia, Iran, Syria, and Turkey. Our results reveal the first genetic signatures of early domestic pigs in the Near Eastern Neolithic core zone. We also demonstrate that these early pigs differed genetically from those in western Anatolia that were introduced to Europe during the Neolithic expansion. In addition, we present a significantly more refined chronology for the introduction of European domestic pigs into Asia Minor that took place during the Bronze Age, at least 900 years earlier than previously detected. By the 5th century AD, European signatures completely replaced the endemic lineages possibly coinciding with the widespread demographic and societal changes that occurred during the Anatolian Bronze and Iron Ages.
Keywords: pig domestication, wild boar, Neolithic, phylogeography
Introduction
The transition from hunting and gathering to agriculture is one of the most important biocultural processes in human history (Diamond and Bellwood 2003). Though this transition took place in numerous locations across the globe (Purugganan and Fuller 2009), the earliest stages of animal domestication in western Eurasia are recorded in the northern Fertile Crescent in the 9th millennium BC (Zeder 2008, 2011). Recent evidence suggests that the establishment of food production was followed by rapid population growth (Bocquet-Appel 2011) and agropastoral economies often spread through demic diffusion (Gignoux et al. 2011). This was certainly the case for Southwest Asia where, following the development of agricultural economies, farmers migrated into Europe during the Neolithic bringing with them domestic crops and livestock (Bramanti et al. 2009).
The increased resolving power of new genetic and morphometric techniques has allowed for the identification of fine-scale population differences across wide temporal and geographic contexts and the capability of tracking these differences through time and space. For example, DNA derived from modern animal (Naderi et al. 2008; Chessa et al. 2009) and plant (Myles et al. 2011; van Heerwaarden et al. 2011) domesticates have been used to unravel geographic origins and dispersal patterns. The use of modern data alone, however, can be problematic. Past domestic populations often underwent dramatic bottlenecks, demographic fluctuations (including complete replacement), and admixture with wild relatives, thus obscuring the genetic signatures of earlier populations (Larson, Albarella, et al. 2007; Larson et al. 2012).
Analyses of ancient DNA (aDNA) have overcome this issue by typing (pre)historic populations and allowing for the direct observation of genetic signatures through time. This approach has generated new insights related to past genetic diversity (Fernandez et al. 2006), wild–domestic hybridization (Bollongino et al. 2008), and human migration (Larson, Albarella, et al. 2007; Larson, Cucchi, et al. 2007). Similarly, novel morphometric methods, including geometric morphometrics (GMM), have been successfully applied to document changes between wild and domestic animals (Larson, Cucchi, et al. 2007) and plants (Terral et al. 2010) and to track the phenotypic evolution of past populations (Cucchi et al. 2009).
Zooarcheological evidence demonstrates that wild boar were domesticated independently in the Near East by at least 8,500 BC (Conolly et al. 2011; Ervynck et al. 2001). By examining pig bones recovered from the Pre-Pottery Neolithic layers at Cayonu Tepesi (10,000–6,300 BC, Erim-Özdoǧan 2011) in southeastern Anatolia, Ervynck et al. (2001) identified a disproportionate decrease in molar tooth size over two millennia. They interpreted this pattern to be the result of a long-term in situ domestication process that led to the emergence of morphologically domestic pigs by 6,800 BC (early Pottery Neolithic). Similar, though contentious, claims for human controlled pig breeding between 8,200 and 7,500 BC have been made at Cafer Höyük (Helmer 2008) and Nevali Çori (Peters et al. 2005) in southeastern Anatolia. The introduction of wild boar to Cyprus by at least 9,700–9,400 BC, however, indicates that humans were actively manipulating wild boar populations for millennia before the emergence of domestic pigs (Vigne et al. 2011; Vigne et al. 2009).
Though the zooarcheological evidence demonstrates that pigs were first domesticated in Southwest Asia, virtually all modern domestic pigs from western Eurasia possess mitochondrial signatures similar (or identical) to European wild boar (Larson et al. 2005). Ancient DNA extracted from early Neolithic domestic pigs in Europe resolved this paradox by demonstrating that early domestic pigs in the Balkans and central Europe shared haplotypes with modern Near Eastern wild boar (Larson, Albarella, et al. 2007). The absence of Near Eastern haplotypes in pre-Neolithic European wild boar suggested that early domestic pigs in Europe must have been introduced from Anatolia by the mid 6th millennium BC before spreading to the Paris basin by the early 4th millennium BC (Larson, Albarella, et al. 2007).
By 3,900 BC, however, virtually all domestic pigs in Europe possessed haplotypes originally only found in European wild boar. This genetic turnover may have resulted from the accumulated introgression of local female wild boar into imported domestic stocks or from an indigenous European domestication process (Larson, Albarella, et al. 2007). After the genetic turnover had taken place in Europe, aDNA from Armenian pigs indicated that European domestic pigs were present in the Near East by the 7th century BC at the end of the Iron Age where they replaced indigenous Near Eastern domestic mtDNA lineages (Larson, Albarella, et al. 2007). Crucially, the archeological record attests to rapid demographic and societal changes during the Late Bronze Age (1,600–1,200 BC) and Iron Age (1,200–600 BC), including large-scale migrations and the expansion of trade and exchange networks across the Mediterranean and the Black Sea region (Sagona and Zimansky 2009).
