Abstract
Sex chromosome evolution is usually seen as a process that, once initiated, will inevitably progress toward an advanced stage of degeneration of the nonrecombining chromosome. However, despite evidence that avian sex chromosome evolution was initiated >100 Ma, ratite birds have been trapped in an arrested stage of sex chromosome divergence. We performed RNA sequencing of several tissues from male and female ostriches and assembled the transcriptome de novo. A total of 315 Z-linked genes fell into two categories: those that have equal expression level in the two sexes (for which Z–W recombination still occurs) and those that have a 2-fold excess of male expression (for which Z–W recombination has ceased). We suggest that failure to evolve dosage compensation has constrained sex chromosome divergence in this basal avian lineage. Our results indicate that dosage compensation is a prerequisite for, not only a consequence of, sex chromosome evolution.
The requirement of fine-tuned regulation of gene expression levels imposes severe constraint on gene dose. Chromosomal abnormalities such as monosomies and trisomies are typically lethal or deleterious, and large deletions causing hemizygosity of single genes can have strong negative impact on fitness (haploinsufficiency) (Hassold and Hunt 2001; Nagaoka et al. 2012). Diverging sex chromosomes are therefore faced with the dilemma of maintaining a balance in gene expression levels between interacting sex-linked and autosomal genes (Ohno 1967). Following cessation of recombination between the X- and the Y-chromosomes (or between the Z- and the W-chromosomes in female heterogametic organisms), and the subsequent degeneration of genes on the Y-chromosome, the dose of X-linked genes in males is only half of what it once was and still is in females. To compensate for this change in gene dose, the evolutionary pressure for adjusting expression levels should be strong. Sex chromosome dosage compensation is well documented in a wide variety of organisms and can evolve rapidly after recombination suppression (Muyle et al. 2012). The molecular mechanisms for how this is achieved vary, reflecting convergent solutions to a common evolutionary problem (Straub and Becker 2007). In mammals, the discovery of X-chromosome inactivation initially brought focus on the equalization of expression levels of sex-linked genes in males and females. However, this ignored the aspect of maintaining the ancestral relationships between expression levels of autosomal and sex-linked genes (Ohno 1967). Recently, empirical evidence from mammals, flies, and nematodes for upregulation of the active X-chromosome in both sexes has led to a model in which equalization of sex-linked and autosomal expression in males drove the evolution of dosage compensation and that gene silencing in females evolved as a secondary response (Deng et al. 2011; He et al. 2011; Kharchenko et al. 2011; Lin et al. 2011; Pessia et al. 2012). However, this inference is a matter of debate, and comparisons of expression levels of mammalian and outgroup orthologs suggest in fact that there has been no overall upregulation of X-linked expression during the evolution of placental mammals (Julien et al. 2012; Lin et al. 2012).
Sex chromosome evolution is usually seen as a process that, once initiated, will inevitably progress toward an advanced stage of degeneration of the nonrecombining chromosome, leading to a largely heteromorphic chromosome pair. Ratite birds (including ostrich and its allies and representing the most basal clade of contemporary birds) constitute a rare example where diverging sex chromosomes have been trapped in a stage of arrest where recombination still occurs over most of the Z- and W-chromosomes (Ogawa et al. 1998) (supplementary figs. S1 and S2, Supplementary Material online), despite evidence that avian sex chromosome evolution was initiated before the split of ratites from other birds more than 120 Ma. Because Z–W differentiation is far-gone in other avian lineages (Nam and Ellegren 2008), this cannot be explained by some inherent characteristic of the ancestral chromosome pair from which Z and W were derived.
It has recently been shown that dosage compensation in birds is far from complete (Ellegren et al. 2007; Itoh et al. 2007, 2010; Wolf and Bryk 2011); in chicken, mean expression level of Z-linked genes is ∼1.5 times higher in males (ZZ) than in females (ZW), with significant variation in sex bias among genes (Ellegren et al. 2007; Itoh et al. 2007; Mank et al. 2008; Mank and Ellegren 2009). Could it be that the absence of a chromosome-wide mechanism for dosage compensation in birds coupled with a failure to compensate the expression level of critical genes in ratites has hindered sex chromosome differentiation in this primitive clade of birds? More generally, does dosage compensation, or rather the absence thereof, impose a constraint to sex chromosome evolution?
