SUMMARY
Small mammals face especially severe thermoregulatory challenges at high altitude because the reduced O2 availability constrains the capacity for aerobic thermogenesis. Adaptive enhancement of thermogenic performance under hypoxic conditions may be achieved via physiological adjustments that occur within the lifetime of individuals (phenotypic plasticity) and/or genetically based changes that occur across generations, but their relative contributions to performance differences between highland and lowland natives are unclear. Here, we examined potentially evolved differences in thermogenic performance between populations of deer mice (Peromyscus maniculatus) that are native to different altitudes. The purpose of the study was to assess the contribution of phenotypic plasticity to population differences in thermogenic performance under hypoxia. We used a common-garden deacclimation experiment to demonstrate that highland deer mice have enhanced thermogenic capacities under hypoxia, and that performance differences between highland and lowland mice persist when individuals are born and reared under common-garden conditions, suggesting that differences in thermogenic capacity have a genetic basis. Conversely, population differences in thermogenic endurance appear to be entirely attributable to physiological plasticity during adulthood. These combined results reveal distinct sources of phenotypic plasticity for different aspects of thermogenic performance, and suggest that thermogenic capacity and endurance may have different mechanistic underpinnings.
KEY WORDS: developmental plasticity, high-altitude adaptation, hypoxia, Peromyscus, thermoregulation
INTRODUCTION
For small homeothermic endotherms, the capacity for metabolic heat production is a primary determinant of survival during periods of prolonged cold stress (Conley and Porter, 1986; Hayes and O'Connor, 1999). Like many complex phenotypic traits, changes in thermogenic performance can be achieved via physiological adjustments that occur within the lifetime of individuals (phenotypic plasticity) and/or genetically based changes that occur across generations (genetic adaptation) (Garland and Carter, 1994; Rezende et al., 2001; Hammond et al., 2002; Storz et al., 2010b; Swanson, 2010). Quantitative genetic studies have demonstrated that thermogenic capacity and similar measures of whole-organism metabolic performance have a heritable basis (Swallow et al., 1998; Dohm et al., 2001; Nespolo et al., 2003; Fontanillas et al., 2005; Nespolo et al., 2005; Sadowska et al., 2005; Wone et al., 2009), indicating that evolutionary changes in such traits could be brought about by directional selection. However, many changes in whole-organism metabolic performance could also occur through reversible physiological adjustments. For example, temperate-zone birds and mammals often exhibit marked changes in thermogenic capacity and cold hardiness with the onset of winter (Merritt, 1995; Liknes and Swanson, 1996; Liknes et al., 2002; Swanson, 2007; Swanson, 2010; Oelkrug et al., 2012). Changes in thermogenic performance can also be induced by environmental conditions experienced during prenatal development (Chappell et al., 2007; Russell et al., 2008) and, in contrast to reversible acclimatization responses, the effects of developmental plasticity may persist throughout postnatal life (Dzialowski et al., 2002; Chappell et al., 2007; Russell et al., 2008). Thus, a central goal of research in evolutionary physiology is to assess the relative contributions of genotypic specialization and phenotypic plasticity in enabling species to cope with changing environmental conditions (Garland and Adolph, 1991; Garland and Carter, 1994; Kingsolver and Huey, 1998; Feder et al., 2000; Storz et al., 2010b). Studies of altitude-related variation in aerobic thermogenic performance are particularly well suited to this goal because ambient temperature and oxygen availability vary predictably as a function of altitude.
The deer mouse (Peromyscus maniculatus) has emerged as a particularly promising study organism for investigations of high-altitude adaptation. This is largely due to the fact that deer mice have the broadest altitudinal distribution of any North American mammal, occurring above 4300 m in mountain ranges of western North America to below sea level in Death Valley, California (Hock, 1964). Deer mice also do not hibernate (Jones et al., 1983) and, as a result, they are highly dependent on aerobic thermogenesis to maintain a constant body temperature during periods of prolonged cold stress (Chappell and Hammond, 2004). Because hypoxia can potentially constrain the metabolic scope for aerobic activity, deer mice face especially severe thermoregulatory challenges in cold, alpine and subalpine environments. Indeed, survivorship studies of free-ranging mice at high altitude have documented strong directional selection on thermogenic capacity (Hayes and O'Connor, 1999), and thermogenic performance is strongly correlated with above-ground activity and foraging behavior in the cold (Sears et al., 2006; Sears et al., 2009). Thus, in high-altitude deer mice, thermogenic performance under hypoxia is clearly an ecologically important trait that has a well-documented connection to Darwinian fitness (Hayes and O'Connor, 1999).
Most previous studies of environmental effects on aerobic performance and hypoxia tolerance in deer mice have focused on interindividual variation among mice collected from a single geographic locality (Chappell, 1985; Hammond et al., 2002; Chappell and Hammond, 2004; Chappell et al., 2007; Russell and Chappell, 2007; Russell et al., 2008; Van Sant and Hammond, 2008; Rezende et al., 2009). Given that deer mice have such a broad altitudinal distribution, it is also of interest to assess the contribution of phenotypic plasticity to population differences in physiological traits that have distinct local optima in different elevational zones. Thus, the purpose of the present study was to assess the contribution of phenotypic plasticity to altitude-related population differences in thermogenic performance under hypoxia.
We used a common-garden deacclimation experiment to characterize the effects of environmental variation during pre- and post-natal life on two aspects of whole-organism thermogenic performance under hypoxia: thermogenic capacity and thermogenic endurance. We show that deer mice that are native to high altitude have elevated thermogenic capacities under hypoxia compared with those that are native to low altitude, and that these differences persist in mice that are born and reared under common-garden conditions, suggesting that they have a genetic basis. Conversely, differences in thermogenic endurance between highland and lowland deer mice appear to be entirely attributable to environmental effects that act during adulthood. These results reveal distinct sources of phenotypic plasticity for different aspects of thermogenic performance, and suggest that thermogenic capacity and endurance may have different mechanistic underpinnings.
MATERIALS AND METHODS
Experimental animals and deacclimation treatments
Adult deer mice, P. maniculatus (Wagner 1845), were live trapped at one high-altitude locality in the Southern Rocky Mountains, the summit of Mount Evans, Clear Creek County, CO, USA (39°35′18″N, 105°38′38″W, 4350 m above sea level, PO2≈95.6 mmHg) and one low-altitude locality in the Great Plains, Nine Mile Prairie, Lancaster County, NE, USA (40°52′12″N, 96°48′20.3″W, 430 m above sea level, PO2≈152.0 mmHg). This pair of high- and low-altitude localities is separated by a linear distance of 770 km. Following capture, high- and low-altitude deer mice were either measured on-site within 1–2 days of capture (in situ treatment; highland mice, N=10; lowland mice, N=10) or they were transferred from collection localities to a common-garden lab environment at the animal research facility at the University of Nebraska (elevation 360 m, PO2≈153.3 mmHg). Mice that were transferred to the common-garden lab environment were assigned to one of two groups. Mice in the first group (highland mice, N=10; lowland mice, N=10) were housed for 6 weeks with a constant ambient temperature (25°C) and light:dark cycle (12 h:12 h). We measured the thermogenic performance of all mice at the conclusion of this 6 week deacclimation period. Mice assigned to the second group were used as the parental stock to produce F1 progeny that were born and reared in the common garden. Once these F1 progeny reached adulthood (75–90 days), we measured thermogenic capacity under hypoxia in 10 full-sibling progeny from a pair of highland parents and 10 full-sibling progeny from a pair of lowland parents. This experimental design allowed us to control for two distinct sources of phenotypic plasticity in thermogenic performance: physiological plasticity during adulthood (through the comparison of wild-caught high- and low-altitude mice that underwent the 6 week deacclimation under common-garden conditions) and developmental plasticity (through comparison of the F1 progeny of wild-caught high- and low-altitude mice that were born and reared under common-garden conditions). Differences in thermogenic performance that persisted in the F1 mice were assumed to represent genetically based differences in thermogenic performance between highland and lowland mice.