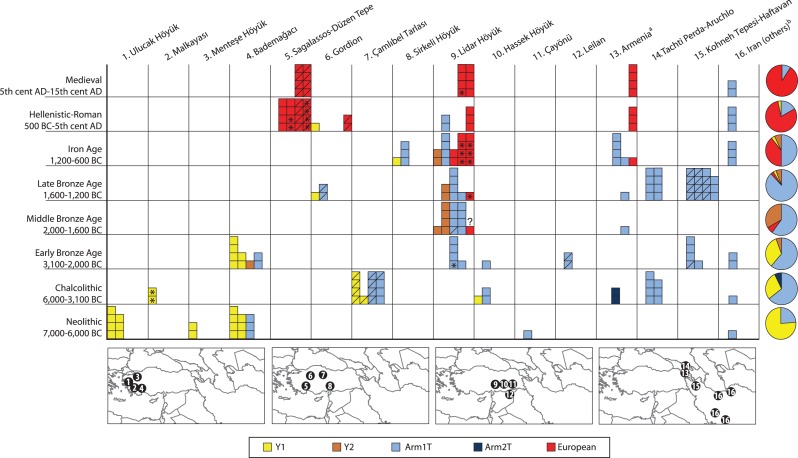
To establish a more precise geographic and temporal framework of mitochondrial Sus haplotypes in Anatolia and to address questions related to the mitochondrial turnover in Armenia at the end of the Iron Age, we obtained mitochondrial sequences from 39 modern wild boar and 393 archeological wild and domestic pigs from 48 Near Eastern sites spanning the Pottery Neolithic (∼7,000 BC) to the 15th century AD from western Turkey to southwestern Iran (fig. 1, supplementary fig. S1a and table S1, Supplementary Material online). We analyzed our novel data alongside previously published ancient and modern sequences (supplementary table S2, Supplementary Material online). In addition, we performed a dental morphological assessment of 46 archeological specimens (with known genetic haplotypes) using traditional osteometric and GMM methods to assess the correlation between genetic and morphometric variation (fig. 2).
Fig. 1.
A spatiotemporal depiction of ancient pig haplotypes. Rows represent eight chronological periods, and columns pertain to sites organized along a longitudinal axis from west to east. Approximate locations of the archeological sites from which the samples are derived are shown as numbered circles on maps beneath the horizontal axis. Asterisks indicate directly AMS-dated samples. The question mark signifies not enough material was available for AMS dating. Slashed boxes indicate samples on which GMM analyses were performed. Pie charts to the right of each row summarize the haplotype frequencies for each chronological period across all sites. Columns pertain to one or two sites except for two columns that consist of several sites: Armenia (Sevkar-4, Areni-1, Khatunarkh, Shengevit, Lchashen, Tmbatir, Pilorpat, Beniamin, and Tsakaektsi) and Iran (Qaleh Rostam, Qare Doyub, Qelīch Qōīneq, Dasht Qal’eh, Doshan Tepe, Malyan, Mehr Ali, Chogha Gavaneh, and Gohar Tepe).
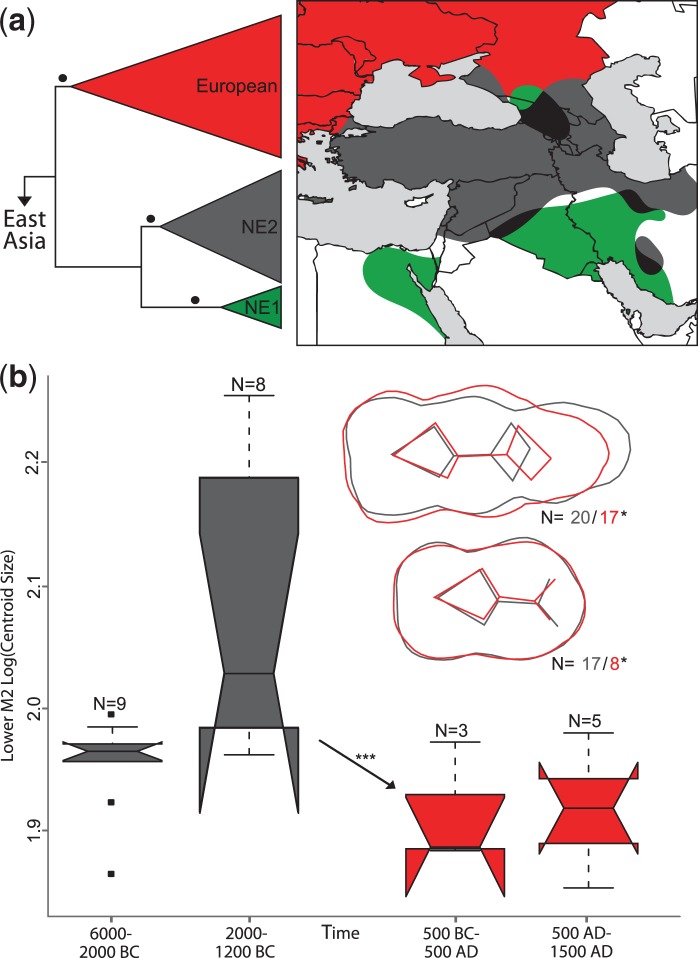
Fig. 2.
