SUMMARY
Survival at cold temperatures is a complex trait, primarily because of the fact that the physiological cause of injury may differ across degrees of cold exposure experienced within the lifetime of an ectothermic individual. In order to better understand how chill-sensitive insects experience and adapt to low temperatures, we investigated the physiological basis for cold survival across a range of temperature exposures from −4 to 6°C in five genetic lines of the fruit fly Drosophila melanogaster. Genetic effects on cold survival were temperature dependent and resulted in a significant genotype–temperature interaction for survival across cold temperature exposures that differ by as little as 2°C. We investigated desiccation as a potential mechanism of injury across these temperature exposures. Flies were dehydrated following exposures near 6°C, whereas flies were not dehydrated following exposures near −4°C. Furthermore, decreasing humidity during cold exposure decreased survival, and increasing humidity during cold exposure increased survival at 6°C, but not at −4°C. These results support the conclusion that in D. melanogaster there are multiple physiological mechanisms of cold-induced mortality across relatively small differences in temperature, and that desiccation contributes to mortality for exposures near 6°C but not for subzero temperatures. Because D. melanogaster has recently expanded its range from tropical to temperate latitudes, the complex physiologies underlying cold tolerance are likely to be important traits in the recent evolutionary history of this fruit fly.
KEY WORDS: Drosophila melanogaster, cold survival, cold tolerance, desiccation, gene–environment interaction
INTRODUCTION
Cold tolerance is likely to be an important trait in the recent evolutionary history of the fruit fly Drosophila melanogaster Meigen 1830 (Schmidt et al., 2005a). Although the species originated in tropical Africa, D. melanogaster has colonized temperate regions across the world over the last 15,000 years (David and Capy, 1988). Like many insects, D. melanogaster freezes at temperatures well below the freezing point of water (Czajka and Lee, 1990). Nevertheless, D. melanogaster dies within hours even at temperatures that do not freeze tissues (Chen and Walker, 1994; Czajka and Lee, 1990; Novitski and Rush, 1949). Despite being susceptible to cold injury, D. melanogaster has been extremely successful in colonizing colder environments, and its range now extends as far north as Finland and as far south as Tasmania (Keller, 2007).
Temperate populations of D. melanogaster tend to be more cold tolerant and more desiccation resistant than tropical and subtropical populations (Bubliy et al., 2002; Davidson, 1990; Karan et al., 1998; Parsons, 1980; Schmidt et al., 2005b). This could indicate that the two traits share a common genetic basis or that temperate environments select for tolerance to both colder and more desiccating environments. However, cold tolerance and desiccation resistance do not always covary with latitude (Da Lage et al., 1989; Hoffmann et al., 2001), suggesting that either the genetic underpinnings of these traits or the selection pressures acting on these traits are separable. Selection for either cold tolerance or desiccation resistance in the laboratory can lead to the evolution of cross-tolerances to both stresses (Bubliy and Loeschcke, 2005), indicating that the two tolerances may share a common physiological or genetic basis or that selection acted to increase a general stress response. However, cold exposure and desiccation induce different gene expression profiles (Sinclair et al., 2007a) and the two traits can evolve independently (MacMillan et al., 2009; Sinclair et al., 2007b), suggesting that the genetic correlation between these traits is not absolute.
A major challenge to understanding how organisms adapt to cold is the degree to which distinct physiological mechanisms of injury contribute to differences in cold tolerance across a range of cold temperatures that will be experienced in the lifetime of an individual. Cold environments present a number of physiological challenges for chill-sensitive ectotherms, such as Drosophila, that are able to avoid ice crystal formation at temperatures below 0°C via supercooling of their body water but nonetheless have decreased performance and survival at temperatures well above their supercooling point. This is because injuries caused by acute exposures to subzero temperatures where chill-susceptible species can survive only for short amounts of time may be distinct from the injuries that accumulate as organisms survive longer at chronic exposure to temperatures above 0°C (reviewed by Lee, 2010). Acute exposure to subzero temperatures may damage cellular membranes. As temperature decreases, cellular membranes become more ordered and rigid (Hazel and Williams, 1990), disrupting the function of membrane-bound proteins (Cossins et al., 1981; Hazel, 1972) and resulting in leakage across the membrane when phase transitions occur (Drobnis et al., 1993). The injuries that accumulate during prolonged exposures to temperatures above zero may also be caused by disruptions of the cell membranes that can lead to a loss of ion homeostasis with consequences for the control of the neuromuscular system (Kostál et al., 2007; Lee, 2010; MacMillan and Sinclair, 2011). At cooler temperatures, rates of reaction also slow and enzymes are less effective catalysts (Hochachka and Somero, 2002).
Desiccation has long been associated with cold injury in insects. For exposures that cause freezing, the increase in electrolyte concentration as ice crystals form may mimic dehydration (Salt, 1961). For cold exposures that do not freeze tissues, desiccation may contribute to cold injury at subzero temperatures during supercooling because the vapor pressure of water is lower for the hemolymph than for the air (Holmstrup et al., 2010; Lundheim and Zachariassen, 1993; Zachariassen et al., 2008). Supercooled insects lose water until the depression of the melting point from colligative properties matches the ambient temperature, and many insects avoid freezing by rapidly losing water during supercooling (Holmstrup, 2011). Desiccation may also contribute to mortality at temperatures above the freezing point of water when individuals experience chill coma, a reversible, quiescent state in which insects are unable to move (Mellanby, 1939). Without the ability to feed or drink, insects in chill coma will eventually die from desiccation or starvation if the exposure is not harsh enough to cause fatal injury through some other physiological mechanism. For these reasons, desiccation may contribute to mortality across a range of cold temperatures.
Here we report that even across relatively small thermal differences there are significant differences in how five wild-type genotypes of D. melanogaster survive cold. These genotype–temperature interaction effects suggest that different genetic and physiological mechanisms underlie cold injury at different temperatures between −4 and 6°C. We find evidence that only particular genotype–temperature combinations result in death from desiccation during cold exposure, indicating that other types of physiological injury are contributing to death across cold temperatures in a genotype-dependent manner. While desiccation contributes to mortality at 6°C, it is not the cause of mortality for subzero exposures. We interpret these results in the context of the colonization of temperate latitudes by D. melanogaster and discuss extensions to other chill-susceptible insects.
MATERIALS AND METHODS
Fly stocks
All experiments included five wild-type, laboratory strains of D. melanogaster obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). Canton-S, Hikone-A-S, Oregon-R-C, Berlin-K and RAL-208 were sampled from different geographic locations and have been in the laboratory for varying numbers of years with differing levels of inbreeding. We have kept these strains at large population sizes to minimize further inbreeding. Flies were cultured in vials on standard cornmeal–agar medium with supplemental yeast. Adult male flies aged 4 to 6 days after eclosion were used in all experiments. Flies were reared at 22°C on a 12 h:12 h light:dark cycle for experiments and for two generations prior to experiments. All experiments were initiated ~6 h into the light cycle to minimize variation caused by circadian rhythm. Because long exposures to carbon dioxide anesthesia are known to affect cold tolerance (Nilson et al., 2006), we minimized the use of carbon dioxide anesthesia for sorting flies to less than 15 min.