Respirometry
We used open-flow respirometry to measure thermogenic capacity as the maximum rate of oxygen consumption (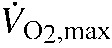 ) elicited by cold exposure. The measurements performed in Lincoln were made in a hypoxic heliox atmosphere (12.6% O2, 87.4% He), which simulates the atmospheric PO2 on the summit of Mount Evans. For the measurements made on the summit of Mount Evans, we used a normoxic heliox atmosphere (21% O2, 79% He). At both localities, heliox gas mixtures were equilibrated to local atmospheric pressure so that all of the experimental animals experienced an equivalent level of hypoxia during the thermogenic trials. The incurrent flow rate of heliox was approximately 450ml min−1 after correction (see below). All of the trials were conducted at ambient temperatures just below freezing (minimum −4°C). Rates of heat loss in heliox are several times greater than in ambient air, which makes it possible to elicit
) elicited by cold exposure. The measurements performed in Lincoln were made in a hypoxic heliox atmosphere (12.6% O2, 87.4% He), which simulates the atmospheric PO2 on the summit of Mount Evans. For the measurements made on the summit of Mount Evans, we used a normoxic heliox atmosphere (21% O2, 79% He). At both localities, heliox gas mixtures were equilibrated to local atmospheric pressure so that all of the experimental animals experienced an equivalent level of hypoxia during the thermogenic trials. The incurrent flow rate of heliox was approximately 450ml min−1 after correction (see below). All of the trials were conducted at ambient temperatures just below freezing (minimum −4°C). Rates of heat loss in heliox are several times greater than in ambient air, which makes it possible to elicit 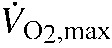 without risking cold injury to experimental animals (Rosenmann and Morrison, 1974). Similar protocols have been used to elicit
without risking cold injury to experimental animals (Rosenmann and Morrison, 1974). Similar protocols have been used to elicit 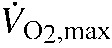 in P. maniculatus in previous studies (Chappell and Hammond, 2004; Rezende et al., 2004; Cheviron et al., 2012). Heliox mixtures were obtained from a commercial supplier (Linweld, Lincoln, NE, USA).
in P. maniculatus in previous studies (Chappell and Hammond, 2004; Rezende et al., 2004; Cheviron et al., 2012). Heliox mixtures were obtained from a commercial supplier (Linweld, Lincoln, NE, USA).
The respirometry setup for the thermogenic trials was identical to that described previously (Cheviron et al., 2012). Briefly, heliox gas mixtures were first equilibrated to atmospheric pressure, and were then pumped into copper coils inside a temperature-control chamber using mass flow controllers (Sable Systems Inc., Las Vegas, NV, USA). The cooled heliox was then pumped into the animal chamber and a baseline (empty) chamber at a rate of approximately 450 ml min−1. The animal and baseline chambers were constructed of thin, airtight polypropylene with an internal volume of 180 ml, which minimized locomotion but still permitted postures normally adopted for shivering. We verified that the temperature inside the temperature-control chamber was identical to that experienced by a mouse inside the animal chamber by using a thermocouple to measure excurrent air at the junction of the excurrent tube and the animal chamber. Excurrent air from the animal and baseline chambers was sampled at a rate of approximately 130 ml min−1; the air was dried with magnesium perchlorate, passed through a CO2 analyzer [CO2 data are reported elsewhere (Cheviron et al., 2012)], scrubbed of CO2 with ascarite, redried with drierite, and passed through an oxygen analyzer (FoxBox, Sable Systems Inc.). We monitored excurrent O2 continuously, and each experimental animal was removed when values showed clear indications of dropping to baseline. We used a rectal thermometer to measure the body temperature of each mouse following the thermogenic trials, and we confirmed that all experimental mice were hypothermic when removed from the chamber. The O2 analyzer was spanned daily with ambient air.
We calculated 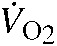 using equation 10.1 in Lighton (Lighton, 2008). Gas conversion factors for two-gas mixtures (helium and oxygen) were calculated, and applied to the flow data prior to
using equation 10.1 in Lighton (Lighton, 2008). Gas conversion factors for two-gas mixtures (helium and oxygen) were calculated, and applied to the flow data prior to 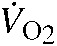 calculations. We measured thermogenic capacity (
calculations. We measured thermogenic capacity (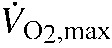 ) as the maximum
) as the maximum 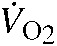 averaged over a continuous 5 min period, and we measured thermogenic endurance as the length of time (min) that individuals maintained >90% of
averaged over a continuous 5 min period, and we measured thermogenic endurance as the length of time (min) that individuals maintained >90% of 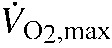 during the thermogenic trials. All experimental protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee (IACUC no. 522).
during the thermogenic trials. All experimental protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee (IACUC no. 522).
Statistics
We tested for differences in body mass between populations and across the deacclimation treatments using a two-way ANOVA design. Body mass varied significantly among the six experimental groups, and was significantly correlated with 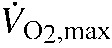 (see Results). To control for the effects of body mass on thermogenic capacity (
(see Results). To control for the effects of body mass on thermogenic capacity (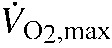 ), we tested for mean differences in thermogenic capacity between samples of highland and lowland deer mice across deacclimation treatments using a two-way ANCOVA design with body mass as a covariate (Packard and Boardman, 1998). In contrast to thermogenic capacity, thermogenic endurance was not correlated with body mass and, as result, we used a two-way ANOVA design to test for differences in thermogenic endurance. Upon detection of significant ANCOVA and ANOVA main effects, we performed post hoc Tukey HSD tests to identify significant pairwise differences between populations and treatments. Finally, we used linear regression to assess the relationship between thermogenic capacity and endurance in each of the experimental groups (in situ, 6 week deacclimation, F1 progeny of deacclimated mice). Consistent with previous studies of thermogenic performance in deer mice (Chappell et al., 2007), sex had no effect on either thermogenic capacity or endurance (data not shown). Thus, the sexes were combined in all analyses. All statistical analyses were performed in either R or JMP 501.
), we tested for mean differences in thermogenic capacity between samples of highland and lowland deer mice across deacclimation treatments using a two-way ANCOVA design with body mass as a covariate (Packard and Boardman, 1998). In contrast to thermogenic capacity, thermogenic endurance was not correlated with body mass and, as result, we used a two-way ANOVA design to test for differences in thermogenic endurance. Upon detection of significant ANCOVA and ANOVA main effects, we performed post hoc Tukey HSD tests to identify significant pairwise differences between populations and treatments. Finally, we used linear regression to assess the relationship between thermogenic capacity and endurance in each of the experimental groups (in situ, 6 week deacclimation, F1 progeny of deacclimated mice). Consistent with previous studies of thermogenic performance in deer mice (Chappell et al., 2007), sex had no effect on either thermogenic capacity or endurance (data not shown). Thus, the sexes were combined in all analyses. All statistical analyses were performed in either R or JMP 501.