Panel (a) depicts a schematic phylogenetic tree derived from an alignment of 267 modern wild boar from western Eurasia. Red, green, and gray triangles refer to the well-supported European, Near Eastern 1 (NE1), and Near Eastern 2 (NE2) clades, respectively. Branches supported by P > 0.99 are indicated by a black circle. A more detailed representation of the tree including support values is presented in supplementary figure S2a, Supplementary Material online. The NE2 clade includes all ancient Near Eastern haplotypes depicted in figure 1. Panel (a) also shows the approximate geographic distribution of modern wild boar belonging to these clades. Areas with overlapping distributions are represented in dark. A more detailed depiction is presented in supplementary figure S2b, Supplementary Material online. Panel (b) presents molar size (M2) and shape (M2 and M3) differences between ancient pigs assigned to European (red) and Near Eastern (gray) mtDNA clades. Differences in shape calculated along linear discriminant analysis (LDA) axes are displayed in overlapping shapes in the upper right. The arrow indicates a statistically significant size reduction in the M2 between European and Near Eastern pigs. Numbers following “N=” represent sample sizes, and single and triple asterisks represent significance to the P < 0.05 and P < 0.01 levels.
Results and Discussion
Genetic Signatures of Early Anatolian Domestic Pigs
Remains of the earliest domestic livestock are found in Southwest Asia ∼9,000–8,000 BC (Zeder 2008). Unlike sheep, goats, and cattle that likely became domesticated through a prey pathway, pigs (like dogs and cats) probably followed a commensal pathway that began with an initial habituation phase before proceeding to a partnership that ended in controlled breeding (Ervynck et al. 2001; Zeder 2012). The protracted time over which pig domestication took place likely included a predomestic management phase that may have been widespread across the region (Vigne 2011).
We first tested the geographic correspondence between archeological and genetic evidence for pig domestication by mapping the geographic distribution of genetic signatures derived from modern wild boar in Anatolia and the Near East (fig. 2). A phylogenetic tree, based on 661 bp (base pairs) of the mitochondrial DNA (mtDNA) control region, revealed a previously observed topology (Larson, Albarella, et al. 2007) that included three well-supported phylogeographic clades: two clades with pigs found exclusively in the Near East (NE1 and NE2) and a European clade. Of the 192 novel ancient sequences (supplementary table S4, Supplementary Material online), all those that possessed one of three Anatolian/Near Eastern mtDNA lineages (Arm1T, Y1, or Y2) (Larson, Albarella, et al. 2007) belonged to the NE2 clade (supplementary fig. S2a, Supplementary Material online). In modern animals, the NE1 clade has been identified only in Near Eastern wild boar (supplementary table S2, Supplementary Material online) and is yet to be found in any modern or ancient domestic pigs. The geographic distributions of the NE1 and NE2 clades overlap only in Iran, Iraq, and in the Caucasus (fig. 2 and supplementary fig. S2b, Supplementary Material online). Given the absence of NE1 boar in Anatolia, and the complete lack of NE1 signatures in modern or ancient domestic pigs, it is plausible that the first domestic pigs in Anatolia belonged to the NE2 clade.
To establish the specific mtDNA lineage of one of the earliest domestic pig populations, we successfully extracted and sequenced DNA from one specimen excavated from an early Pottery Neolithic layer (∼6,800–6,500 BC) at Çayönü Tepesi, representing the final stages of the proposed in situ domestication process (Hongo and Meadow 1998; Ervynck et al. 2001). This specimen possessed the Arm1T haplotype that (along with the Y1 haplotype) is the dominant signature in other Neolithic and Bronze Age Anatolian Sus remains (fig. 1).
We then contrasted the frequencies of NE2 lineages across Southwest Asia (fig. 1). Like numerous other pig clades distributed across the Old World (Larson et al. 2005), the distributions of Y1 and Arm1T are geographically partitioned. Y1 is significantly more frequent in western Anatolia (Fisher’s exact test; P < 0.001), whereas Arm1T has a much wider distribution and dominates in southeastern Anatolia, Armenia, Syria, Georgia, and Iran (Fisher’s exact test; P < 0.001) (fig. 1). Despite the limited sample size, the combined zooarcheological and genetic data suggest that at least the Arm1T lineage was present in the first domestic pigs in western Eurasia.
Anatolian Origins of European Neolithic Pigs
A previous DNA study of modern and ancient, wild and domestic pigs demonstrated that the earliest domestic pigs in Europe possessed one of two NE2 clade haplotypes: Y1 or Y2. Because both of these haplotypes clustered with others found in modern Anatolian and Near Eastern wild boar, the authors concluded that Y1 and Y2 lineages were indigenous to Anatolia and were later transported into Europe by migrating farmers at the onset of the European Neolithic. The lack of ancient Anatolian samples, however, precluded a direct demonstration of that assertion (Larson, Albarella, et al. 2007).