Cold mortality curves
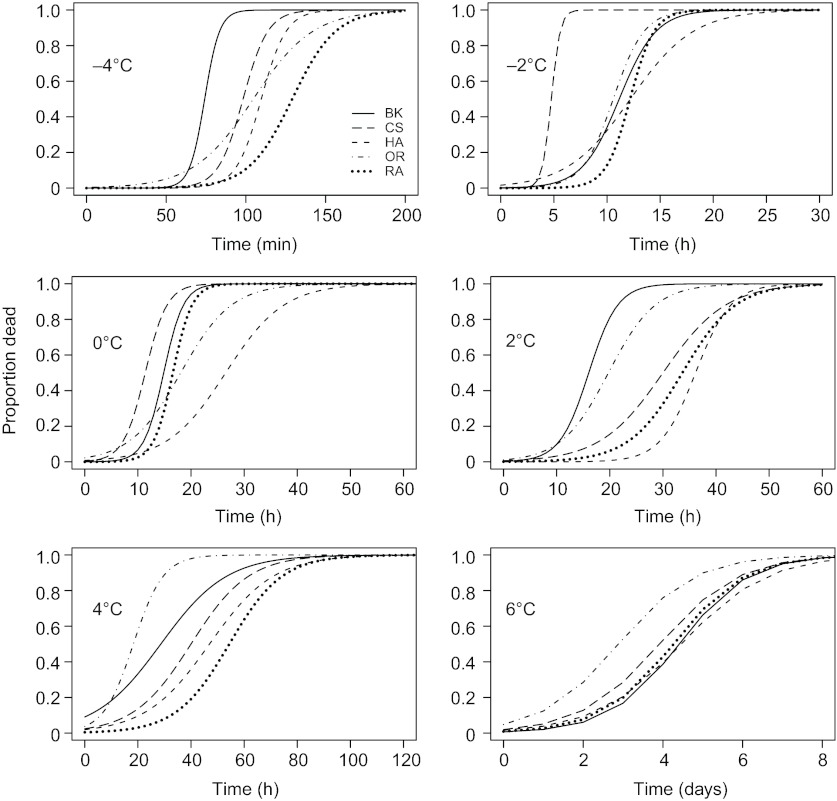
Survival was scored and mortality curves were fit for each genotype at temperatures ranging from −4 to 6°C at 2°C intervals (supplementary material TableS1). Pools of 10 adult male flies were sorted into 10 mm diameter glass test tubes. The test tubes were placed at low temperatures in a cold bath that could regulate temperature with a precision of ±0.1°C. Because all flies were in chill coma after cold exposure, flies were transferred to inverted food vials to recover, and we then scored the proportion of flies alive after a 3-day recovery at 22°C. To generate mortality curves at each temperature, survival was scored for each genotype for at least five time points with at least five replicate test tubes containing 10 flies per time point. Flies sampled at each time point were independent samples of 10 males. Time points were chosen using information from the literature and from our own preliminary data such that we would sample vials with complete, intermediate and no survival. Supplementary material Fig. S1 shows the complete survival data and time points from which the curves in Fig. 1 were fit.
Fig. 1.
Fitted mortality curves for five Drosophila melanogaster genotypes at six exposure temperatures from −4 to 6°C. Genotypes are Berlin-K (BK), Canton-S (CS), Hikone-A-S (HK), Oregon-R-C (OR) and Raleigh-208 (RA). Curves were fit using logistic regression with survival data from ~400 individual male flies of the same genotype per curve.
Flies from at least three different sibling cohorts and two non-overlapping generations were used to generate mortality curves for each genotype in order to ensure that the estimates were robust to micro-environmental effects. For each cohort, all genotypes were exposed to a single temperature in parallel on the same days to avoid confounding differences between genotypes with day-to-day variation in laboratory conditions. Each mortality curve was estimated from ~400 flies per genotype. Curves were fit to the data using logistic regression in the R statistical package version 2.10.0 (R Development Core Team, 2008). From these curves we estimated the duration of exposure predicted to cause 50% mortality (LT50), with 95% confidence intervals.
Water content
To determine whether flies that die from cold exposure have water contents similar to or distinct from flies that die from desiccation, we measured the water content of 15 individual males of each genotype that were exposed to each cold temperature for a duration corresponding to the LT50 value for the genotype. Approximately 50% of flies should survive an LT50 exposure, but the majority of flies should be either near death or recently dead. Thus, measuring the water content following an LT50 exposure approximates water content at or near the time of death. Immediately following cold exposure, flies were stored at −80°C for less than 24 h and then weighed to determine wet mass. Flies were allowed to thaw for 2–3 min before weighing. We then dried the flies at 55°C overnight and weighed dry mass. Storing flies at −80°C for 24 h has very little effect on the mass of a fly and is consistent across genotypes, decreasing mass by less than 3.5% (supplementary material Fig. S2). Masses were measured to the nearest 0.001 mg using a Sartorius ME5 microbalance (Sartorius, Goettingen, Germany). We quantified the water content of flies after LT50 exposure as the difference between their wet and dry masses (Gibbs et al., 1997). Water contents were converted to a proportion by dividing by the wet mass.
In order to compare the water contents of flies after an LT50 cold exposure with those of flies that died from desiccation, we measured the water content of flies that died from desiccation. We placed individual male flies from each genotype in vials with Drierite desiccant (Xenia, OH, USA) at 22°C and monitored them every hour until death. At death, flies were stored at −80°C and water contents were measured as described above. Fifteen flies were measured for each genotype. Water contents were also measured for fed, hydrated control males of the same age, but with no LT50 or desiccant exposure.
Cold survival under altered humidity
We measured survival at 6 and −4°C with altered humidity to determine whether low water contents following LT50 exposures were the cause of mortality. For the high humidity treatment, pools of ten 4- to 6-day-old adult males were placed in test tubes containing a moist paper towel with a rayon ball separating the flies from the paper towel. Pools of flies for the low humidity treatment were placed in test tubes containing Drierite desiccant with a rayon ball separating the flies from the desiccant. The sides of the test tubes were wiped down to remove any residues left behind by the desiccant. Control flies were placed in test tubes with a rayon ball separating the flies from the bottom of the test tube. For each genotype, all three humidity treatments were run in parallel so as not to confound treatment effects with any day-to-day variation in laboratory conditions. For 6°C exposures, 80 flies per treatment were exposed to low temperature in a cold bath for a duration corresponding to the calculated LT50 for each genotype. Water content was measured as described immediately following the cold exposures in 15 flies for each genotype and treatment.