RESULTS
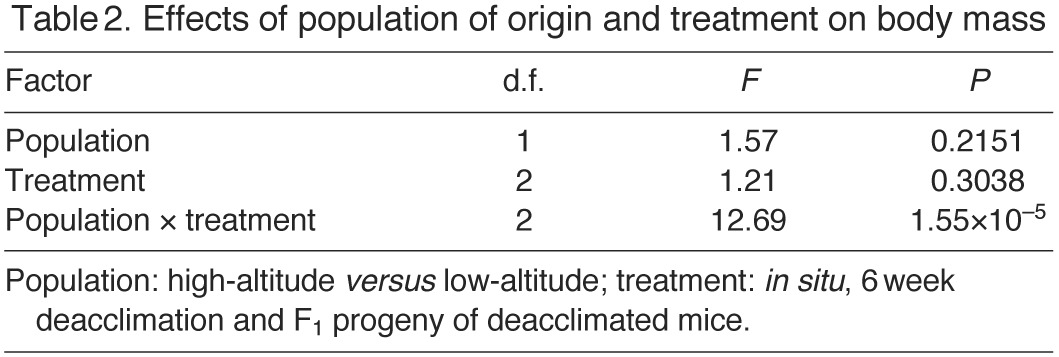
There was significant variation in body mass among experimental groups (Tables 1, 2). The highland mice were significantly larger than the lowland mice in the in situ group, and this difference persisted in the 6 week deacclimation group, although it was not statistically significant (Table 1). Interestingly, this relationship was reversed in the F1 mice, with the lowland mice being significantly larger than highlanders, which resulted in a significant population × treatment interaction (Table 2). This reversal likely reflects sampling error; it is not attributable to biased sex ratios in the population samples, as we measured equal numbers of F1 males (N=5/group) and females (N=5/group). Variation in body mass was also significantly correlated with thermogenic capacity (uncorrected 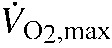 values)
(r2=0.081, P=0.028), but not thermogenic endurance (r2=0.006, P=0.57). Thus, we statistically controlled for body mass by using an ANCOVA design in the analysis of thermogenic capacity. We did not correct for body mass in the analysis of thermogenic endurance.
values)
(r2=0.081, P=0.028), but not thermogenic endurance (r2=0.006, P=0.57). Thus, we statistically controlled for body mass by using an ANCOVA design in the analysis of thermogenic capacity. We did not correct for body mass in the analysis of thermogenic endurance.
Table 1.
Altitudinal variation in body mass of deer mice across three treatments
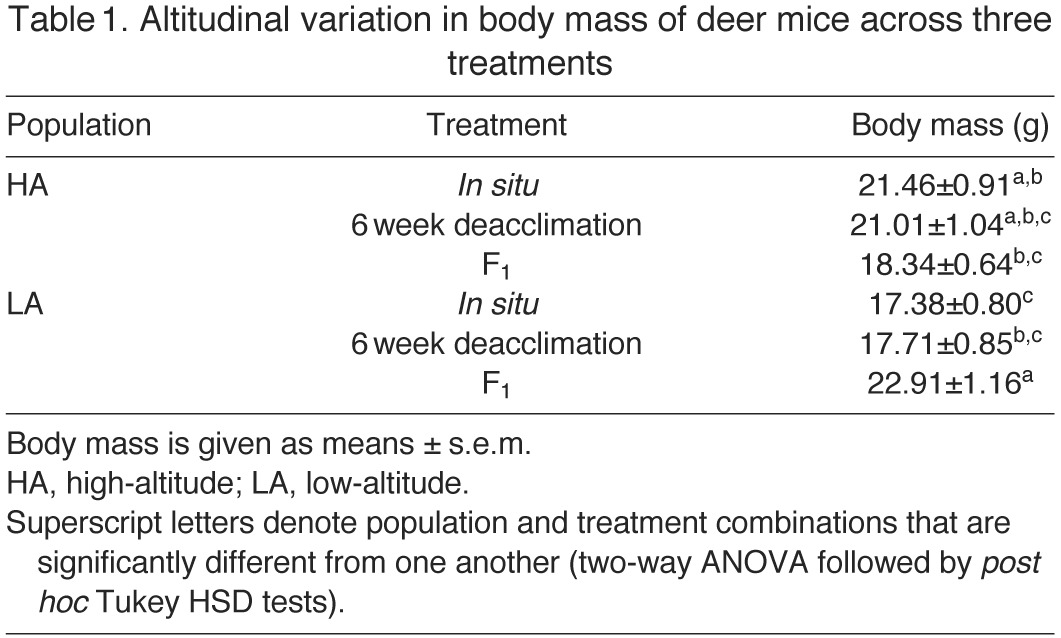
Table 2.
Effects of population of origin and treatment on body mass
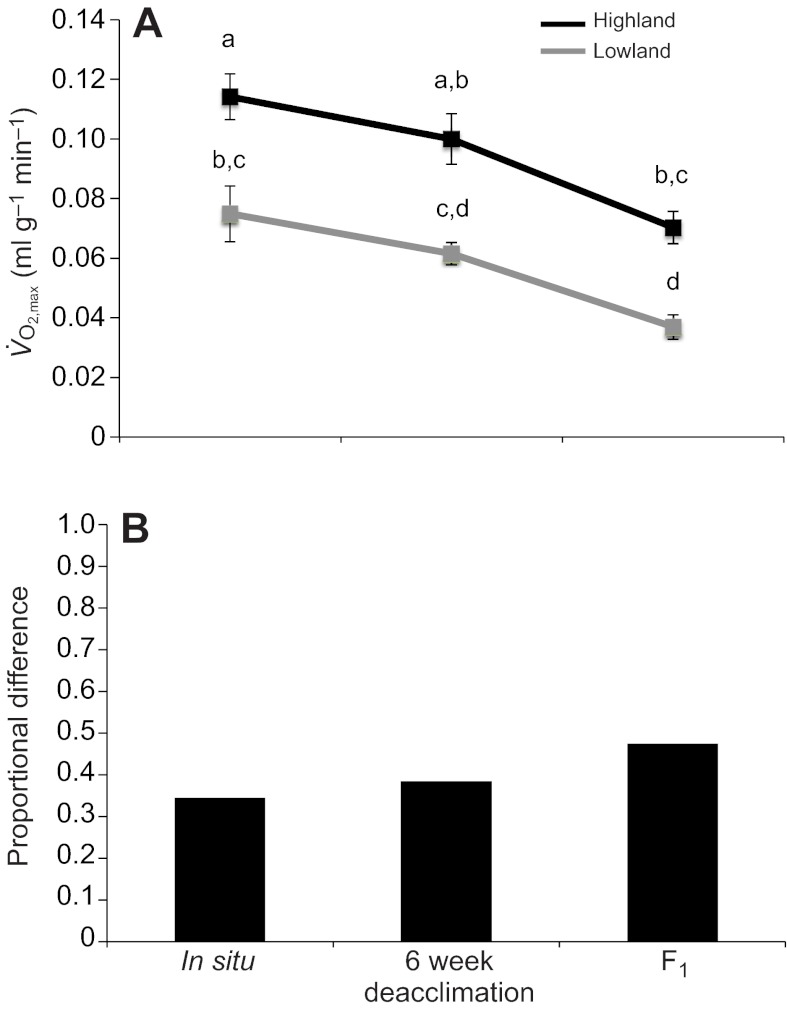
There were significant differences in thermogenic capacity between high- and low-altitude mice (ANCOVA F1,59=60.17, P=2.74×10−10; Table 3) and among treatment groups (ANCOVA F2,58=21.46, P=1.49×10−10; Table 3). Within each experimental group (in situ, 6 week deacclimation and F1), highland deer mice had significantly higher thermogenic capacities than their lowland counterparts (Fig. 1A), but the proportional difference in thermogenic capacity between highland and lowland mice increased across the deacclimation treatments (Fig. 1B). Specifically, the proportional difference in thermogenic capacity was greatest in the high- versus low-altitude comparison between the lab-reared F1 progeny of wild-caught mice, and smallest in comparisons between highland and lowland mice that were tested at the site of capture.