The ancient Anatolian data presented here reveal that both morphologically wild and domestic Neolithic pigs (distinguished using logarithmically indexed linear osteometrics) possessed Y1 haplotypes (fig. 1, supplementary fig. S3a and table S5, Supplementary Material online) and were present at three archeological sites in western Anatolia: Bademağacı (6,400–6,100 BC) (De Cupere et al. 2008), Ulucak Höyük (6,400–5,900 BC) (Çakırlar 2012), and Menteşe Höyük (∼6,000 BC). The presence of these lineages corroborates the supposition that the earliest domestic pigs in Europe originated from populations originally domesticated in the Near East, conclusively linking the Neolithization of Europe with Neolithic cultures of western Anatolia (Larson, Albarella, et al. 2007; Özdoǧan 2005).
The Y1 haplotype does not appear to be associated with either wild boar or early domestic pigs in eastern parts of Anatolia, and it is completely absent in Iranian and Caucasian pigs where the Arm1T lineage dominates. Intriguingly, though Arm1T is present in early domestic pigs in eastern Turkey, this lineage has yet to be identified in either ancient or modern European pigs. This temporal and geographic pattern (fig. 1) could be the result of two different processes. First, it is possible that genetically differentiated wild boar populations in eastern and western Anatolia were domesticated independently. More likely, however, is a scenario in which southeastern Anatolian wild boar were initially domesticated and subsequently transported west out of the Neolithic “core zone” (Özdoğan 2011). Then, following admixture with female wild boar indigenous to western Turkey, they acquired the local Y1 lineage that prevailed over the Arm1T lineage in this area.
The route along which domestic pigs traveled to arrive in western Anatolia remains unknown. The presence of morphologically domestic pig remains by the 7th millennium BC (Pottery Neolithic layers) at the site of Yumuktepe, in south-central Turkey (Buitenhuis and Caneva 1998), and at the early 7th millennium BC layers of Ulucak (Çakırlar 2012; Çilingiroğlu 2012) near the eastern Aegean coast, contrasted with the general dearth of pigs during the same period in central Anatolia (Conolly et al. 2011), however, suggest that one of the possible routes was along the Mediterranean coast.
Timing and Nature of the Anatolian Pig Turnover
A previous study (Larson, Albarella, et al. 2007) demonstrated that domestic pigs with mitochondrial haplotypes predominantly found in Europe replaced mitochondrial lineages in Armenia that possibly originated from the early domestic swine herds in the Neolithic core zone by 700 BC. Because that study did not include ancient pigs from central or western Anatolia, the scale and timing of this proposed eastward dispersal and replacement by European domestic pigs remained unresolved.
The temporal and geographic distribution of genetic haplotypes presented in our study demonstrates that the first AMS radiocarbon-dated pig with European ancestry (haplotype A) appeared almost 1,000 years earlier than the Armenian samples, in a Late Bronze Age context (∼1,600–1,440 BC) at Lidar Höyük (fig. 1). An apparently even earlier Middle Bronze Age specimen from the same site also possessed a European signature, but a direct radiocarbon date for this specimen could not be obtained.
Our data also show that European pigs are unlikely to have arrived in Anatolia before 2,000 BC since the Early Bronze Age layers at Bademağacı and Lidar Höyük (in southwestern and southeastern Anatolia, respectively) only possess indigenous Near Eastern pig lineages. The frequency of pigs with European ancestry increased rapidly from the 12th century BC onwards, and by the 5th century AD, domestic pigs possessing a Near Eastern genetic signature had all but disappeared across Anatolia and the southern Caucasus. Though we did not detect European signatures in the ancient Iranian samples (fig. 1), the eastward spread of European lineages may have continued into Iran later than the Iron Age, since European lineages have been found in wild caught modern Iranian samples (Larson, Albarella, et al. 2007).
If European pig haplotypes were present in Anatolia at <5% before the Middle Bronze Age, our sample size (binomial distribution, n = 73, confidence interval = 95%) would not have allowed us to detect them. To assess the possibility that haplotypes so far found exclusively in Europe were indigenous to Anatolia and the Near East, we analyzed the morphometric differentiation in molar size and shape between archeological samples that possessed European and Anatolian/Near Eastern genetic signatures. Single interbreeding populations have been shown to possess deeply divergent mitochondrial haplotypes (e.g., yaks [Guo et al. 2006]) demonstrating that maternal genetic differentiation alone is not sufficient to infer geographic separation. Statistically significant phenotypic differences between pigs possessing Anatolian/Near Eastern and European haplotypes, however, would indicate that the two populations had been evolving in isolation from one another and that pigs with a European genetic signature were not present in Anatolia before being introduced by people.
A GMM analysis of 46 pigs with known genetic signatures revealed significant differences in both molar size (P < 0.01) and shape (P < 0.05) between European and Anatolian/Near Eastern pigs (fig. 2 and supplementary table S6, Supplementary Material online). European pigs possessed overall smaller teeth and proportionally shorter and laterally widened third molars. The concordance between genetic and GMM signatures strongly suggests that pigs possessing European and Anatolian/Near Eastern mtDNA lineages are morphologically different and that European pigs were, therefore, introduced to Anatolia. The DNA evidence suggests that this process may have taken place (at the latest) during the Middle to Late Bronze Age, at least 900 years earlier than previously inferred.