To address this issue, and given that there have been no previous large-scale studies of dosage compensation in ratites, we performed RNA sequencing of brain and liver from pools of male and female ostriches and assembled the transcriptome de novo. Contigs were mapped to the chicken reference set of genes, which led to the identification of 7,314 1:1 ostrich orthologs. Chromosome painting has revealed that the avian Z-chromosome is syntenic across all investigated bird species, including ratites (Shetty et al. 1999; Nishida-Umehara et al. 2007), and this is also supported by available evidence from sequenced avian genomes (Warren et al. 2010). Physical mapping of individual genes further corroborates Z-chromosome conservation between ostrich and chicken (nine genes have been mapped to the ostrich Z-chromosome; Ogawa et al. 1998; Nishida-Umehara et al. 2007), albeit with intrachromosomal rearrangements (supplementary fig. S2, Supplementary Material online). A total of 315 of the genes identified as ostrich orthologs to chicken genes map to the chicken Z-chromosome, and in the following, we consider these Z-linked also in ostrich.
The male-to-female expression ratio of Z-linked genes in ostriches showed a bimodal distribution with peaks corresponding to an equal ratio between sexes (log2 ∼ 0) and a 2-fold excess of male expression (log2 ∼ 1) (fig. 1A, brain; see supplementary fig. S3, Supplementary Material online, for liver). When we mapped the genes according to their physical position on the chicken Z-chromosome (taking into account that chicken and ostrich Z-chromosomes are not colinear), distinct clusters with respect to the sex bias in gene expression were apparent, and these clusters closely corresponded to the bimodality observed for the whole set of genes (fig. 1B, supplementary fig. S3, Supplementary Material online). Four clusters, containing 222 genes, had a median male-to-female expression ratio of log2 = 0.005 in brain and log2 = 0.106 in liver. Another four clusters, together containing 93 genes had a median ratio of log2 = 0.931 in brain and log2 = 1.028 in liver.
Fig. 1.
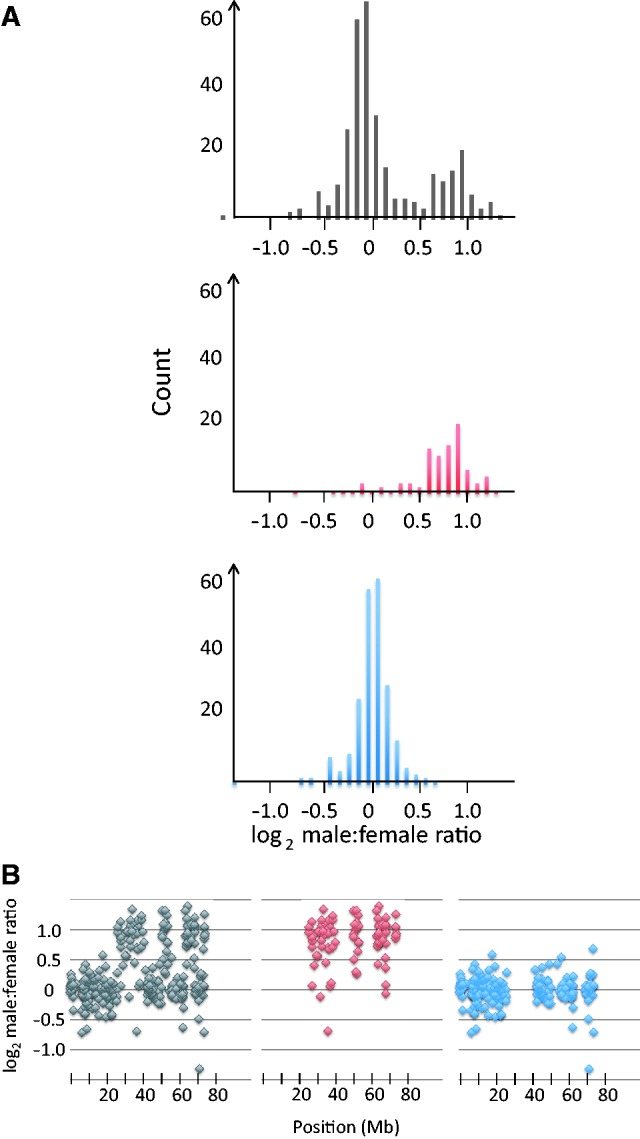
Distribution of the male-to-female expression ratio in ostrich brain for Z-chromosome genes. (A) Distribution of the male:female expression ratio for all genes (black), 93 genes from clusters with a 2-fold excess of male expression (red), and 222 genes from clusters with equal expression in males and females (blue). (B) Expression ratio plotted according to start position on the chicken Z-chromosome showing all genes (black), genes from clusters with a 2-fold excess of male expression (red), and genes from clusters with equal expression in males and females (blue).