Table 3.
Effects of body mass, population of origin and treatment on thermogenic capacity
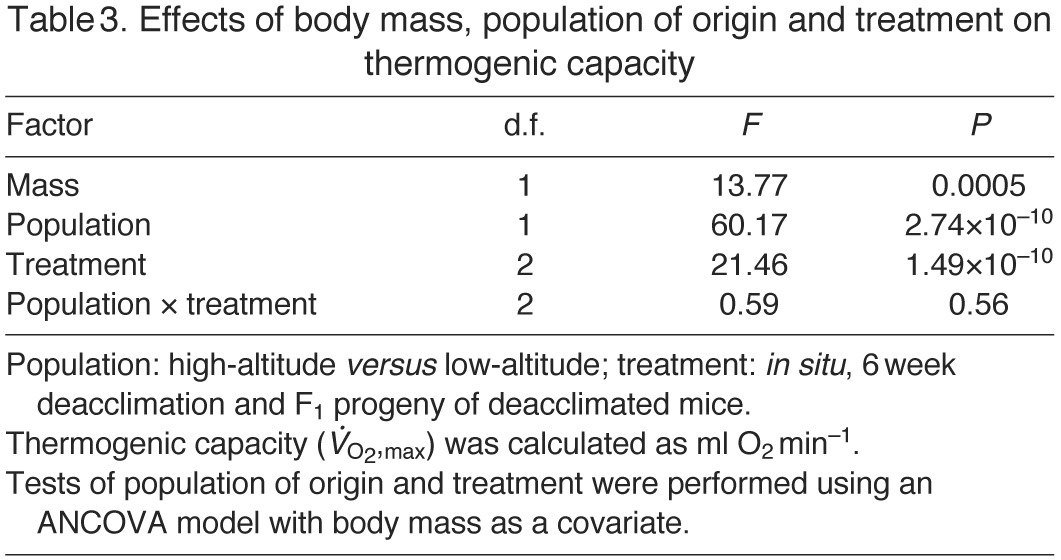
Fig. 1.
(A) Mass-specific maximum rates of oxygen consumption (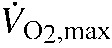 ) under hypoxic cold stress for highland and lowland deer mice (Peromyscus maniculatus) across the three treatments (in situ, 6 week deacclimation and F1 progeny of deacclimated mice). Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference
) under hypoxic cold stress for highland and lowland deer mice (Peromyscus maniculatus) across the three treatments (in situ, 6 week deacclimation and F1 progeny of deacclimated mice). Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference 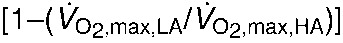 in
in 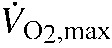 between highland (HA) and lowland (LA) deer mice across treatments.
between highland (HA) and lowland (LA) deer mice across treatments.
However, within population samples, thermogenic capacity decreased across the three treatments, demonstrating that thermogenic capacity is highly plastic. The interaction between population of origin and treatment was not significant (F2,58=0.59, P=0.56) (Table 3), indicating that the decline in thermogenic capacity across the three treatments was qualitatively similar for highland and lowland mice (Fig. 1B).
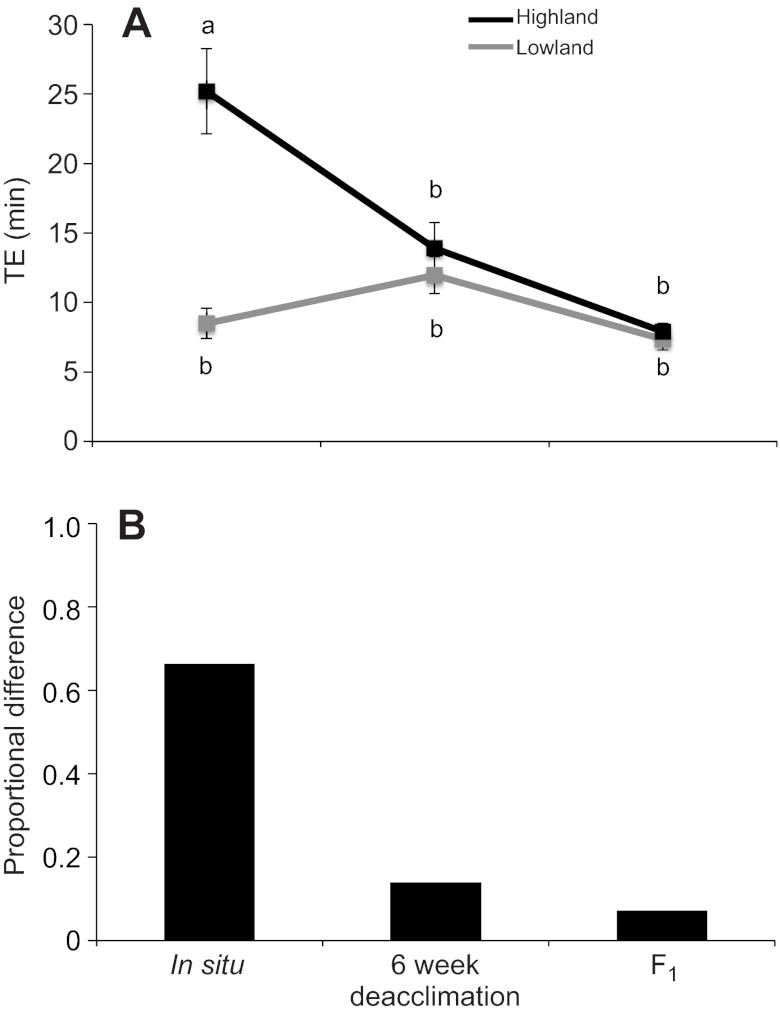
We found a fundamentally different pattern for thermogenic endurance. Although both population of origin (ANOVA F1,59=22.03, P=1.87×10−5) and acclimation treatment (ANOVA F1,59=15.4, P=5.01×10−6) also had significant effects on endurance (Table 4), this result was largely driven by differences between the in situ groups. After the 6 week deacclimation period, the difference in thermogenic endurance between the highland and lowland deer mice essentially disappeared (Fig. 2). Thus, in contrast to thermogenic capacity, the difference in thermogenic endurance between the in situ highland and lowland mice appears to be entirely attributable to physiological plasticity during adulthood.
Table 4.
Effects of population of origin and treatment on thermogenic endurance
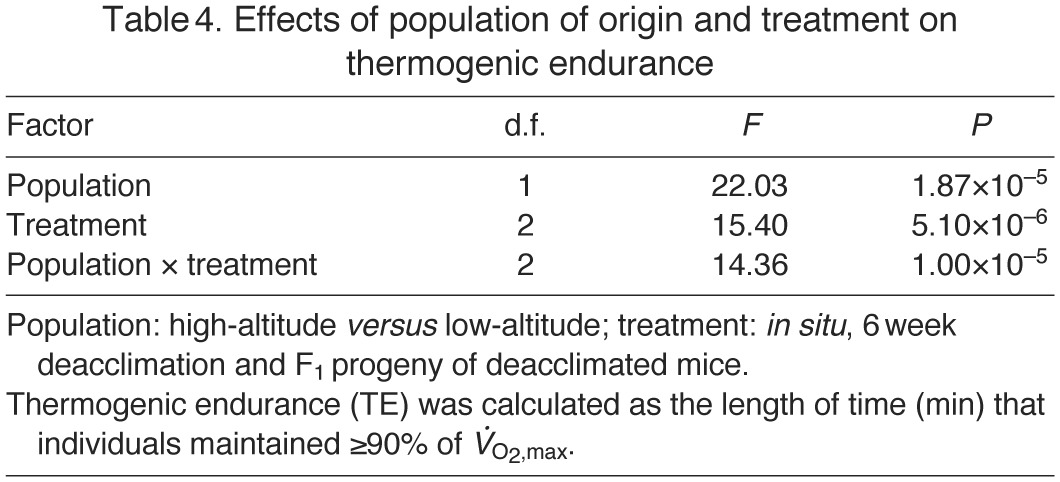
Fig. 2.