Establishing a more precise temporal and geographic pattern for the initial introduction and subsequent dominance of European pigs allows for the turnover to be assessed in its cultural context, though the limited archeological coverage of pigs in western Anatolia precludes a definitive identification of an entrance route. Minoans and Mycenaeans may have initially introduced pigs during the Bronze Age when they colonized the western Anatolian coast from the 16th to 12th centuries BC. Alternatively, pigs may have been imported by the Hittites (Seeher 2011) whose kingdom extended from central Anatolia to the northern Levant from the 17th to 13th centuries BC (Bryce 2005). The lack of pigs possessing European signatures in Bronze Age contexts from sites in Georgia suggests that pigs did not arrive via the Caucasus (fig. 1). Regardless of the exact routes of their arrival, European domestic pigs were deliberately introduced into Anatolia. Within two millennia, European mitochondrial lineages had replaced their Near Eastern domestic counterparts that were present, and grew in frequency in the early domestic herds of the Near East over the previous 6,000 years.
Conclusions
This study addresses questions regarding the origins and dispersal of domestic pigs in Southwest Asia by combining genetic and morphometric analyses often on the same archeological samples. The data presented here add to the growing body of evidence suggesting that pig domestication was a complex, nonlinear process that took place over several millennia and involved multiple Southwest Asian wild boar populations (Ervynck et al. 2001; Peters et al. 2005; Vigne et al. 2009).
More specifically, our data suggest a narrative that begins with the domestication of pigs in Southwest Asia, at Upper Tigris sites including Çayönü Tepesi (Ervynck et al. 2001) and possibly Upper Euphrates sites including Cafer Höyük (Helmer 2008) and Nevali Çori (Peters et al. 2005). Early domestic pigs likely possessed at least the Arm1T haplotype (indigenous to Southwest Asia) and dispersed with humans as the Neolithic expanded away from these centers. Once introduced to western Anatolia, domestic swineherds acquired a mitochondrial signature (Y1) associated with the local wild boar most likely through admixture. The eastern Anatolian mitochondrial lineage (Arm1T) became less frequent likely as a result of this admixture process, small population sizes, and genetic drift.
This same turnover pattern was evident after pigs possessing domestic Y1 lineages were subsequently transported west into Europe as far as the Paris Basin (Larson, Albarella, et al. 2007). Once domestic pigs orientating from southeastern Anatolia but possessing the western Anatolian Y1 haplotype arrived in Europe, they acquired European wild boar genetic signatures and lost the Y1 haplotype through introgression of resident wild boar mitochondria into the imported domestic pig population. From at least the beginning of the Late Bronze Age, and possibly several centuries before, domestic pigs of European wild boar origin now all carrying European wild boar mtDNA lineages were introduced to Anatolia. On this occasion, however, swineherds did not take on the genetic characteristics of the local populations. Instead, by the 5th century AD, European domestic pig haplotypes had completely replaced the endemic Y1 and Arm1T lineages.
The movement of domestic pigs from western Anatolia into Europe is consistent with recent aDNA studies of human remains that support a demic diffusion model of the initial Central European Neolithic (Bramanti et al. 2009). Whether the back migration of European pigs into Anatolia reflects human migration or trade and exchange remains unclear. Addressing these and other questions can be accomplished by incorporating both mitochondrial and nuclear markers in combination with large-scale morphological analyses.
Material and Methods
Ancient Samples
We analyzed 393 ancient pig bone and tooth specimens excavated from 48 Anatolian archeological sites (supplementary fig. S1a and table S1, Supplementary Material online). All dates are reported in calibrated radiocarbon years BC. The ages of the archeological remains ranged from the 10th millennium BC to the medieval era and were determined using direct accelerator mass spectrometry (AMS) radiocarbon dating (Beta Analytic Inc. and University of Oxford), stratigraphic associations with AMS dates, and contextual archeological evidence. Samples dated at Oxford were treated using standard protocols as described by Brock et al. (2010).
Genetic Analyses
Analyses were carried out in aDNA facilities in three separate institutions: the Forensic Genetics and Molecular Archeology department in Leuven (Belgium), the Department of Archaeology at Durham University (United Kingdom), and the Institute of Anthropology in Mainz (Germany) using standard contamination precautions (Gilbert et al. 2005). Two ∼120 bp fragments of the control region of the mitochondrial genome were amplified (Larson, Albarella, et al. 2007) and sequenced. Some fragments were cloned (supplementary table S3, Supplementary Material online). Larger control region fragments (up to ∼800 bp) were generated from DNA extracts of 39 modern wild boar from the greater geographic region (supplementary table S2, Supplementary Material online). Modern sequences were generated at the Department of Animal Sciences, Universitat Autònoma de Barcelona (Spain). A maximum likelihood (ML) tree was created from an alignment of 661 bp of the control region of 267 modern wild boar using PhyML (Guindon and Gascuel 2003) in Geneious 5.5 (Drummond et al. 2011) (supplementary text, Supplementary Material online). Variations in substitution model and analytical framework did not affect the topology of the main clades. Details regarding methods, contamination avoidance procedures, authentication, and phylogenetic analyses are described in the supplementary text, Supplementary Material online.