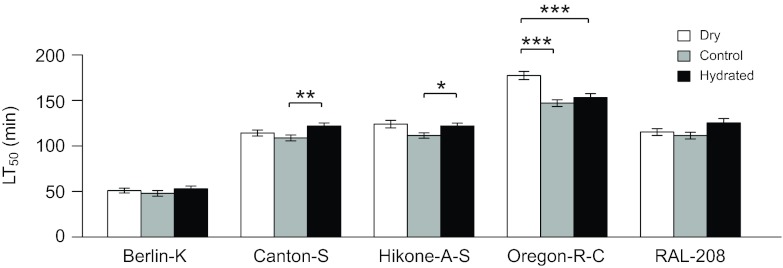
Unlike 6°C exposures, at −4°C the mortality curves are very steep and the transition from 0 to 100% mortality occurs rapidly. Because of this, we generated full mortality curves for each genotype at −4°C for each humidity treatment (supplementary material TableS4). Each mortality curve was generated from five time points with data from 60 flies per time point (N=300 flies per mortality curve). All three humidity treatments for each genotype were run in parallel to control for laboratory conditions. Percent survival was scored in each vial after a 3-day recovery and LT50 was estimated as described above. Adding desiccant at −4°C will decrease the amount of moisture in the air surrounding flies. Increasing the humidity via a moist paper towel presents more of a challenge due to the formation of ice. It is not possible to eliminate the vapor pressure deficit during supercooling because excess water in the air will form ice crystals on available surfaces. However, when the vapor pressure of water in the air is less than the vapor pressure of ice, there will be a net movement of water from the solid phase to the vapor phase. Under these conditions, the presence of ice will increase the amount of water in the air surrounding the fly and decrease the vapor pressure deficit between the insect and the air, relative to the control condition.
Statistical analyses
Statistical analyses were performed with R statistical package version 2.10.0 (R Development Core Team, 2008). Logistic regression models were fit using the glm function and post hoc tests were performed using the TukeyHSD function. LT50 (median survival time) and 95% confidence intervals were calculated using the dose.p function from the MASS package (Venables and Ripley, 2002). Post hoc tests for logistic regression models were performed using the glht function in the multcomp package (Hothorn et al., 2008).
RESULTS
Cold survival across temperatures and genotypes
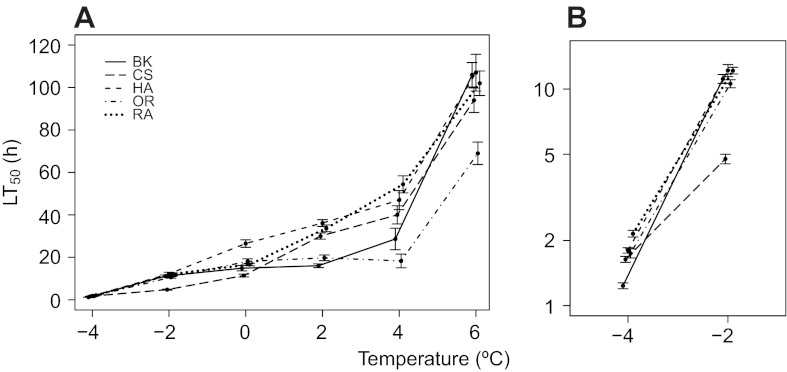
Both genotype and temperature had strong effects on survival (Table 1, Figs 1, 2; supplementary material Tables S2, S3). There were large differences in survival time across the range of temperatures measured; flies at −4°C survived on the order of minutes, with flies living for hours when exposed to 0–4°C and for days at 6°C. If flies experience all low temperatures as a single type of stress or injury, then genotypes with higher survival at one temperature should have higher survival at all temperatures. However, mortality curves generated for each genotype at each of six low temperatures (Fig. 1) revealed large genotypic effects on survival that changed in rank order across temperatures that differed by as little as 2°C (Fig. 2), indicative of temperature-dependent genetic effects (i.e. genotype–temperature interactions) (Table 1). The most cold-tolerant genotype at one temperature was not the most cold-tolerant genotype across all temperatures. For example, Canton-S had a significantly higher survival time than Berlin-K at −4°C (P<0.001), 2°C (P<0.001) and 4°C (P=0.004), while Berlin-K had a significantly higher survival time than Canton-S at −2°C (P<0.001), 0°C (P<0.001) and 6°C (P=0.013) (P-values from Tukey's post hoc tests). This suggests that the injury experienced by individual flies that differed in genotype was not the same across these low temperatures, and that different physiological and genetic mechanisms likely mediate cold tolerance across temperature differences as small as 2°C.
Table 1.
Logistic regression analysis of survival across temperatures
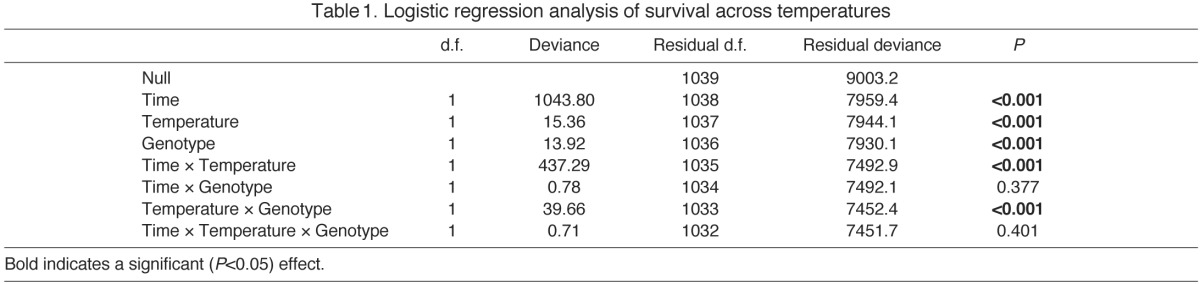
Fig. 2.
Genotype–temperature effects on cold survival in Drosophila melanogaster. (A) The LT50 (median survival time ± 95% CI) at each of six exposure temperatures from −4 to 6°C was estimated from mortality curves for each of five genotypes: Berlin-K (BK), Canton-S (CS), Hikone-A-S (HK), Oregon-R-C (OR) and Raleigh-208 (RA). The genotype–temperature interaction can be seen visually by the crossing reaction norms that connect genotype LT50 estimates across cold temperature exposures. (B) A close up reveals crossing genotype reaction norms even at the coldest temperatures. Temperature is plotted on a log scale.