(A) Thermogenic endurance (TE, the length of time that individuals maintained >90% of 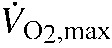 ) under hypoxic cold stress for highland and lowland deer mice across the three treatments. Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference [1–(TE,LA/TE,HA)] in thermogenic endurance between highland and lowland deer mice across treatments.
) under hypoxic cold stress for highland and lowland deer mice across the three treatments. Different letters denote population and treatment combinations that are significantly different from one another. Data are presented as means ± 1 s.e.m. (B) The proportional difference [1–(TE,LA/TE,HA)] in thermogenic endurance between highland and lowland deer mice across treatments.
In contrast to the deacclimation changes in thermogenic capacity, the highland and lowland mice exhibited distinct patterns of change in thermogenic endurance across the three experimental treatments, as indicated by a significant population × treatment interaction (F2,58=14.36, P=1×10−5) (Table 4). In the highland mice, thermogenic endurance decreased steadily across treatments (Fig. 2A). By contrast, endurance in the lowland 6 week deacclimation group was slightly higher than that in both the lowland in situ and lowland F1 groups, although this difference was not significant.
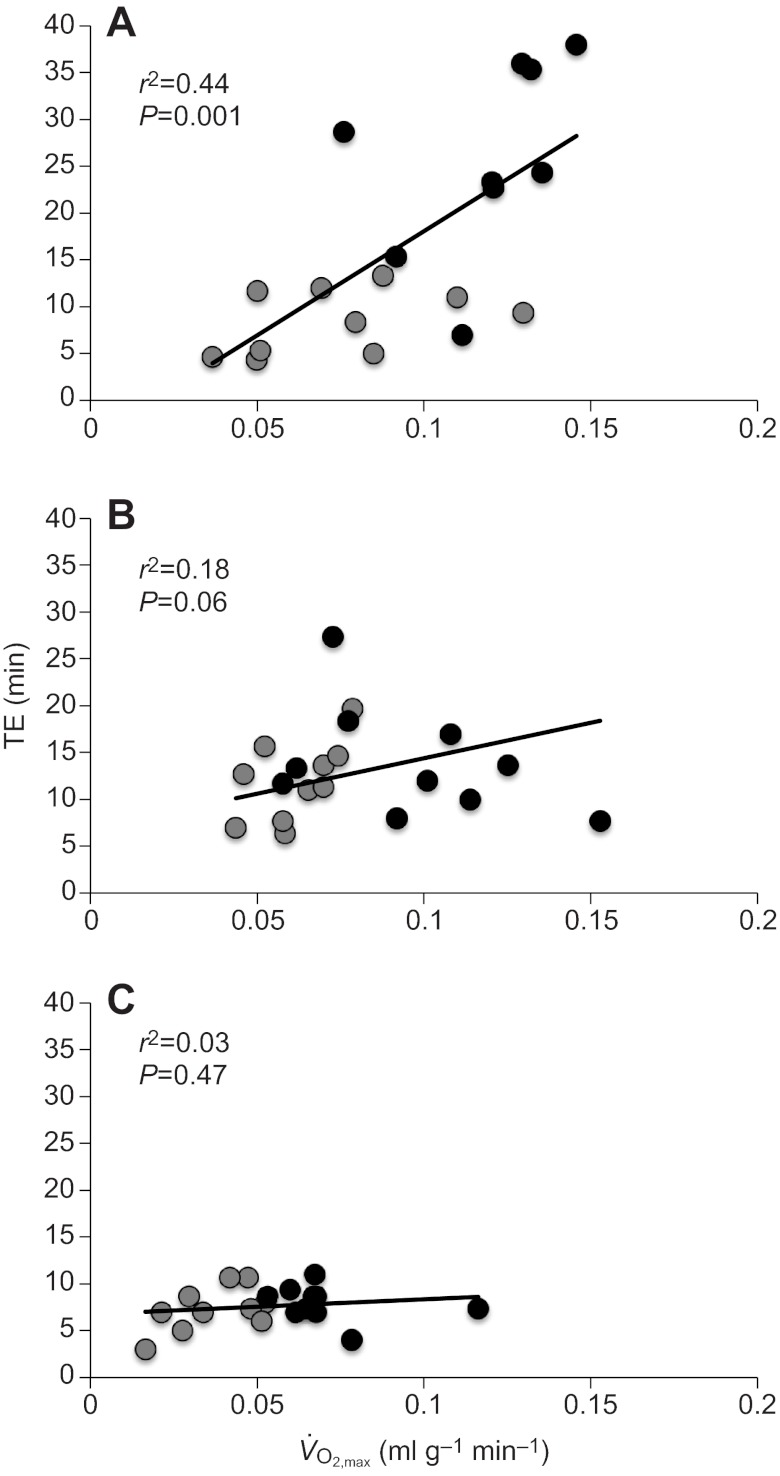
For the in situ mice, thermogenic capacity was strongly and positively correlated with thermogenic endurance (r2=0.44, P=0.001) (Fig. 3A). Although this correlation approached significance in the 6 week deacclimation group (r2=0.17, P=0.063) (Fig. 3B), there was no relationship between thermogenic capacity and endurance for the F1 mice (r2=0.03; P=0.467) (Fig. 3C). This pattern mainly reflects the strong influence of environmental effects on thermogenic endurance, and it demonstrates that there is no ineluctable functional linkage between thermogenic capacity and endurance.
Fig. 3.
Correlations between thermogenic endurance (TE) and 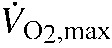 across the three treatments: (A) in situ, (B) 6 week deacclimation, (C) F1. Black and gray symbols represent highland and lowland deer mice, respectively.
across the three treatments: (A) in situ, (B) 6 week deacclimation, (C) F1. Black and gray symbols represent highland and lowland deer mice, respectively.
DISCUSSION
Thermogenic capacity and endurance are constrained by the reduced PO2 at high altitude (Hayes, 1989; Ward et al., 1995; Chappell and Hammond, 2004), and this hypoxic challenge can be offset through a combination of genetic adaptation, physiological plasticity and/or developmental plasticity. Our experiments were designed to assess the contribution of phenotypic plasticity to population differences in thermogenic performance under hypoxia, and to distinguish between physiological adjustments that occur during adulthood and prenatal development.
The results from our experiments on the in situ groups revealed that highland deer mice have greater overall thermogenic capacity and endurance under hypoxia relative to lowland deer mice (in situ groups; Figs 1, 2). The common-garden deacclimation experiment revealed that although a fraction of the in situ performance differences in both thermogenic capacity and endurance could be attributed to phenotypic plasticity, the relative contributions and sources of plasticity were different for each of the two traits. Thermogenic capacity decreased across the three treatments, but trait values for the highland mice were significantly higher than those of the lowland mice in each treatment (Fig. 1A). These results suggest that differences in thermogenic capacity have a genetic basis, but the upper limits of capacity are affected by environmental influences during both pre- and post-natal life.