Reproducible aDNA sequences were obtained from 192 of 393 specimens (48.9%, supplementary table S4, Supplementary Material online). As expected for ancient samples (Smith et al. 2003), we observed an inverse correlation between aDNA success frequency and sample age in nine time bins (Spearman’s rank correlation r2 = 0.87, P < 0.001; supplementary fig. S1b, Supplementary Material online). All sequences have been deposited in GenBank (JX893958–JX894188). Variable positions of the two concatenated ANC1 and ANC2 fragments are presented in supplementary table S4, Supplementary Material online. As shown in the ML tree (supplementary fig. S2a, Supplementary Material online), the majority of the diagnostic variation is present in the ANC1 fragment (Larson, Albarella, et al. 2007). Given the greater resolving power of this fragment, the relatively low variation within the ANC2 fragment, and to be consistent with the terminology developed by Larson et al. (Larson, Albarella, et al. 2007), haplotype assignments for each specimen were based on the ANC1 terminology. Additional information regarding haplotype assignment and terminology is present in the supplementary text, Supplementary Material online. The haplotype distribution across Southwest Asia was tested using a Fisher’s exact test. Western Anatolia included sites from Ulucak Höyük to Çamlibel Tarlası and eastern Anatolia included sites from Sirkeli Höyük to Çayönü.
Morphometric Analyses
A total of 62 mandibular molars (25 M2 and 37 M3) from 46 ancient specimens were analyzed using traditional biometrical and GMM approaches from standardized photographs taken from the occlusal view (supplementary fig. S3b, Supplementary Material online). For the traditional metrical approach, we measured maximum length and width metrics (two for the M2 and three for the M3, supplementary fig. S3b, Supplementary Material online). Two-dimensional GMM methods (based on coordinates) were used to separately analyze size and shape variables. In total, we analyzed 7 landmarks (homologous points) for the M2, 8 for the M3, 68 sliding semilandmarks (points along the outline of the tooth) for the M2, and 91 for the M3 (Cucchi et al. 2011; Evin et al. forthcoming) (supplementary fig. S3b, Supplementary Material online).
Differences between ancient pigs assigned to Near Eastern mtDNA clade NE2 and European mtDNA clades were tested using traditional metric and GMM approaches that analyzed both shape and log-transformed centroid size. Traditional measurements were analyzed using a log-shape ratio (LSR) approach (Mosimann and James 1979) that allowed a separation of shape and isometric size. Differences between clades were tested with Kruskal–Wallis tests for size indices (centroid and isometric) paired with boxplots and multivariate analyses of variance for shape measures (from GMM and LSR) coupled with linear discriminant analyses paired with leave-one-out cross-validation percentages. Details are described in the supplementary text, Supplementary Material online.
Supplementary Material
Supplementary text, figures S1–S3, and tables S1–S6 are available at Molecular Biology and Evolution online (http://mbe.oxfordjournals.org/).
Acknowledgments
The authors thank Marcel Amills, Universitat Autònoma de Barcelona and Hikmet Ün, Etlik Veterinary Control Central Research Institute, Ankara (Turkey), for modern wild boar samples, Helen Everett, AHVLA-Weybridge (UK), for DNA extraction, and Eske Willerslev and Tom Gilbert (both at the Center for GeoGenetics, Denmark) for assistance with sequencing and comments on the manuscript. They also thank Boris Gasparyan and Mary M. Voigt, College of William and Mary (USA), for access to ancient samples. This work was supported by grants from the Natural Environment Research Council (NE/F003382/1), the Leverhulme Trust (F/00 128/AX), the Belgian Program on Inter-University Poles of Attraction (IAP 6/22), the Research Fund of the KU Leuven (BOF-GOA 07/02), the Hercules Foundation (AKUL/09/16), and the Deutsche Archäologisches Institut (DAI). G.L. was supported by an RCUK Academic Fellowship, J.P. was supported by the German Science Foundation (PE 424/10-1), M.W. received a Methusalem grant from the Flemish Ministry for Science Policy, and C.G. was supported by the German Archaeological Institute, Berlin (Research Cluster 1, Project 8999539). All research within the framework of the Sagalassos Project was carried out by the Centre for Archeological Sciences of the KU Leuven. Archeozoological research in Iran was supported by the Iranian Cultural Heritage, Handicraft, and Tourism Organization (ICHHTO); the UMR 7209 of the CNRS/MNHN; the British Institute for Persian Studies; the Cultural Service of the French Embassy in Iran; and the University of Edinburgh. Interdisciplinary research into the Neolithic of Southwest Iran was supported by a joint French Japanese Cooperation project (04.2.444) and JSPS. The archaeozoological study of Neolithic Ulucak has been funded through an individual fieldwork grant from the Institute for Aegean Prehistory since 2008 and C.C. was supported by the Belgian Federal Science Policy Office (postdoctoral fellowship to non-EU researchers). The authors also wish to thank the assistant director of Ulucak excavation, Çiler Çilingiroğlu, for collaboration.