The fact that large part of the ostrich Z-chromosome still recombine with the W-chromosome (Pigozzi and Solari 1997) implies that females have two copies of many Z-linked genes but only a single copy of others. In light of the observed bimodality in the extent of sex-biased gene expression, we hypothesized that the nonrandom distribution of sex bias in gene expression along the chromosome can be explained by that clusters of genes with no sex bias correspond to regions that do recombine (two gene copies in females, i.e., equal gene dose in the two sexes), whereas clusters of genes with a 2-fold excess of male expression correspond to regions that do not recombine (one gene copy in females, i.e., double gene dose in males). If correct, this would mean that, overall, nonrecombining Z-chromosome genes in ostrich are not dosage compensated. It should be noted that there is only one recombining and one nonrecombining region on ratite Z-chromosomes (Pigozzi and Solari 1999), as is the general pattern for diverging sex chromosomes. However, because we plotted genes according to their position on the chicken Z-chromosome and because several chromosomal rearrangements (inversions) have occurred since the split of ostrich and chicken lineages (Tsuda et al. 2007), we would not expect to see all recombining genes forming one cluster and all nonrecombining genes another. It could be noted that a similar pattern with alternating clusters of genes with 2-fold excess of male expression and genes with equal expression levels in the two sexes is seen when the data are plotted according to the physical position of genes on a songbird Z-chromosome (Ellegren et al. 2012), showing that our results cannot be explained by an assembly artifact in chicken (supplementary fig. S4, Supplementary Material online).
Our transcriptome sequencing provided expression data for four of the nine genes mapped to the ostrich Z-chromosome, three from the recombining region (ATP5A1, GHR, and ZOV3) and one from the nonrecombining region (ACO1). If our hypothesis that there is no dosage compensation on the ostrich Z-chromosome was correct, we would expect genes from the former category to show no sex difference in expression, whereas genes from the latter category would show 2-fold higher expression in males than in females. In all four cases, the observed male-to-female expression ratio fulfilled the predictions from our hypothesis. Specifically, the three recombining genes (expected ratio close to log2 = 0) had ratios of −0.299, 0.023, and 0.142, respectively, whereas the nonrecombining gene (expected ratio close to log2 = 1) had a ratio of 1.070.
Although genes on nonrecombining sex chromosomes typically tend to degenerate and become lost, at least some genes that have ceased to recombine between Z and W could still have an active and paralogous (gametologous) copy on the W chromosome, as is the case in other birds. Such genes, if they exist in ostrich, would be expected to occur in the clusters with 2-fold excess of male expression. We stringently searched for female-specific alleles and indeed found evidence for W-linked alleles in 12 of 105 genes (11.2%) in the regions with male-biased expression. In contrast, there were only 1 of 223 genes (0.04%) showing signs of W-linkage in the clusters with equal levels of male and female expression (Fisher exact test, P = 0.000007).
To test the hypothesis that sex-biased gene expression correlates with gene dose, we assessed the degree of polymorphism of Z-linked genes. For genes with only one copy in females, data from female pools should contain fewer polymorphic sites than male pools. Moreover, genes with only one copy in females should contain fewer polymorphic sites than genes in female pools with two copies. By classifying genes according to which type of male-to-female expression rate ratio cluster they reside in, both predictions are fulfilled. There were a total of 229 single-nucleotide polymorphisms (SNPs) in male pools for genes from clusters with a 2-fold excess of male expression, compared with 174 segregating sites for the same set of genes in female pools (Wilcoxon’s rank test, P = 0.0077). For genes from clusters with equal expression in males and females, there was no such difference (630 vs. 641; P = 0.85). Moreover, for female pools, there were on average 3.54 SNPs per gene from clusters with no sex bias in gene expression, compared with 2.35 SNPs per gene from clusters with 2-fold excess of male expression (Mann–Whitney U test, P = 0.0038). The corresponding numbers in male pools were 3.48 and 3.09, respectively (P = 0.556). We thus conclude that our data are compatible with a scenario in which gene dose is directly manifested in the expression level of sex-linked genes, that is, that there is no dosage compensation.