In contrast to thermogenic capacity, the in situ differences in thermogenic endurance were no longer evident after the 6 week deacclimation period, suggesting that the elevated thermogenic endurance of highland deer mice is largely attributable to acclimatization to hypoxic cold stress during adulthood. Although aerobic capacity and endurance tend to be positively correlated in animals (Bennett, 1991), the mechanistic link between these two performance measures is unclear. Like total aerobic capacity, thermogenic capacity appears to be generally correlated with measures of thermogenic endurance in birds (Swanson, 2001; Swanson and Liknes, 2006; Swanson and Garland, 2009). The relationship between thermogenic capacity and endurance is less well studied in mammals, but there is some evidence for a positive correlation between maximal aerobic capacity and exercise endurance in rodents (Bennett, 1991; Rezende et al., 2006). Our results suggest that under hypoxic conditions, this correlation is at least partially dependent on acclimation history (Fig. 3).
Given the observed differences in the plasticity of thermogenic capacity and thermogenic endurance under hypoxia, our results also suggest that these performance measures may have different mechanistic underpinnings. A number of factors are known to contribute to thermogenic capacity under hypoxia in deer mice. These include the relative sizes of thermogenic and respiratory organs (Rezende et al., 2009), differences in ventilatory traits (Rezende et al., 2004), blood–O2 transport (Chappell and Snyder, 1984; Chappell et al., 1988; Hammond et al., 2001; Hammond et al., 2002), and the capacity for lipid oxidation (Cheviron et al., 2012). Although each of these traits displays a degree of phenotypic plasticity, blood–O2 transport is associated with genetic differences in hemoglobin–O2 affinity (Snyder et al., 1982; Chappell and Snyder, 1984; Snyder, 1985; Chappell et al., 1988; Storz et al., 2007; Storz et al., 2009; Storz et al., 2010a), and population differences in the capacity for metabolizing lipid fuels between highland and lowland deer mice persist for at least 6 weeks of low-altitude deacclimation (Cheviron et al., 2012). Thus, genetically based differences in hemoglobin function and metabolic capacities may contribute to the consistently elevated thermogenic capacities of high-altitude deer mice.
The mechanistic underpinnings of thermogenic endurance have received less empirical attention in deer mice, but studies from other rodents suggest that aerobic endurance is influenced by a number of factors including muscle fiber composition and respiratory capacity (Hoppeler et al., 1973; Hoppeler and Lindstedt, 1985; Kraemer et al., 1995), mitochondrial density and function (Holloszy and Coyle, 1984; Hoppeler and Lindstedt, 1985; Chow et al., 2007), decreased rates of glucose and glycogen utilization (Holloszy and Coyle, 1984) and increased rates of lipid oxidation (Holloszy and Coyle, 1984; Bjorntorp, 1991; McClelland et al., 1994; Henriksson and Hickner, 1996; Bangsbo et al., 2006; Weber, 2011). All of these traits are highly plastic. Although these muscular phenotypes may enhance shivering endurance, rodents also rely heavily on non-shivering thermogenesis to regulate body temperature. Brown adipose tissue (BAT) is the primary site of non-shivering thermogenesis, and the mass of BAT depots in rodents is known to decrease dramatically with warm acclimation and seasonal acclimatization (Didow and Hayward, 1969; Himms-Hagen, 1985; Rafael et al., 1985; Klaus et al., 1988; Cannon and Nedergaard, 2004). Regression of BAT depots over the course of the 6 week deacclimation period and the concomitant decrease in non-shivering thermogenic capacities could lead to an increased reliance on shivering thermogenesis in the warm-acclimated mice. An increased reliance on shivering thermogenesis would not only reduce total thermogenic capacity but also could compound the effects of muscular changes associated with inactivity that decrease thermogenic endurance, and could therefore help explain the rapid reduction in thermogenic endurance in high-altitude deer mice.
Finally, variation in body mass may also have important consequences for thermoregulatory performance. In principle, larger mice should have a thermoregulatory advantage because of their decreased surface area to volume ratio and the concomitant reduction in rates of heat loss (Bartholomew, 1968; Conley and Porter, 1986). However, variation in body mass is not sufficient to explain all the observed performance differences between high- and low-altitude deer mice. First, although body mass was significantly and positively associated with thermogenic capacity, there was no relationship between body mass and endurance. Second, although body mass did not vary systematically across the treatment groups (Table 1), the highland mice always had higher thermogenic capacities (Fig. 1). Finally, we statistically controlled for the correlation between body mass and thermogenic capacity in all comparisons between highland and lowland mice. Taken together, these results suggest that population differences in body mass do not fully account for the observed performance differences between high- and low-altitude deer mice.
Interestingly, our estimates of hypoxic thermogenic capacity were considerably lower than those reported in highland deer mice from the White Mountains of eastern California (Chappell et al., 2007). For wild-caught mice sampled and measured at 3800 m (analogous to our in situ treatment), Chappell and colleagues reported an uncorrected mean 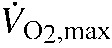 of 5.87 ml min−1 (Chappell et al., 2007), which is nearly twice as high as our mean
of 5.87 ml min−1 (Chappell et al., 2007), which is nearly twice as high as our mean 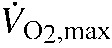 for the in situ highland mice measured at 4350 m (supplementary material Table S1). These apparent differences do not seem to stem from methodological differences as our studies employed highly similar protocols. It is possible that the apparent differences in thermogenic capacity could stem from differences in the test altitude (3800 versus 4350 m), and the differences could also reflect geographic variation in thermogenic performance among P. maniculatus populations. Highland deer mice from the White Mountains (subspecies P. m. sonoriensis) are genetically distinct from the highland Colorado mice (subspecies P. m. rufinus) and the lowland Nebraska mice (subspecies P. m. nebracensis) (Gering et al., 2009). Given that these subspecies/phylogroups vary with respect to body size and many other multifactorial traits (Osgood, 1909), it would not be surprising if they also varied in measures of whole-animal physiological performance such as thermogenic capacity. Detailed study of the underlying mechanisms of this geographic variation in thermogenic performance is likely to be a fruitful area of future research.
for the in situ highland mice measured at 4350 m (supplementary material Table S1). These apparent differences do not seem to stem from methodological differences as our studies employed highly similar protocols. It is possible that the apparent differences in thermogenic capacity could stem from differences in the test altitude (3800 versus 4350 m), and the differences could also reflect geographic variation in thermogenic performance among P. maniculatus populations. Highland deer mice from the White Mountains (subspecies P. m. sonoriensis) are genetically distinct from the highland Colorado mice (subspecies P. m. rufinus) and the lowland Nebraska mice (subspecies P. m. nebracensis) (Gering et al., 2009). Given that these subspecies/phylogroups vary with respect to body size and many other multifactorial traits (Osgood, 1909), it would not be surprising if they also varied in measures of whole-animal physiological performance such as thermogenic capacity. Detailed study of the underlying mechanisms of this geographic variation in thermogenic performance is likely to be a fruitful area of future research.
Recent years have witnessed a surge of interest in studies that explore the mechanistic underpinnings of adaptive trait variation in natural populations (Dalziel et al., 2009; Storz and Wheat, 2010). Much of this work has focused on genetically fixed traits, while mechanisms of physiological and developmental plasticity have received less attention (but see Whitehead et al., 2011). Our experimental results revealed the contributions of phenotypic plasticity to observed population differences in thermogenic capacity under hypoxia, a measure of whole-organism physiological performance that has a well-documented connection to Darwinian fitness (Hayes and O'Connor, 1999). After controlling for both physiological and developmental sources of plasticity, the highland and lowland deer mice exhibited persistent differences in thermogenic capacity, suggesting that genetically based physiological differentiation between populations may reflect local adaptation to different elevational zones.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Carling, J. Projecto-Garcia, I. Revsbech, A. Runck and D. Tufts for assistance with fieldwork, and D. Eddy, H. Hoppeler, G. McClelland, G. Scott and two anonymous reviewers for helpful comments on an earlier version of the manuscript.