References
- Bocquet-Appel JP. When the world’s population took off: the springboard of the Neolithic Demographic Transition. Science. 2011;333:560–561. doi: 10.1126/science.1208880. [DOI] [PubMed] [Google Scholar]
- Bollongino R, Elsner J, Vigne JD, Burger J. Y-SNPs do not indicate hybridisation between European aurochs and domestic cattle. PLoS One. 2008;3:e3418. doi: 10.1371/journal.pone.0003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramanti B, Thomas MG, Haak W, et al. (16 co-authors) Genetic discontinuity between local hunter-gatherers and central Europe’s first farmers. Science. 2009;326:137–140. doi: 10.1126/science.1176869. [DOI] [PubMed] [Google Scholar]
- Brock F, Higham TFG, Ditchfield P, Bronk Ramsey C. Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (ORAU) Radiocarbon. 2010;52:103–112. [Google Scholar]
- Bryce T. The kingdom of the Hittites. Oxford: Oxford University Press; 2005. [Google Scholar]
- Buitenhuis H, Caneva I. Early animal breeding in southeastern Anatolia: Mersin-Yumuktepe. In: Anreiter P, Bartosiewicz L, Jerem E, Meids W, editors. Man and the animal world. Budapest (Hungary): Archaeolingua; 1998. pp. 122–130. [Google Scholar]
- Çakırlar C. Evolution of animal husbandry in Neolithic Central-West Anatolia, the archaeozoological record from Ulucak Höyük (ca. 7040–5660 cal. BC, İzmir, Turkey) Anatolian Studies. 2012;62:1–33. [Google Scholar]
- Chessa B, Pereira F, Arnaud F, et al. (33 co-authors) Revealing the history of sheep domestication using retrovirus integrations. Science. 2009;324:532–536. doi: 10.1126/science.1170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çilingiroğlu Ç. The Neolithic pottery of Ulucak in Aegean Turkey: organization of production, interregional comparisons, and relative chronology. British Archaeological Reports (International Series) 2012:2426. [Google Scholar]
- Conolly J, Colledge S, Dobney K, Vigne J-D, Peters J, Stopp B, Manning K, Shennan S. Meta-analysis of zooarchaeological data from SW Asia and SE Europe provides insight into the origins and spread of animal husbandry. J Archaeol Sci. 2011;38:538–545. [Google Scholar]
- Cucchi T, Fujita M, Dobney K. New insights into pig taxonomy, domestication, and human dispersal in Island South East Asia: molar shape analysis of Sus remains from Niah Caves, Sarawak. Int J Osteoarchaeol. 2009;19:508–530. [Google Scholar]
- Cucchi T, Hulme-Beaman A, Yuan J, Dobney K. Early Neolithic pig domestication at Jiahu, Henan Province, China: clues from molar shape analyses using geometric morphometric approaches. J Archaeol Sci. 2011;38:11–22. [Google Scholar]
- De Cupere B, Duru R, Umurtak G. Animal husbandry at the Early Neolithic to Early Bronze Age site of Bademağacı (Antalya province, SW Turkey): evidence from the faunal remains. In: Vila E, Gourichon L, Choyke AM, Buitenhuis H, editors. Proceedings of the 8th International Symposium on the Archaeozoology of Southwestern Asia and Adjacent Areas. Lyon (France): Archéorient, Maison de l’Orient et de la Méditerranée; 2008. pp. 367–405. [Google Scholar]
- Diamond J, Bellwood P. Farmers and their languages: the first expansions. Science. 2003;300:597–603. doi: 10.1126/science.1078208. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, et al. (15 co-authors) Geneious v5.4. 2011. Available from: http://www.geneious.com/ (last accessed December 10, 2012)
- Erim-Özdoǧan A. Çayönü. In: Özdoǧan M, Basglen N, editors. 2011. The Neolithic in Turkey: New Excavations & New Research: The Tigris Basin. Istanbul: Archaeology and Art Publications. p. 185–269. [Google Scholar]
- Ervynck A, Dobney K, Hongo H, Meadow R. Born free? New evidence for the status of Sus scrofa at Neolithic Çayönü Tepesi (southeastern Anatolia, Turkey) Paléorient. 2001;27:47–73. [Google Scholar]
- Evin A, Cucchi T, Cardini A, Strand Vidarsdottir U, Larson G, Dobney K. The long and winding road: identifying pig domestication through molar size and shape. J Archaeol Sci. 2013;40:735–743. [Google Scholar]
- Fernandez H, Hughes S, Vigne JD, Helmer D, Hodgins G, Miquel C, Hanni C, Luikart G, Taberlet P. Divergent mtDNA lineages of goats in an Early Neolithic site, far from the initial domestication areas. Proc Natl Acad Sci U S A. 2006;103:15375–15379. doi: 10.1073/pnas.0602753103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci U S A. 2011;108:6044–6049. doi: 10.1073/pnas.0914274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Bandelt HJ, Hofreiter M, Barnes I. Assessing ancient DNA studies. Trends Ecol Evol. 2005;20:541–544. doi: 10.1016/j.tree.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Guo S, Savolainen P, Su J, Zhang Q, Qi D, Zhou J, Zhong Y, Zhao X, Liu J. Origin of mitochondrial DNA diversity of domestic yaks. BMC Evol Biol. 2006;6:73. doi: 10.1186/1471-2148-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer D. Révision de la faune de Cafer Höyük (Malatya, Turquie): apports des méthodes de l’analyse des mélanges et de l’analyse de Kernel à la mise en évidence de la domestication. In: Vila E, Gourichon L, Choyke AM, Buitenhuis H, editors. Archaeozoology of the Near East VIII: Proceedings of the 8th International Symposium on the Archaeozoology of Southwestern Asia and Adjacent Areas. Lyon (France): Maison de l’Orient et de la Méditerraneé; 2008. pp. 169–195. [Google Scholar]