Absolute expression level of Z-linked genes in males did not differ significantly from the level of autosomal expression in males, either for genes from Z-chromosome clusters with a 2-fold excess of male expression (Mann–Whitney U test, P = 0.190) or for genes from clusters with equal expression in male and females (P = 0.364) (supplementary fig. S5, Supplementary Material online). Similarly, absolute expression level in females of Z-linked genes from clusters with equal expression in male and females did not differ significantly from the level of autosomal expression in females (P = 0.346). In contrast, median absolute expression level in females of genes from clusters with a 2-fold excess of male expression was only 64% of median autosomal expression (P = 0.002). These observations are consistent with a reduction in female gene expression levels for those Z-linked genes that have ceased to recombine.
Nonratite birds are hemizygous for the majority of all Z-linked genes. The intermediate nature of sex bias in expression of these genes in chicken and other species (i.e., with a mean male-to-female ratio of ∼1.5) has been interpreted as indicating that although there is no chromosome-wide mechanism like sex chromosome inactivation, some compensation occurs on demands as per gene-by-gene basis (Mank and Ellegren 2009; Itoh et al. 2010). Alternatively, incomplete dosage compensation may potentially be the result of accumulation of male-beneficial genes on the avian Z-chromosome (Ellegren 2011) in which case male-biased expression reflects the resolution of past sexual antagonism (Naurin et al. 2010). Comparison of expression levels in ostrich and chicken orthologs sheds further light on these issues. Assuming that the expression levels of Z-linked genes that still recombine in ostrich represent the ancestral state, we find that expression level of the majority of these genes have evolved from a state of no sex bias to a state of male-biased expression following sex chromosome divergence in the chicken lineage (fig. 2A). This suggests that Z-chromosome genes by their nature are not generally male beneficial, at least not ancestrally. Moreover, for most Z-linked genes that we infer as not recombining in ostrich, the sex bias in gene expression is less pronounced in chicken than in ostrich (fig. 2B). This supports the notion that some compensation of sex-linked gene expression has evolved in the chicken lineage.
Fig. 2.

Comparison of the male:female (M:F) expression ratio in brain for orthologous Z-chromosome genes in ostrich and chicken. (A) Genes from clusters on the ostrich Z-chromosome with an equal male:female expression ratio, and (B) genes from clusters on the ostrich Z-chromosome with a 2-fold excess of male expression. Chicken data are without replicates, likely explaining the larger variation among genes for this species.
Similar to Julien et al. (2012) and Lin et al. (2012), we also compared expression of sex-linked ostrich genes with their orthologs in the human genome (levels of gene expression are generally conserved across divergent vertebrates [Brawand et al. 2011], which we confirm for the ostrich–human comparison: R2 = 0.31, P < 0.001). Avian sex chromosomes evolved from a different pair of autosomes than the mammalian X- and Y-chromosomes (Fridolfsson et al. 1998). Genes that are sex linked in birds are thus autosomal in mammals and vice versa. Human expression data from orthologs to avian sex-linked genes can therefore be taken as an indication of ancestral gene expression levels before avian sex chromosome divergence. We found that genes from clusters with a 2-fold excess of male expression in ostrich are not male biased in human (median male:female ratio, log2 = −0.028). Similarly, genes from clusters with equal expression level in the two sexes in ostrich are not male biased in human (log2 = −0.298). These observations corroborate conclusions from the ostrich–chicken comparison.