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/216/7/1160/DC1
FUNDING
This work was supported by grants to J.F.S. from the National Institutes of Health/National Heart Lung and Blood Institute [grant nos R01 HL087216 and HL087216-S1] and the National Science Foundation [grant no. IOS-0949931]. Deposited in PMC for release after 12 months.
REFERENCES
- Bangsbo J., Mohr M., Poulsen A., Perez-Gomez J., Krustrup P. (2006). Training and testing the elite athlete. J. Exerc. Sci. Fit. 4, 1-14 [Google Scholar]
- Bartholomew G. A. (1968). Body temperature and energy metabolism. In Animal Function Principles and Adaptions (ed. Gordon M. S.). New York, NY: MacMillan; [Google Scholar]
- Bennett A. F. (1991). The evolution of activity capacity. J. Exp. Biol. 160, 1-23 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. (1991). Importance of fat as a support nutrient for energy: metabolism of athletes. J. Sports Sci. 9 Suppl., 71-76 [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277-359 [DOI] [PubMed] [Google Scholar]
- Chappell M. A. (1985). Effects of ambient temperature and altitude on ventilation and gas exchange in deer mice (Peromyscus maniculatus). J. Comp. Physiol. B 155, 751-758 [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Hammond K. A. (2004). Maximal aerobic performance of deer mice in combined cold and exercise challenges. J. Comp. Physiol. B 174, 41-48 [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Snyder L. R. G. (1984). Biochemical and physiological correlates of deer mouse alpha-chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. USA 81, 5484-5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A., Hayes J. P., Snyder L. R. G. (1988). Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus), physiology of beta-globin variants and alpha-globin recombinants. Evolution 42, 681-688 [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Hammond K. A., Cardullo R. A., Russell G. A., Rezende E. L., Miller C. (2007). Deer mouse aerobic performance across altitudes: effects of developmental history and temperature acclimation. Physiol. Biochem. Zool. 80, 652-662 [DOI] [PubMed] [Google Scholar]
- Cheviron Z. A., Bachman G. C., Connaty A. D., McClelland G. B., Storz J. F. (2012). Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc. Natl. Acad. Sci. USA 109, 8635-8640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. S., Greenlund L. J., Asmann Y. W., Short K. R., McCrady S. K., Levine J. A., Nair K. S. (2007). Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J. Appl. Physiol. 102, 1078-1089 [DOI] [PubMed] [Google Scholar]
- Conley K. E., Porter W. P. (1986). Heat loss from deer mice (Peromyscus): evaluation of seasonal limits to thermoregulation. J. Exp. Biol. 126, 249-269 [DOI] [PubMed] [Google Scholar]
- Dalziel A. C., Rogers S. M., Schulte P. M. (2009). Linking genotypes to phenotypes and fitness: how mechanistic biology can inform molecular ecology. Mol. Ecol. 18, 4997-5017 [DOI] [PubMed] [Google Scholar]
- Didow L., Hayward J. (1969). Seasonal variations in the mass and composition of brown adipose tissue in the meadow vole, Microtus pennsylvanicus. Can. J. Zool. 47, 547-555 [Google Scholar]
- Dohm M. R., Hayes J. P., Garland T., Jr (2001). The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159, 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzialowski E. M., von Plettenberg D., Elmonoufy N. A., Burggren W. W. (2002). Chronic hypoxia alters the physiological and morphological trajectories of developing chick embryos. Comp. Biochem. Physiol. 131A, 713-724 [DOI] [PubMed] [Google Scholar]
- Feder M. E., Bennett A. F., Huey R. B. (2000). Evolutionary physiology. Annu. Rev. Ecol. Syst. 31, 315-341 [Google Scholar]
- Fontanillas P., Dépraz A., Giorgi M. S., Perrin N. (2005). Nonshivering thermogenesis capacity associated to mitochondrial DNA haplotypes and gender in the greater white-toothed shrew, Crocidura russula. Mol. Ecol. 14, 661-670 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Adolph S. C. (1991). Physiological differentiation of vertebrate populations. Annu. Rev. Ecol. Syst. 22, 193-229 [Google Scholar]
- Garland T., Jr, Carter P. A. (1994). Evolutionary physiology. Annu. Rev. Physiol. 56, 579-621 [DOI] [PubMed] [Google Scholar]
- Gering E. J., Opazo J. C., Storz J. F. (2009). Molecular evolution of cytochrome b in high- and low-altitude deer mice (genus Peromyscus). Heredity 102, 226-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond K. A., Szewczak J., Król E. (2001). Effects of altitude and temperature on organ phenotypic plasticity along an altitudinal gradient. J. Exp. Biol. 204, 1991-2000 [DOI] [PubMed] [Google Scholar]
- Hammond K. A., Chappell M. A., Kristan D. M. (2002). Developmental plasticity in aerobic performance in deer mice (Peromyscus maniculatus). Comp. Biochem. Physiol. 133A, 213-224 [DOI] [PubMed] [Google Scholar]
- Hayes J. P. (1989). Altitudinal and seasonal effects on aerobic metabolism of deer mice. J. Comp. Physiol. B 159, 453-459 [DOI] [PubMed] [Google Scholar]
- Hayes J. P., O'Connor C. S. (1999). Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53, 1280-1287 [DOI] [PubMed] [Google Scholar]
- Henriksson J., Hickner R. (1996). Skeletal muscle adaptation to endurance training. In Intermittent High Intensity Exercise (ed. MacLeod D., Maughan R., Williams C., Madeley C., Sharp J., Nutton R.). London: E. & F. N. Spon; [Google Scholar]
- Himms-Hagen J. (1985). Brown adipose tissue metabolism and thermogenesis. Annu. Rev. Nutr. 5, 69-94 [DOI] [PubMed] [Google Scholar]
- Hock R. (1964). Physiological responses of deer mice to various native altitudes. In The Physiological Effects of High Altitude (ed. Weihe W. H.). New York, NY: Macmillan; [Google Scholar]
- Holloszy J. O., Coyle E. F. (1984). Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 56, 831-838 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Lindstedt S. L. (1985). Malleability of skeletal muscle in overcoming limitations: structural elements. J. Exp. Biol. 115, 355-364 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Luth H., Claassen H., Weibel E. R., Howald H. (1973). The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflügers Archiv. 344, 217-232 [DOI] [PubMed] [Google Scholar]
- Jones J. K., Jr, Armstrong D. M., Hoffmann R. S., Jones C. (1983). Mammals of the Northern Great Plains. Lincoln, NE: University of Nebraska Press; [Google Scholar]
- Kingsolver J. G., Huey R. B. (1998). Selection and evolution of morphological and physiological plasticity in thermally varying environments. Am. Zool. 38, 545-560 [Google Scholar]
- Klaus S., Heldmaier G., Ricquier D. (1988). Seasonal acclimation of bank voles and wood mice: nonshivering thermogenesis and thermogenic properties of brown adipose tissue mitochondria. J. Comp. Physiol. B 158, 157-164 [DOI] [PubMed] [Google Scholar]
- Kraemer W. J., Patton J. F., Gordon S. E., Harman E. A., Deschenes M. R., Reynolds K., Newton R. U., Triplett N. T., Dziados J. E. (1995). Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J. Appl. Physiol. 78, 976-989 [DOI] [PubMed] [Google Scholar]
- Lighton J. R. B. (2008). Measuring Metabolic Rates: A Manual for Scientists. New York, NY: Oxford University Press; [Google Scholar]