Water content following cold exposure
We used LT50 exposures to provide a snapshot of the physiological state of flies of different genotypes near death from cold exposure. We compared the water contents of flies given an LT50 cold exposure with the water contents of flies that died from desiccation to determine whether the hydration state of flies dying from cold was consistent with that of flies that we knew had died from desiccation at room temperature (Fig. 3). If desiccation is the cause of mortality, water content following cold exposure should be similar to that of desiccated flies and significantly lower than that of unstressed, hydrated controls. For exposures at temperatures near −4°C, most genotypes had water contents that were significantly higher than desiccated flies and not significantly different from hydrated controls (Fig. 3). All genotypes had water contents that were significantly higher than those of desiccated flies for exposures below 0°C (Fig. 3). For exposures at temperatures near 6°C, most genotypes had water contents that were significantly lower than those of hydrated controls (Fig. 3), and some genotypes had water contents that were not significantly different from those of desiccated flies (Fig. 3). Thus, the overall trend across genotypes was for flies to have less water following exposures to higher cold temperatures, leading us to hypothesize that desiccation contributes to mortality at temperatures near 6°C, but not at subzero exposures.
Fig. 3.
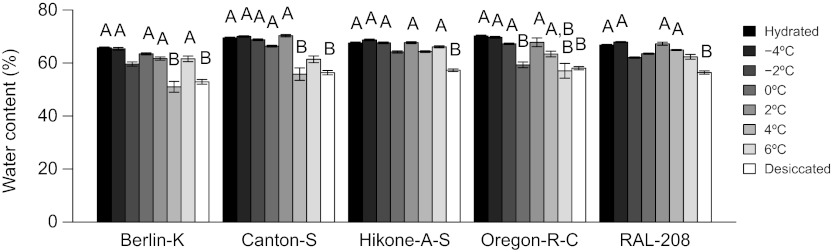
Water content of individual adult Drosophila melanogaster following LT50 exposures at each of six temperatures compared with desiccated and hydrated control flies. Percentage of water (mean ± s.e.m.) was measured by subtracting dry mass from wet mass, dividing by wet mass and multiplying by 100. Bars labeled A were not significantly different from hydrated flies (Tukey's post hoc test, P>0.05). Bars labeled B were not significantly different from desiccated flies (Tukey's post hoc test, P>0.05). Bars labeled A,B were not significantly different from hydrated or desiccated flies (Tukey's post hoc test, P>0.05). Unlabeled bars were significantly different from both hydrated and desiccated flies (Tukey's post hoc test, P<0.05).
Cold survival at high and low humidity
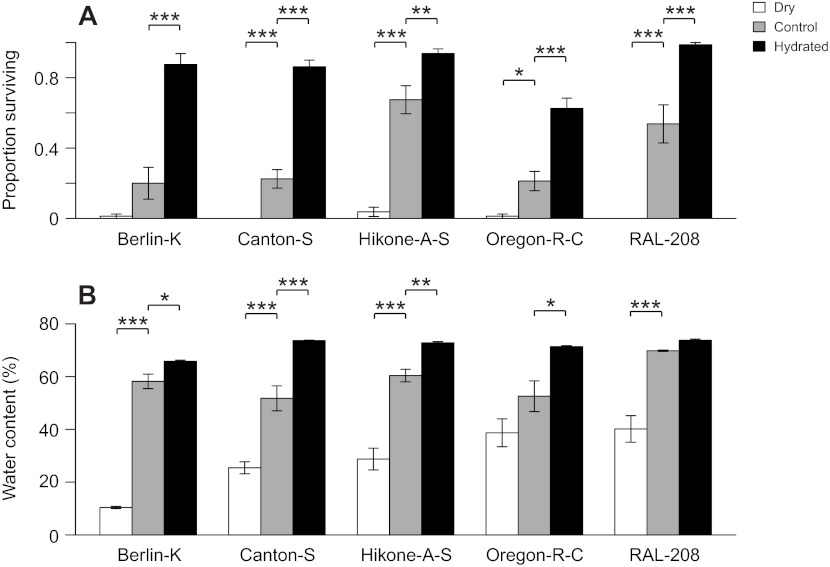
If flies are dying from desiccation during cold exposures, then altering the humidity during cold exposure should alter the rate of water loss and affect how long flies survive the cold. Given the patterns of water content described above, we predicted that altered humidity would affect survival at 6°C, where flies appear to be desiccated when dying, but not at −4°C, where flies appeared to be hydrated when dying. Consistent with these predictions, all genotypes had higher survival when humidity was increased and lower survival when humidity was decreased at 6°C (Fig. 4A). This pattern was reflected in the water contents of flies following the 6°C LT50 exposure; water contents were increased relative to controls after cold exposure at higher humidity and decreased relative to controls after cold exposure at lower humidity (Fig. 4B). This indicates that mortality is caused by desiccation at 6°C. However, desiccation does not contribute to mortality at −4°C. At −4°C, increasing humidity had little to no effect on survival, and when decreased humidity had a significant effect, it increased cold survival (Fig. 5). These results confirm that desiccation contributes to mortality at 6°C but not at −4°C. The temperature-dependent genetic effects that we have observed reflect physiological differences in the cause of death at low temperatures, and desiccation is one such cause.
Fig. 4.
Effects of altered humidity on survival and water content of Drosophila melanogaster at 6°C. (A) Proportion (mean ± s.e.m.) of flies surviving a 6°C LT50 in dry, control or hydrated conditions. (B) Water content (mean ± s.e.m.) of flies surviving a 6°C LT50 in dry, control or hydrated conditions. *P<0.05, **P<0.01, ***P<0.001 (Tukey's post hoc test).
Fig. 5.
Effects of altered humidity on survival and water content of Drosophila melanogaster at −4°C. The LT50 (median survival time ± 95% CI) was estimated for all five genotypes at −4°C with decreased (dry), control and increased (hydrated) humidities. *P<0.05, **P<0.01, ***P<0.001 (Tukey's post hoc test).
DISCUSSION
It has been previously suggested that cold, but non-freezing, temperature exposures might produce two qualitatively different types of cold injury depending on the exposure temperature (Chen and Walker, 1994). Three pieces of evidence support this conclusion: (1) mortality occurs within minutes or hours at subzero temperatures but takes days to occur at higher temperatures (Chen and Walker, 1994); (2) acclimation treatments that increase survival at subzero temperatures do not increase survival at 0°C (Chen and Denlinger, 1992); and (3) selection for survival at −7°C results in higher survival at −7°C but not 0°C, while selection at 0°C increases survival at both temperatures (Chen and Walker, 1994). While these two types of cold exposure are often interpreted to represent differences in the physiological mechanism of injury (Lee, 2010; Nedved, 2000; Sinclair and Roberts, 2005), these different temperature exposures have also been interpreted as a single physiological mechanism of injury acting at different rates (Morris and Watson, 1984). Our findings support the theory that both genetic effects and the mechanisms of physiological injury that cause death differ across a range of cold temperature exposures, although the interesting possibility remains that similar cellular structures (e.g. the cell membranes) are compromised across this range of cold.
