Abstract
Background
Much ado has been made about obesity’s health impact, largely founded on simple patient weight and circulating adipose-derived mediator levels. Paradoxically, a “healthy obese” state exists, but substantial knowledge gaps also exist regarding human adipose-phenotype determinants. Surgical major amputation (AMP) patients are the “sickest-of-the-sick”. Conversely, elective knee-replacement (TKR) is reserved for patients who expect continued health and longevity. To delineate human adipose biology variability and clinical determinants, we studied fresh subcutaneous adipose from AMP patients, using TKR patients as controls. We hypothesized that AMP patients would display a pro-inflammatory adipokine signature, and that certain clinical conditions (diabetes, hypertension, hyperlipidemia, high BMI, uremia) would independently drive elevated adipose inflammation.
Methods
AMP (n=29) and TKR (n=20) adipose and clinical data were collected prospectively, and protein was isolated and analyzed for eight adipose-related mediators. Statistical analyses included Wilcoxon-rank sum, Fischer’s exact and multiple linear-regression modeling of clinical parameter predictors of mediator expression.
Results
IL-6, IL-8, leptin, resistin, and PAI-1 were differentially expressed (up to 200-fold) between AMP/TKR cohorts. Key clinical parameters which associated with protein levels of adipose-phenotype included age, sex, hypertension, hyperlipidemia, congestive heart failure, cerebrovascular disease, renal disease, and warfarin, statin, and insulin use, with simple BMI failing to be predictive.
Conclusions
AMP-patients display adiposopathy, with a pro-inflammatory adipose-phenotypic signature compared to TKR-controls. BMI fails to predict phenotype, yet other clinical conditions such as age, hyperlipidemia, and renal insufficiency do drive adipokine expression. Understanding human adipose-phenotypic determinants stands as a fundamental priority when future studies dissect the interplay between adipose biology and surgical diseases/outcomes.
Introduction
Adipose tissue has emerged as a pivotal effector of mammalian homeostasis beyond its historic role as an inert energy depot.1, 2 While classical clinical evaluation of adiposity utilizes total fat volume (e.g. body mass index (BMI), percent body fat, distribution, etc.), these measurements do not always correlate well with clinical phenotypes.3 In recent years the term “adiposopathy” has been coined to represent immune and metabolic derangements in adipose tissue. 4 Substantial knowledge gaps exist regarding the dynamics of adipose phenotype and the true role of adipose-related signaling networks in disease. The literature to date largely builds on serum circulating biomarker levels or animal models of human disease, and direct interrogation of clinically relevant human adipose tissue has been relatively limited.5–9
Patients progressing to clinical need for major amputation are considered the “sickest of the sick”, usually with advanced stages of diseases such as diabetes, renal insufficiency, and atherosclerosis.10 Few surgical procedures rival major lower extremity amputation for thirty day morbidity and mortality.10–12 Conversely, elective orthopedic procedures for osteoarthritis (such as hip and knee replacements) are offered to selected similarly aged patients who are well enough to withstand such elective surgical procedures, and who are predicted to maintain overall health and longevity sufficient to derive benefit from surgery.
Thus to advance understanding of the spectrum and determinants of human adipose biology, we compared key lower extremity adipose tissue protein components from major amputation patients with fat collected from patients undergoing elective orthopedic procedures. Use of these real-world clinical specimens offers insights into the variability and clinical determinants of human adipose phenotypes. We hypothesized that there would be more variation between the two patient cohorts than within the groups, and that patients undergoing leg amputation would display a relatively higher pro-inflammatory adipokine signature and lower levels of anti-inflammatory marker adiponectin. Finally, we hypothesized that clinical conditions such as diabetes, hypertension, hyperlipidemia, body mass index, and uremia would correlate positively with adipose inflammation.
Materials and Methods
Patients undergoing lower extremity major amputation (below knee or above knee) or elective orthopedic total knee replacement at a single institution were prospectively identified via procedures approved by the local institutional review board (IRB). Informed consent was obtained from the control elective orthopedic cohort. The amputation patients were enrolled under an IRB approved protocol that allowed us to collect de-identified medical information and tissue from the amputated limb without informed consent. Indications for amputation included unreconstructable critical limb ischemia and non-salvageable foot. All patients in the control cohort underwent elective knee replacement for osteoarthritis.
All samples were collected intraoperatively by trained surgeons. Two grams of subcutaneous adipose tissue were collected from the amputated limb and immediately flash frozen in liquid nitrogen, then stored at −80 °C until the time of analysis. The adipose samples were from the proximal end of the specimen, i.e. near a level where the clinical evaluation supported enough blood supply for healing, and remote from ischemic and infected tissues. Proteins were isolated from the samples in ice-cold Dulbecco’s phosphate buffered saline with Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), homogenized, and centrifuged (2,000g × 5 minutes) to remove gross debris. The homogenates were next centrifuged once more (10,000g × 10 minutes). The supernatant was then collected for quantitative protein analysis via multiple antigen flow microparticle bead assay Luminex (Luminex Corporation, Austin, TX) according to the manufacturer’s instructions, for eight key biologic mediators: interleukin-6 (IL-6), interleukin-8 (IL-8), leptin, tumor necrosis factor-α (TNF-α), adiponectin, resistin, and plasminogen activator inhibitor-1(PAI-1). Quantities of the biologic mediators were normalized to total protein as determined via a Bradford protein assay.