- Hongo H, Meadow R. Pig exploitation at Neolithic Çayönü Tepesi (Southeastern Anatolia) In: Nelson SM, editor. Ancestors for the pigs: pigs in prehistory. Philadelphia (PA): Penn Press; 1998. pp. 77–98. [Google Scholar]
- Larson G, Albarella U, Dobney K, et al. (19 co-authors) Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci U S A. 2007;104:15276–15281. doi: 10.1073/pnas.0703411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson G, Cucchi T, Fujita M, et al. (32 co-authors) Phylogeny and ancient DNA of Sus provides insights into neolithic expansion in Island Southeast Asia and Oceania. Proc Natl Acad Sci U S A. 2007;104:4834–4839. doi: 10.1073/pnas.0607753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson G, Dobney K, Albarella U, et al. (13 co-authors) Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science. 2005;307:1618–1621. doi: 10.1126/science.1106927. [DOI] [PubMed] [Google Scholar]
- Larson G, Karlsson EK, Perri A, et al. (20 co-authors) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci U S A. 2012;109:8878–8883. doi: 10.1073/pnas.1203005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann JE, James FC. New statistical methods for allometry with application to Florida red-winged blackbirds. Evolution. 1979;33:444–459. doi: 10.1111/j.1558-5646.1979.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Myles S, Boyko AR, Owens CL, et al. (12 co-authors) Genetic structure and domestication history of the grape. Proc Natl Acad Sci U S A. 2011;108:3530–3535. doi: 10.1073/pnas.1009363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi S, Rezaei HR, Pompanon F, et al. (12 co-authors) The goat domestication process inferred from large-scale mitochondrial DNA analysis of wild and domestic individuals. Proc Natl Acad Sci U S A. 2008;105:17659–17664. doi: 10.1073/pnas.0804782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdoǧan M. The expansion of the Neolithic way of life: what we know and what we do not know. In: Lichter C, editor. 2005. How did farming reach Europe? Anatolian-European relations from the second half of the 7th through the first half of the 6th millennium cal BC (Proceedings of the International Workshop Istanbul, 20–22 May 2004; Byzas 2): 13–27. Istanbul: Deutsches Archäologisches Institut. [Google Scholar]
- Özdoğan M. Archaeological evidence on the westward expansion of farming communities from eastern Anatolia to the Aegean and the Balkans. Curr Anthropol. 2011;52:S415–S430. [Google Scholar]
- Peters J, von den Driesch A, Helmer D. The upper Euphrates-Tigris basin: cradle of agro-pastoralism? In: Vigne JD, Peters J, Helmer D, editors. The first steps of animal domestication: new archaeological approaches. Proceedings of the 9th ICAZ Conference. Oxford (United Kingdom): Oxbow Books; 2005. pp. 96–124. [Google Scholar]
- Purugganan MD, Fuller DQ. The nature of selection during plant domestication. Nature. 2009;457:843–848. doi: 10.1038/nature07895. [DOI] [PubMed] [Google Scholar]
- Sagona AG, Zimansky P. New York: Routledge; 2009. Ancient Turkey. [Google Scholar]
- Seeher J. The plateau: the Hittites. In: Steadman SR, McMahon JG, editors. The Oxford handbook of ancient Anatolia: 10,000–323 B.C.E. New York: Oxford University Press; 2011. pp. 376–392. [Google Scholar]
- Smith CI, Chamberlain AT, Riley MS, Stringer C, Collins MJ. The thermal history of human fossils and the likelihood of successful DNA amplification. J Hum Evol. 2003;45:203–217. doi: 10.1016/s0047-2484(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Terral J-F, Tabard E, Bouby L, et al. (15 co-authors) Evolution and history of grapevine (Vitis vinifera) under domestication: new morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient European cultivars. Ann Bot. 2010;105:443–455. doi: 10.1093/aob/mcp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerwaarden J, Doebley J, Briggs WH, Glaubitz JC, Goodman MM, de Jesus Sanchez Gonzalez J, Ross-Ibarra J. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc Natl Acad Sci U S A. 2011;108:1088–1092. doi: 10.1073/pnas.1013011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne JD. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C R Biol. 2011;334:171–181. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Vigne JD, Carrère I, Briois F, Guilaine J. The early process of mammal domestication in the Near East. Curr Anthropol. 2011;52:S4. [Google Scholar]
- Vigne JD, Zazzo A, Saliege JF, Poplin F, Guilaine J, Simmons A. Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. Proc Natl Acad Sci U S A. 2009;106:16135–16138. doi: 10.1073/pnas.0905015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder MA. Domestication and early agriculture in the Mediterranean Basin: origins, diffusion, and impact. Proc Natl Acad Sci U S A. 2008;105:11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder M. The origins of agriculture in the Near East. Curr Anthropol. 2011;52:S221–S235. [Google Scholar]
- Zeder MA. The domestication of animals. Journal of Anthropological Research. 2012;68:161–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.