It is known that cold survival in D. melanogaster differs between genetic strains, for acute versus chronic cold exposure and with different acclimation treatments (Chen and Walker, 1994; Rajamohan and Sinclair, 2008). Here we investigated basal cold survival in five genotypes across six densely sampled cold temperatures, allowing us to quantify strong effects of genotype–temperature interactions on cold survival across a relatively continuous gradient of cold exposure. Furthermore, we show that this genotype–environment interaction for cold survival is caused by differences in physiological injury across this gradient. Across genotypes, water content following potentially lethal exposures tended to decrease with increasing temperatures. For most genotypes, flies dying from 6°C exposures had water contents identical to those of flies that died from desiccation. Combined with the strong effect of humidity on survival at 6°C, but not at −4°C, these data indicate that desiccation contributes to mortality for milder cold exposures but not for subzero exposures in D. melanogaster. This pattern supports the conclusion that there are at least two classes of cold injury in D. melanogaster, with desiccation contributing to death at milder, cold temperatures. This is consistent with the time scales at which flies are dying across these temperatures. In the presence of desiccant, genotypes had median survival times of ~10 to 20 h at room temperature, and some individual flies survived for 1–2 days (data not shown). Flies exposed to temperatures from 0 to 6°C survived for 1–4 days, consistent with the expectation that desiccation survival times should be longer at lower temperature (Da Lage et al., 1989) and in the absence of desiccant. The rate of water loss would have to be 10 or 20 times higher for desiccation to cause mortality at −4°C, as flies only survive for a few hours under these extreme conditions.
These findings may reveal why investigations of cross-tolerances for desiccation and cold in D. melanogaster using different temperature exposures have yielded conflicting results. Selection for survival at −5°C does not increase desiccation resistance (MacMillan et al., 2009), and selection for desiccation resistance does not increase cold survival for exposures near −5°C (Sinclair et al., 2007b). The lack of a correlated response to selection suggests that desiccation does not contribute to mortality for exposures near −5°C, and we find that flies show no sign of dehydration following potentially lethal −4°C exposures. Selection for desiccation resistance increases cold survival at 0.5°C, and selection for cold resistance at 0.5°C increases survival at low humidity (Bubliy and Loeschcke, 2005). In our experiments, three of the five genotypes were partially or fully dehydrated following the 0°C exposure and had water contents significantly lower than hydrated controls. While there are likely to be additional reasons for the different results obtained by artificial selection experiments in the literature (Gibbs, 2002; Harshman and Hoffmann, 2000), we suggest that differences in exposure temperature explain some of these differences. Furthermore, our findings indicate that mechanisms of desiccation resistance, such as increased glycogen stores (Gefen et al., 2006), likely contribute to the evolution of cold tolerance in temperate natural populations that regularly experience cold temperatures above 0°C.
Even if the proximate cause of mortality at 0°C is not dehydration, we find that partial dehydration is occurring in most genotypes at temperatures near 0°C. This may magnify other types of cold injury and affect other fitness components of flies that are able to survive a single bout of cold exposure. Partial dehydration increases mortality at low temperature in the springtail, Orchesella cincta, even when the loss of water is not sufficient to cause death on its own (Nedved et al., 1998). Partial dehydration also has negative effects on life history traits in insects, such as the fecundity of mosquitos (Benoit et al., 2010; Canyon et al., 1999). In D. melanogaster, less desiccation-resistant males have lower mating success in arid environments (Gefen and Gibbs, 2009). Yet, female fecundity is not lowered by desiccation (Albers and Bradley, 2006; Sepulveda et al., 2008), suggesting that the physiological consequences of bouts of desiccating cold exposure may be sex-specific. Thus, the selection pressure experienced by flies at temperatures near 0°C may increase desiccation resistance due to the correlated effects of dehydration on reproductive success, regardless of whether mortality is due to dehydration. Selection in natural populations likely acts on suites of traits that not only affect survival during cold exposure, but also mediate the lasting effects of cold injury on fitness traits once the cold exposure has passed. For example, the female reproductive-diapause phenotype in D. melanogaster is at higher frequency in high-latitude populations and is correlated with a suite of stress resistance traits that include higher cold survival and starvation resistance (Schmidt et al., 2005a; Schmidt et al., 2005b).
The differences among genotypes in water content after cold exposure, particularly at temperatures just above 0°C, indicate that flies of different genotypes may be dying from different physiological causes at the same temperature. For example, flies of the Hikone-A genotype were hydrated when dying across the entire range of cold exposures, while the water contents of the Berlin-K, Oregon-R and Canton-S genotypes dying at 4°C were similar to those of desiccated flies. This suggests that Hikone-A individuals die from injury other than desiccation at a temperature at which other genotypes are dying from desiccation. This may be because Hikone-A is a more desiccation-resistant genotype, allowing for increased survival at temperatures where the capacity to resist desiccation becomes increasingly important for survival. Yet Hikone-A was not the most cold-tolerant genotype at all non-subzero temperatures, highlighting the physiological complexity of surviving cold. Thus, while types of cold injury may differ across a range of low temperatures, in any given population or species, different genotypes experiencing the same thermal environment may also die from distinct or combined physiological stresses.
While differential contributions of desiccation to survival contribute to the genotype–temperature effects that we observed, our findings also indicate that other physiological and genetic mechanisms are responding to cold injury in populations of flies that regularly experience subzero temperatures. We observed a complex pattern of genotype survival times across temperatures that differ by as little as 2°C and across temperatures from −4 to 4°C where flies are not entirely dying from desiccation. These observations suggest that there may be more than just two classes of cold injury. In other words, the genetic effects do not fall into two qualitatively different categories that would be indicative of two genetic mechanisms responding to two qualitatively different types of cold injury (i.e. desiccated and non-desiccated). Other physiological mechanisms implicated in cold injury without freezing include loss of membrane fluidity (Lee et al., 2006; Overgaard et al., 2008; Shreve et al., 2007), oxidative stress (Joanisse and Storey, 1996; Lalouette et al., 2011; Rojas and Leopold, 1996), loss of ion homeostasis (Kostál et al., 2004; Kostál et al., 2007), protein misfolding (Rinehart et al., 2007) and the induction of cell death pathways (Yi et al., 2007), any of which could potentially contribute to the differential mortality of genotypes across temperatures. At the lowest temperatures, we cannot rule out the possibility that inoculative freezing caused by ice crystal formation on the external cuticle of flies contributes to mortality (Lee, 2010). However, if flies are dying from inoculative freezing, the effects are not immediate; flies sampled after 1 h at −4°C have nearly 100% survival. Further investigation of these sources of injury across a range of cold temperatures will provide insight into the underlying basis for the physiological and genetic complexity of cold tolerance that we have observed.