Our observations raise the interesting possibility that the lack of dosage compensation in ostriches is instrumental rather than coincidental to the arrested stage of sex chromosome evolution. We thus hypothesize that if there is no regulatory mechanism available to compensate the halved dose of sex-linked genes in the hemizygous sex, sex chromosome evolution will be heavily constrained. Under this scenario, mutations (e.g., structural rearrangements) that reduce recombination between partly differentiated sex chromosomes should be highly deleterious. However, a caveat behind this reasoning is that gene dose may not be directly affected by, for example, an inversion event. Perhaps, there are fitness effects immediately associated with rearrangements, for example, due to impaired cis-regulatory functions. However, some differentiation between the Z- and W-chromosomes have obviously occurred, and the limited sex chromosome divergence seen in ratites may in this sense represent what is tolerable in the absence of dosage compensation. Indeed, dosage compensation should only be expected to evolve in genes for which the maintenance of ancestral expression level is vital for proper interaction with other genes.
Because the mechanistic basis for the partial dosage compensation seen in other birds is not known, it is difficult to speculate about what could have hindered ratites for >100 My to develop compensatory mechanisms to deal with differences in sex-linked gene dose. Whatever is the cause, we note that the evolutionary resistance to dosage compensation in ratite birds is in stark contrast to rapid de novo evolution of dosage compensation seen in evolutionarily young sex chromosome systems (Muyle et al. 2012) and to the rapid adjustment of expression levels following neoX formation via autosome—X-chromosome fusions (Marin et al. 1996).
Materials and Methods
Materials and methods are presented as supplementary information, Supplementary Material online.
Supplementary Material
Supplementary information, table S1, and figures S1–S5 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Acknowledgments
The authors thank Linnéa Smeds for bioinformatics support, Henrik Kaessmann and Anamaria Necsulea for access to raw data, Gunnar Sahlin at Sahlins Struts, Borlänge for help with samples and sampling, and members of the Ellegren laboratory for helpful discussions. Sequencing was performed at the SNP&SEQ Technology Platform of Uppsala University. Computational work was performed at the Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) of Uppsala University, supported by the Swedish National Infrastructure for Computing (SNIC). This work was supported by a European Research Council Advanced Investigator Grant, a Knut and Alice Wallenberg Foundation Wallenberg Scholar Grant, and the Swedish Research Council to H.E., and the European Union Seventh Framework Programme (grant number 253511 to S.A.).
References
- Brawand D, Soumillon M, Necsulea A, et al. (18 co-authors) The evolution of gene expression levels in mammalian organs. Nature. 2011;478:343–348. doi: 10.1038/nature10532. [DOI] [PubMed] [Google Scholar]
- Deng XX, Hiatt JB, Nguyen DK, et al. (16 co-authors) Evidence for compensatory upregulation of expressed X-linked genes in mammals, Caenorhabditis elegans and Drosophila melanogaster. Nat Genet. 2011;43:1179–1128. doi: 10.1038/ng.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H. Emergence of male-biased genes on the chicken Z-chromosome: sex-chromosome contrasts between male and female heterogametic systems. Genome Res. 2011;21:2082–2086. doi: 10.1101/gr.119065.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Hultin-Rosenberg L, Brunstrom B, Dencker L, Kultima K, Scholz B. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Smeds L, Burri R, et al. (12 co-authors) The genomic landscape of species divergence in Ficedula flycatchers. Nature. 2012;491:756–760. doi: 10.1038/nature11584. [DOI] [PubMed] [Google Scholar]
- Fridolfsson AK, Cheng H, Copeland NG, Jenkins NA, Liu HC, Raudsepp T, Woodage T, Chowdhary B, Halverson J, Ellegren H. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc Natl Acad Sci U S A. 1998;95:8147–8152. doi: 10.1073/pnas.95.14.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To ERR (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- He X, Chen X, Xiong Y, Chen Z, Wang X, Shi S, Wang X, Zhang J. He et al. reply. Nat Genet. 2011;43:1171–1172. [Google Scholar]