- Liknes E. T., Swanson D. L. (1996). Seasonal variation in cold tolerance, basal metabolic rate, and maximal capacity for thermogenesis in white-breasted nuthatches (Sitta carolinensis) and downy woodpeckers (Picoides pubscens), two unrelated arboral temperate residents. J. Avian Biol. 27, 279-288 [Google Scholar]
- Liknes E. T., Scott S., Swanson D. L. (2002). Seasonal acclimatization in the American goldfinch revisted: to what extent do metabolic rates vary seasonally? Condor 104, 548-557 [Google Scholar]
- McClelland G. B., Zwingelstein G., Taylor C. R., Weber J. M. (1994). Increased capacity for circulatory fatty acid transport in a highly aerobic mammal. Am. J. Physiol. 266, R1280-R1286 [DOI] [PubMed] [Google Scholar]
- Merritt J. (1995). Seasonal thermogenesis and changes in body mass of masked shrews, Sorex cinereus. J. Mammal. 76, 1020-1035 [Google Scholar]
- Nespolo R. F., Bacigalupe L. D., Bozinovic F. (2003). Heritability of energetics in a wild mammal, the leaf-eared mouse (Phyllotis darwini). Evolution 57, 1679-1688 [DOI] [PubMed] [Google Scholar]
- Nespolo R. F., Bustamante D. M., Bacigalupe L. D., Bozinovic F. (2005). Quantitative genetics of bioenergetics and growth-related traits in the wild mammal, Phyllotis darwini. Evolution 59, 1829-1837 [PubMed] [Google Scholar]
- Oelkrug R., Meyer C. W., Heldmaier G., Mzilikazi N. (2012). Seasonal changes in thermogenesis of a free-ranging afrotherian small mammal, the Western rock elephant shrew (Elephantulus rupestris). J. Comp. Physiol. B 182, 715-727 [DOI] [PubMed] [Google Scholar]
- Osgood W. H. (1909). Revision of the mice of the American genus Peromyscus. North American Fauna 28, 1-285 [Google Scholar]
- Packard G. C., Boardman T. J. (1998). The misuse of ratios, indices, and percentages in ecophysiological research. Physiol. Zool. 61, 1-9 [Google Scholar]
- Rafael J., Vsiansky P., Heldmaier G. (1985). Seasonal adaptation of brown adipose tissue in the Djungarian hamster. J. Comp. Physiol. B 155, 521-528 [DOI] [PubMed] [Google Scholar]
- Rezende E. L., Silva-Durán I., Fernando Novoa F., Rosenmann M. (2001). Does thermal history affect metabolic plasticity?: a study in three Phyllotis species along an altitudinal gradient. J. Therm. Biol. 26, 103-108 [DOI] [PubMed] [Google Scholar]
- Rezende E. L., Chappell M. A., Hammond K. A. (2004). Cold-acclimation in Peromyscus: temporal effects and individual variation in maximum metabolism and ventilatory traits. J. Exp. Biol. 207, 295-305 [DOI] [PubMed] [Google Scholar]
- Rezende E. L., Gomes F. R., Malisch J. L., Chappell M. A., Garland T., Jr (2006). Maximal oxygen consumption in relation to subordinate traits in lines of house mice selectively bred for high voluntary wheel running. J. Appl. Physiol. 101, 477-485 [DOI] [PubMed] [Google Scholar]
- Rezende E. L., Hammond K. A., Chappell M. A. (2009). Cold acclimation in Peromyscus: individual variation and sex effects in maximum and daily metabolism, organ mass and body composition. J. Exp. Biol. 212, 2795-2802 [DOI] [PubMed] [Google Scholar]
- Rosenmann M., Morrison P. (1974). Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am. J. Physiol. 226, 490-495 [DOI] [PubMed] [Google Scholar]
- Russell G. A., Chappell M. A. (2007). Is BMR repeatable in deer mice? Organ mass correlates and the effects of cold acclimation and natal altitude. J. Comp. Physiol. B 177, 75-87 [DOI] [PubMed] [Google Scholar]
- Russell G. A., Rezende E. L., Hammond K. A. (2008). Development partly determines the aerobic performance of adult deer mice, Peromyscus maniculatus. J. Exp. Biol. 211, 35-41 [DOI] [PubMed] [Google Scholar]
- Sadowska E. T., Labocha M. K., Baliga K., Stanisz A., Wróblewska A. K., Jagusiak W., Koteja P. (2005). Genetic correlations between basal and maximum metabolic rates in a wild rodent: consequences for evolution of endothermy. Evolution 59, 672-681 [PubMed] [Google Scholar]
- Sears M. W., Hayes J. P., O'Connor C. S., Geluso K., Sedinger J. S. (2006). Individual variation in thermogenic capacity affects above-ground activity of high-altitude deer mice. Funct. Ecol. 20, 97-104 [Google Scholar]
- Sears M. W., Hayes J. P., Banta M. R., McCormick D. (2009). Out in the cold: physiological capacity influences behavior in deer mice. Funct. Ecol. 23, 774-783 [Google Scholar]
- Snyder L. R. G. (1985). Low P50 in deer mice native to high altitude. J. Appl. Physiol. 58, 193-199 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G., Born S., Lechner A. J. (1982). Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir. Physiol. 48, 89-105 [DOI] [PubMed] [Google Scholar]
- Storz J. F., Wheat C. W. (2010). Integrating evolutionary and functional approaches to infer adaptation at specific loci. Evolution 64, 2489-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Sabatino S. J., Hoffmann F. G., Gering E. J., Moriyama H., Ferrand N., Monteiro B., Nachman M. W. (2007). The molecular basis of high-altitude adaptation in deer mice. PLoS Genet. 3, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., Moriyama H., Weber R. E., Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106, 14450-14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E., Fago A. (2010a). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Scott G. R., Cheviron Z. A. (2010b). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125-4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow J. G., Garland T., Jr, Carter P. A., Zhan W.-Z., Sieck G. C. (1998). Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J. Appl. Physiol. 84, 69-76 [DOI] [PubMed] [Google Scholar]
- Swanson D. L. (2001). Are summit metabolism and thermogenic endurance correlated in winter-acclimatized passerine birds? J. Comp. Physiol. B 171, 475-481 [DOI] [PubMed] [Google Scholar]
- Swanson D. L. (2007). Cold hardiness and summit metabolism in North American kinglets during fall migration. Acta Zoologica Sinica 53, 600-606 [Google Scholar]
- Swanson D. L. (2010). Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr. Ornithol. 17, 75-129 [Google Scholar]
- Swanson D. L., Garland T., Jr (2009). The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution 63, 184-194 [DOI] [PubMed] [Google Scholar]
- Swanson D. L., Liknes E. T. (2006). A comparative analysis of thermogenic capacity and cold tolerance in small birds. J. Exp. Biol. 209, 466-474 [DOI] [PubMed] [Google Scholar]
- Van Sant M. J., Hammond K. A. (2008). Contribution of shivering and nonshivering thermogenesis to thermogenic capacity for the deer mouse (Peromyscus maniculatus). Physiol. Biochem. Zool. 81, 605-611 [DOI] [PubMed] [Google Scholar]
- Ward M., Milledge J., West J. (1995). High Altitude Physiology and Medicine. London: Chapman Hall Medical; [Google Scholar]
- Weber J. M. (2011). Metabolic fuels: regulating fluxes to select mix. J. Exp. Biol. 214, 286-294 [DOI] [PubMed] [Google Scholar]
- Whitehead A., Roach J. L., Zhang S., Galvez F. (2011). Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc. Natl. Acad. Sci. USA 108, 6193-6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone B., Sears M. W., Labocha M. K., Donovan E. R., Hayes J. P. (2009). Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proc. Biol. Sci. 276, 3695-3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.