Some of the proposed cellular mechanisms of cold injury may either result from or be similar to physiological injuries caused by desiccation. Dehydration results in the increased concentration of solutes within cells and the hemolymph. Cold exposure has been associated with a loss of ion homeostasis caused by leaky membranes (Drobnis et al., 1993) and the inability to regulate ion homeostasis at the organismal level during cold exposure (Kostál et al., 2004; Kostál et al., 2007; MacMillan and Sinclair, 2011). The loss of ion homeostasis during cold exposure may have effects that are similar to the effect of dehydration on ion balance. Ion imbalance may affect protein folding (Record et al., 1998), and the association of heat shock protein expression with both cold and desiccation may be the result of a shared response to misfolded proteins (Benoit et al., 2010; Burton et al., 1988; Hayward et al., 2004; Kostál et al., 2009; Petersen et al., 1990; Rajamohan and Sinclair, 2008; Sinclair et al., 2007a). Both extreme dehydration and low temperature can have similar effects on membrane fluidity (Crowe et al., 1992). However, D. melanogaster does not survive the levels of extreme dehydration that are typically necessary to significantly affect membrane fluidity (Crowe et al., 1992). Thus, we might expect that the adaptations that confer tolerance of cold injury may share a common genetic basis with desiccation tolerance.
Although simple, sudden low temperature exposures do not mimic the variable and complex conditions experienced by flies in nature, these experiments do inform our understanding of cold tolerance in natural populations. The physiological and genetic complexity that we find underlying cold tolerance across temperatures suggest that there are unlikely to be genotypes that are superior at surviving the full range of cold injuries that may be experienced even across a fairly narrow range of temperatures. Thus, the evolution of cold tolerance is likely to involve tradeoffs that favor generalist strategies that maximize fitness across a range of cold temperatures at latitudes with seasonal cooling. Furthermore, natural populations experience fluctuating thermal environments, and bouts of extreme cold may be interrupted by milder conditions with an opportunity to repair injuries. Our findings from single temperature exposures inform our understanding of the transient injuries that would need to be repaired in a fluctuating environment, and highlight the large role that partial dehydration will likely play in the physiological fitness costs of temperatures that fluctuate around 0°C. Multiple sub-lethal exposures to low temperature are known to decrease fecundity in D. melanogaster (Marshall and Sinclair, 2010). While this has been interpreted as a tradeoff in the allocation of energy stores that maximizes fitness, the loss of reproductive fitness may also be due to accumulated injuries of transient dehydration stress during these exposures (Benoit et al., 2010). Even if dehydration is not the primary cause of death, desiccation resistance mechanisms likely contribute to increased fitness in natural populations of D. melanogaster experiencing transient subzero exposures, in addition to the clear advantages of desiccation resistance that we find for flies at cold temperatures above 0°C.
Additionally, insects in nature likely acclimate to cooling thermal environments during their lifetime to increase cold tolerance. Interestingly, the beneficial effects of cold acclimation treatments can differ across temperatures (Chen and Denlinger, 1992; Chen and Walker, 1994; Rajamohan and Sinclair, 2008). Although our study only measured basal cold survival, our results provide a possible explanation for the differential effects of acclimation on cold survival. If different physiological mechanisms of injury underlie survival across cold exposures, we might expect that not all cold acclimation treatments will have the same effects on survival across temperatures. Consistent with this, cold acclimation treatments do not all have the same effects on gene expression (Goto, 2000; Goto, 2001; Qin et al., 2005; Sinclair et al., 2007a) or on physiological traits (Overgaard et al., 2005; Overgaard et al., 2006; Overgaard et al., 2007; Overgaard et al., 2008; Tomcala et al., 2006). For example, exposure to 0°C results in a gene expression profile different from that induced by desiccation (Sinclair et al., 2007a). Acclimation at 0°C increases survival at −5°C (Czajka and Lee, 1990), which would not be expected to cause injury by desiccation based on our results at −4°C. Thus, it is logical for acclimation at 0°C to have a different gene expression profile from desiccation. Although a profile of gene expression does not take into account all of the physiological changes that occur, we would predict that acclimation at 0°C does not increase survival at 6°C based on this data. A more thorough investigation of the differential effects of these acclimation treatments on survival will inform our understanding of the physiological mechanisms of injury across temperatures and the plastic responses that protect against injury.
These results also have implications for the evolution of cold-tolerance strategies in other small ectotherms. There has been a great deal of research on freezing as a critical temperature and cold-tolerance strategies for either avoiding freezing or tolerating freezing (Bale, 1993). However, D. melanogaster does not freeze until temperatures are near −20°C (Czajka and Lee, 1990). The identification of different physiological causes of death at different temperatures above −20°C indicates that there are other critical temperatures between freezing and chill coma at which the physiological cause of death changes. Elucidating the physiological mechanisms of injury at these temperatures, the effect of acclimation on these critical temperatures, the adaptations to these physiologically different stresses, and any tradeoffs between adaptive strategies is likely to uncover novel strategies for maximizing fitness in cold environments. The frequency and duration with which an environment crosses these threshold temperatures will influence the cold-tolerance strategies favored by natural selection.
Supplementary Material
ACKNOWLEDGEMENTS
We thank undergraduate researchers Elizabeth Eggleston, Rob Gassert and Anna Guanzon for assistance, and the Bloomington Drosophila Stock Center for providing flies. We thank Brandon Cooper, Allen Gibbs, Luke Hoekstra, Brent Lockwood and two anonymous reviewers for comments that improved this manuscript.
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/216/7/1174/DC1
FUNDING
This research was supported by research funds from Indiana University to K.L.M. R.L.K. was supported on the Indiana University Genetics, Molecular and Cellular Sciences Training Grant T32-GM007757 funded by the National Institutes of Health. Deposited in PMC for release after 12 months.