- Itoh Y, Melamed E, Yang X, et al. (13 co-authors) Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Replogle K, Kim YH, Wade J, Clayton DF, Arnold AP. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 2010;20:512–518. doi: 10.1101/gr.102343.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien P, Brawand D, Soumillon M, Necsulea A, Liechti A, Schütz F, Daish T, Grützner F, Kaessmann H. Mechanisms and evolutionary patterns of mammalian and avian dosage compensation. PLoS Biol. 2012;10:e1001328. doi: 10.1371/journal.pbio.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko PV, Xi RB, Park PJ. Evidence for dosage compensation between the X chromosome and autosomes in mammals. Nat Genet. 2011;43:1167–1169. doi: 10.1038/ng.991. [DOI] [PubMed] [Google Scholar]
- Lin F, Xing K, Zhang J, He X. Expression reduction in mammalian X chromosome evolution refutes Ohno's hypothesis of dosage compensation. Proc Natl Acad Sci U S A. 2012;109:11752–11757. doi: 10.1073/pnas.1201816109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Halsall JA, Antczak P, O'Neill LP, Falciani F, Turner BM. Relative overexpression of X-linked genes in mouse embryonic stem cells is consistent with Ohno's hypothesis. Nat Genet. 2011;43:1169–1170. doi: 10.1038/ng.992. [DOI] [PubMed] [Google Scholar]
- Mank J, Hultin-Rosenberg L, Webster M, Ellegren H. The unique genomic properties of sex-biased genes: insights from avian microarray data. BMC Genomics. 2008;9:148. doi: 10.1186/1471-2164-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE, Ellegren H. All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity. 2009;102:312–320. doi: 10.1038/hdy.2008.116. [DOI] [PubMed] [Google Scholar]
- Marin I, Franke A, Bashaw GJ, Baker BS. The dosage compensation system of Drosophila is co-opted by newly evolved X chromosomes. Nature. 1996;383:160–163. doi: 10.1038/383160a0. [DOI] [PubMed] [Google Scholar]
- Muyle A, Zemp N, Deschamps C, Mousset S, Widmer A, Marais GAB. Rapid de novo evolution of X chromosome dosage compensation in Silene latifolia, a plant with young sex chromosomes. PLoS Biol. 2012;10:e1001308. doi: 10.1371/journal.pbio.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Ellegren H. The chicken (Gallus gallus) Z chromosome contains at least three nonlinear evolutionary strata. Genetics. 2008;180:1131–1136. doi: 10.1534/genetics.108.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naurin S, Hansson B, Bensch S, Hassequist D. Why does dosage compensation differ between XY and ZW taxa? Trends Genet. 2010;26:15–20. doi: 10.1016/j.tig.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Nishida-Umehara C, Tsuda Y, Ishijima J, Ando J, Fujiwara A, Matsuda Y, Griffin DK. The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chromosome Res. 2007;15:721–734. doi: 10.1007/s10577-007-1157-7. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Murata K, Mizuno S. The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. Proc Natl Acad Sci U S A. 1998;95:4415–4418. doi: 10.1073/pnas.95.8.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Sex chromosomes and sex linked genes. Berlin (Germany): Springer Verlag; 1967. [Google Scholar]
- Pessia E, Makino T, Bailly-Bechet M, McLysaght A, Marais GAB. Mammalian X chromosome inactivation evolved as a dosage-compensation mechanism for dosage-sensitive genes on the X chromosome. Proc Natl Acad Sci U S A. 2012;109:5346–5351. doi: 10.1073/pnas.1116763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigozzi MI, Solari AJ. Extreme axial equalization and wide distribution of recombination nodules in the primitive ZW pair of Rhea americana (Aves, Ratitae) Chromosome Res. 1997;5:421–428. doi: 10.1023/a:1018404610973. [DOI] [PubMed] [Google Scholar]
- Pigozzi MI, Solari AJ. The ZW pairs of two paleognath birds from two orders show transitional stages of sex chromosome differentiation. Chromosome Res. 1999;7:541–551. doi: 10.1023/a:1009241528994. [DOI] [PubMed] [Google Scholar]
- Shetty S, Griffin DK, Graves JAM. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999;7:289–295. doi: 10.1023/a:1009278914829. [DOI] [PubMed] [Google Scholar]
- Straub T, Becker PB. Dosage compensation: the beginning and end of generalization. Nat Rev Genet. 2007;8:47–57. doi: 10.1038/nrg2013. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Nishida-Umehara C, Ishijima J, Yamada K, Matsuda Y. Comparison of the Z and W sex chromosomal architectures in elegant crested tinamou (Eudromia elegans) and ostrich (Struthio camelus) and the process of sex chromosome differentiation in palaeognathous birds. Chromosoma. 2007;116:159–173. doi: 10.1007/s00412-006-0088-y. [DOI] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, (81 co-authors) The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JBW, Bryk J. General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC Genomics. 2011;12:91. doi: 10.1186/1471-2164-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