REFERENCES
- Albers M. A., Bradley T. J. (2006). Fecundity in Drosophila following desiccation is dependent on nutrition and selection regime. Physiol. Biochem. Zool. 79, 857-865 [DOI] [PubMed] [Google Scholar]
- Bale J. S. (1993). Classes of insect cold-hardiness. Funct. Ecol. 7, 751-753 [Google Scholar]
- Benoit J. B., Patrick K. R., Desai K., Hardesty J. J., Krause T. B., Denlinger D. L. (2010). Repeated bouts of dehydration deplete nutrient reserves and reduce egg production in the mosquito Culex pipiens. J. Exp. Biol. 213, 2763-2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubliy O. A., Loeschcke V. (2005). Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 18, 789-803 [DOI] [PubMed] [Google Scholar]
- Bubliy O. A., Riihimaa A., Norry F. M., Loeschcke V. (2002). Variation in resistance and acclimation to low-temperature stress among three geographical strains of Drosophila melanogaster. J. Therm. Biol. 27, 337-344 [Google Scholar]
- Burton V., Mitchell H. K., Young P., Petersen N. S. (1988). Heat shock protection against cold stress of Drosophila melanogaster. Mol. Cell. Biol. 8, 3550-3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canyon D. V., Hii J. L. K., Müller R. (1999). Adaptation of Aedes aegypti (Diptera: Culicidae) oviposition behavior in response to humidity and diet. J. Insect Physiol. 45, 959-964 [DOI] [PubMed] [Google Scholar]
- Chen C. P., Denlinger D. L. (1992). Reduction of cold injury in flies using an intermittent pulse of high-temperature. Cryobiology 29, 138-143 [Google Scholar]
- Chen C. P., Walker V. K. (1994). Cold-shock and chilling tolerance in Drosophila. J. Insect Physiol. 40, 661-669 [Google Scholar]
- Cossins A. R., Bowler K., Prosser C. L. (1981). Homeoviscous adaptation and its effect upon membrane-bound proteins. J. Therm. Biol. 6, 183-187 [Google Scholar]
- Crowe J. H., Hoekstra F. A., Crowe L. M. (1992). Anhydrobiosis. Annu. Rev. Physiol. 54, 579-599 [DOI] [PubMed] [Google Scholar]
- Czajka M. C., Lee R. E., Jr (1990). A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J. Exp. Biol. 148, 245-254 [DOI] [PubMed] [Google Scholar]
- Da Lage J. L., Capy P., David J. R. (1989). Starvation and desiccation tolerance in Drosophila melanogaster adults: effects of environmental temperature. J. Insect Physiol. 35, 453-457 [Google Scholar]
- David J. R., Capy P. (1988). Genetic variation of Drosophila melanogaster natural populations. Trends Genet. 4, 106-111 [DOI] [PubMed] [Google Scholar]
- Davidson J. K. (1990). Nonparallel geographic patterns for tolerance to cold and desiccation in Drosophila melanogaster and Drosophila simulans. Aust. J. Zool. 38, 155-161 [Google Scholar]
- Drobnis E. Z., Crowe L. M., Berger T., Anchordoguy T. J., Overstreet J. W., Crowe J. H. (1993). Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J. Exp. Zool. 265, 432-437 [DOI] [PubMed] [Google Scholar]
- Gefen E., Gibbs A. G. (2009). Interactions between environmental stress and male mating success may enhance evolutionary divergence of stress-resistant Drosophila populations. Evolution 63, 1653-1659 [DOI] [PubMed] [Google Scholar]
- Gefen E., Marlon A. J., Gibbs A. G. (2006). Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation. J. Exp. Biol. 209, 3293-3300 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G. (2002). Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comp. Biochem. Physiol. 133A, 781-789 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Chippindale A. K., Rose M. R. (1997). Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821-1832 [DOI] [PubMed] [Google Scholar]
- Goto S. G. (2000). Expression of Drosophila homologue of senescence marker protein-30 during cold acclimation. J. Insect Physiol. 46, 1111-1120 [DOI] [PubMed] [Google Scholar]
- Goto S. G. (2001). A novel gene that is up-regulated during recovery from cold shock in Drosophila melanogaster. Gene 270, 259-264 [DOI] [PubMed] [Google Scholar]
- Harshman L. G., Hoffmann A. A. (2000). Laboratory selection experiments using Drosophila: what do they really tell us? Trends Ecol. Evol. 15, 32-36 [DOI] [PubMed] [Google Scholar]
- Hayward S. A. L., Rinehart J. P., Denlinger D. L. (2004). Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. J. Exp. Biol. 207, 963-971 [DOI] [PubMed] [Google Scholar]
- Hazel J. R. (1972). Effect of temperature acclimation upon succinic dehydrogenase activity from epaxial muscle of common goldfish (Carassius auratus L.). II. Lipid reactivation of soluble enzyme. Comp. Biochem. Physiol. B 43, 863-882 [DOI] [PubMed] [Google Scholar]
- Hazel J. R., Williams E. E. (1990). The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29, 167-227 [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Somero G. N. (2002). Biochemical Adaptation: Mechanism and Process in Physiological Evolution. New York: Oxford University Press; [Google Scholar]
- Hoffmann A. A., Hallas R., Sinclair C., Mitrovski P. (2001). Levels of variation in stress resistance in drosophila among strains, local populations, and geographic regions: patterns for desiccation, starvation, cold resistance, and associated traits. Evolution 55, 1621-1630 [DOI] [PubMed] [Google Scholar]
- Holmstrup M., Bayley M., Pedersen S. A., Zachariassen K. E. (2010). Interactions between cold, desiccation and enivronmental toxins. In Low Temperature Biology of Insects (ed. Denlinger D. L., Lee R. E.), pp. 166-187 New York: Cambridge University Press; [Google Scholar]
- Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biom. J. 50, 346-363 [DOI] [PubMed] [Google Scholar]
- Joanisse D. R., Storey K. B. (1996). Oxidative stress and antioxidants in overwintering larvae of cold-hardy goldenrod gall insects. J. Exp. Biol. 199, 1483-1491 [DOI] [PubMed] [Google Scholar]
- Karan D., Dahiya N., Munjal A. K., Gibert P., Moreteau B., Parkash R., David J. R. (1998). Desiccation and starvation tolerance of adult Drosophila: opposite latitudinal clines in natural populations of three different species. Evolution 52, 825-831 [DOI] [PubMed] [Google Scholar]
- Keller A. (2007). Drosophila melanogaster's history as a human commensal. Curr. Biol. 17, R77-R81 [DOI] [PubMed] [Google Scholar]
- Kostál V., Tollarová-Borovanská M. (2009). The 70 kDa heat shock protein assists during the repair of chilling injury in the insect, Pyrrhocoris apterus. PLoS ONE 4, e4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostál V., Vambera J., Bastl J. (2004). On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J. Exp. Biol. 207, 1509-1521 [DOI] [PubMed] [Google Scholar]
- Kostál V., Renault D., Mehrabianová A., Bastl J. (2007). Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: role of ion homeostasis. Comp. Biochem. Physiol. 147A, 231-238 [DOI] [PubMed] [Google Scholar]
- Lalouette L., Williams C. M., Hervant F., Sinclair B. J., Renault D. (2011). Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. 158A, 229-234 [DOI] [PubMed] [Google Scholar]
- Lee R. E. (2010). A primer on insect cold tolerance. In Low Temperature Biology of Insects (ed. Denlinger D. L., Lee R. E.), pp. 3-34 New York: Cambridge University Press; [Google Scholar]
- Lee R. E., Jr, Damodaran K., Yi S. X., Lorigan G. A. (2006). Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology 52, 459-463 [DOI] [PubMed] [Google Scholar]
- Lundheim R., Zachariassen K. E. (1993). Water balance of over-wintering beetles in relation to strategies for cold tolerance. J. Comp. Physiol. B 163, 1-4 [Google Scholar]
- MacMillan H. A., Sinclair B. J. (2011). The role of the gut in insect chilling injury: cold-induced disruption of osmoregulation in the fall field cricket, Gryllus pennsylvanicus. J. Exp. Biol. 214, 726-734 [DOI] [PubMed] [Google Scholar]
- MacMillan H. A., Walsh J. P., Sinclair B. J. (2009). The effects of selection for cold tolerance on cross-tolerance to other environmental stressors in Drosophila melanogaster. Insect Sci. 16, 263-276 [Google Scholar]
- Marshall K. E., Sinclair B. J. (2010). Repeated stress exposure results in a survival-reproduction trade-off in Drosophila melanogaster. Proc. R. Soc. Lond. B 277, 963-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby K. (1939). Low temperature and insect activity. Proc. R. Soc. Lond. B 127, 473-487 [Google Scholar]
- Morris G. J., Watson P. F. (1984). Cold shock injury – a comprehensive bibliography. Cryo Letters 5, 352-372 [Google Scholar]
- Nedved O. (2000). Snow white and the seven dwarfs: a multivariate approach to classification of cold tolerance. Cryo Letters 21, 339-348 [PubMed] [Google Scholar]
- Nedved O., Lavy D., Verhoef H. A. (1998). Modelling the time-temperature relationship in cold injury and effect of high-temperature interruptions on survival in a chill-sensitive collembolan. Funct. Ecol. 12, 816-824 [Google Scholar]
- Nilson T. L., Sinclair B. J., Roberts S. P. (2006). The effects of carbon dioxide anesthesia and anoxia on rapid cold-hardening and chill coma recovery in Drosophila melanogaster. J. Insect Physiol. 52, 1027-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitski E., Rush G. (1949). Viability and fertility of Drosophila exposed to sub-zero temperatures. Biol. Bull. 97, 150-157 [PubMed] [Google Scholar]
- Overgaard J., Sørensen J. G., Petersen S. O., Loeschcke V., Holmstrup M. (2005). Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J. Insect Physiol. 51, 1173-1182 [DOI] [PubMed] [Google Scholar]
- Overgaard J., Sorensen J. G., Petersen S. O., Loeschcke V., Holmstrup M. (2006). Reorganization of membrane lipids during fast and slow cold hardening in Drosophila melanogaster. Physiol. Entomol. 31, 328-335 [Google Scholar]
- Overgaard J., Malmendal A., Sørensen J. G., Bundy J. G., Loeschcke V., Nielsen N. C., Holmstrup M. (2007). Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J. Insect Physiol. 53, 1218-1232 [DOI] [PubMed] [Google Scholar]
- Overgaard J., Tomcala A., Sørensen J. G., Holmstrup M., Krogh P. H., Simek P., Kostál V. (2008). Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J. Insect Physiol. 54, 619-629 [DOI] [PubMed] [Google Scholar]
- Parsons P. A. (1980). Parallel climatic races for tolerances to high-temperature desiccation stress in two Drosophila species. J. Biogeogr. 7, 97-101 [Google Scholar]
- Petersen N. S., Young P., Burton V. (1990). Heat shock mRNA accumulation during recovery from cold shock in Drosophila melanogaster. Insect Biochem. 20, 679-684 [Google Scholar]
- Qin W., Neal S. J., Robertson R. M., Westwood J. T., Walker V. K. (2005). Cold hardening and transcriptional change in Drosophila melanogaster. Insect Mol. Biol. 14, 607-613 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- Rajamohan A., Sinclair B. J. (2008). Short-term hardening effects on survival of acute and chronic cold exposure by Drosophila melanogaster larvae. J. Insect Physiol. 54, 708-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Zhang W. T., Anderson C. F. (1998). Analysis of effects of salts and uncharged solutes on protein and nucleic acid equilibria and processes: a practical guide to recognizing and interpreting polyelectrolyte effects, Hofmeister effects, and osmotic effects of salts. Adv. Protein Chem. 51, 281-353 [DOI] [PubMed] [Google Scholar]
- Rinehart J. P., Li A., Yocum G. D., Robich R. M., Hayward S. A. L., Denlinger D. L. (2007). Upregulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 104, 11130-11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R. R., Leopold R. A. (1996). Chilling injury in the housefly: evidence for the role of oxidative stress between pupariation and emergence. Cryobiology 33, 447-458 [Google Scholar]
- Salt R. W. (1961). Principles of insect cold-hardiness. Annu. Rev. Entomol. 6, 55-74 [Google Scholar]
- Schmidt P. S., Matzkin L., Ippolito M., Eanes W. F. (2005a). Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution 59, 1721-1732 [PubMed] [Google Scholar]
- Schmidt P. S., Paaby A. B., Heschel M. S. (2005b). Genetic variance for diapause expression and associated life histories in Drosophila melanogaster. Evolution 59, 2616-2625 [PubMed] [Google Scholar]
- Sepulveda S., Shojaeian P., Rauser C. L., Jafari M., Mueller L. D., Rose M. R. (2008). Interactions between injury, stress resistance, reproduction, and aging in Drosophila melanogaster. Exp. Gerontol. 43, 136-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve S. M., Yi S. X., Lee R. E., Jr (2007). Increased dietary cholesterol enhances cold tolerance in Drosophila melanogaster. Cryo Letters 28, 33-37 [PubMed] [Google Scholar]
- Sinclair B. J., Roberts S. P. (2005). Acclimation, shock and hardening in the cold. J. Therm. Biol. 30, 557-562 [Google Scholar]
- Sinclair B. J., Gibbs A. G., Roberts S. P. (2007a). Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol. Biol. 16, 435-443 [DOI] [PubMed] [Google Scholar]
- Sinclair B. J., Nelson S., Nilson T. L., Roberts S. P., Gibbs A. G. (2007b). The effect of selection for desiccation resistance on cold tolerance of Drosophila melanogaster. Physiol. Entomol. 32, 322-327 [Google Scholar]
- Tomcala A., Tollarová M., Overgaard J., Simek P., Kostál V. (2006). Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: triggering by low temperature, desiccation and diapause progression. J. Exp. Biol. 209, 4102-4114 [DOI] [PubMed] [Google Scholar]
- Venables W. N., Ripley B. D. (2002). Modern Applied Statistics with S, 4th edn. New York: Springer; [Google Scholar]
- Yi S. X., Moore C. W., Lee R. E., Jr (2007). Rapid cold-hardening protects Drosophila melanogaster from cold-induced apoptosis. Apoptosis 12, 1183-1193 [DOI] [PubMed] [Google Scholar]
- Zachariassen K. E., Li N. G., Laugsand A. E., Kristiansen E., Pedersen S. A. (2008). Is the strategy for cold hardiness in insects determined by their water balance? A study on two closely related families of beetles: Cerambycidae and Chrysomelidae. J. Comp. Physiol. B 178, 977-984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.