Demographic and clinical data were collected retrospectively from patients’ online medical records. Endpoints recorded included age at surgery, gender, race, body mass index, diagnoses of diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, cerebrovascular disease, renal disease, or pulmonary disease, any history of current or former tobacco use, and use of the following pharmaceutical agents: anti-platelet therapy, warfarin, calcium channel blockers, beta blockers, ACE inhibitors/angiotensin receptor blockers, HMG-CoA reductase inhibitors, insulin, and oral steroids.
All statistical analyses were performed with the Stata 12 software package (StataCorp, College Station, TX). Patient demographics as well as sample mediator levels from the two groups were compared for significance using Wilcoxon-rank-sum tests. A linear regression model was utilized to determine which clinical parameters were predictive of the mediators examined and assess the relative strength of the predictive ability (denoted by the coefficient β). Significant results were defined as those with α ≤ 0.05.
Results
Adipose samples were collected from 29 patients who underwent lower extremity amputation and 20 patients who underwent total knee replacement. Patient demographics and baseline characteristics as collected at the time of surgery are listed in Table I. Diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, cerebrovascular disease, renal disease, and pulmonary disease were defined as documented diagnosis in clinical records. The amputation cohort was older than the control cohort overall and had a greater incidence of diabetes mellitus, cardiac, cerebrovascular, renal, and pulmonary disease. In addition, this cohort took calcium channel blockers, beta blockers, statins, and insulin at higher frequencies and displayed a lower overall BMI and a more frequent history of smoking.
Table I.
Descriptive statistics; P by Rank Sum or Fisher’s exact test
|
Mean or Weighted Frequency (SE)
|
P | ||
|---|---|---|---|
| Amputation (n = 29) | Control (n = 20) | ||
| Age | 70.7 years (±2.7) | 63.5 years (±2.2) | 0.029 |
| Race: | 0.596 | ||
| White | 16 (55.17%) | 14 (70.00%) | |
| Black | 8 (27.59%) | 4 (20.00%) | |
| Hispanic | 5 (17.24%) | 2 (10.00%) | |
| Female | 9 (31.03%) | 11 (55.00%) | 0.140 |
| BMI | 24.7 kg/m2 (±1.2) | 34.2 kg/m2 (±1.8) | <0.0001 |
| Diabetes Mellitus | 19 (65.52%) | 5 (25.00%) | 0.006 |
| Hypertension | 27 (93.10%) | 15 (75.00%) | 0.087 |
| Hyperlipidemia | 20 (68.97%) | 10 (50.00%) | 0.149 |
| CAD | 17 (58.62%) | 3 (15.00%) | 0.002 |
| CHF | 7 (24.14%) | 0 (0.00%) | 0.018 |
| CVA | 8 (27.59%) | 0 (0.00%) | 0.010 |
| Renal Disease | 12 (41.38%) | 0 (0.00%) | 0.001 |
| Pulmonary Disease | 9 (31.03%) | 0 (0.00%) | 0.005 |
| Smoking History | 18 (62.07%) | 5 (25.00%) | 0.006 |
| Antiplatelet Therapy | 22 (75.86%) | 14 (70.00%) | 0.446 |
| Warfarin | 9 (27.58%) | 11 (55.00%) | 0.051 |
| Calcium Channel Blocker | 10 (34.48%) | 1 (5.00%) | 0.015 |
| Beta Blocker | 24 (82.76%) | 3 (15.00%) | <0.0001 |
| ACE Inhibitor/ARB | 12 (41.38%) | 12 (60.00%) | 0.161 |
| Statin | 22 (75.87%) | 9 (45.00%) | 0.029 |
| Insulin | 18 (62.07%) | 3 (15.00%) | 0.001 |
| NSAID | 23 (79.31%) | 17 (85%) | 0.455 |
| Steroid | 3 (10.34%) | 0 (0.00%) | 0.198 |
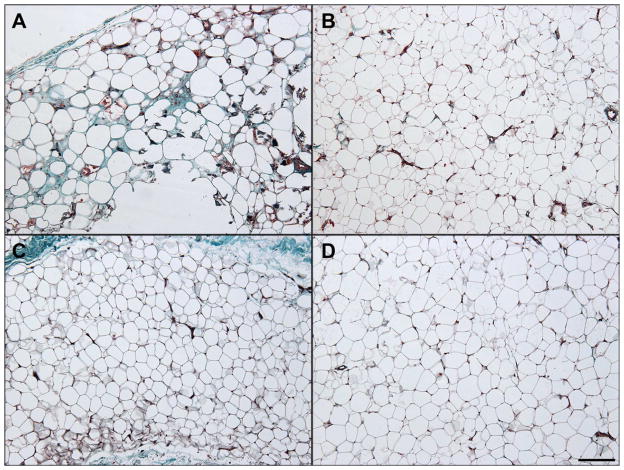
Representative Masson-stained microscopic sections from the two cohorts are shown in Figure I. While some fields in the amputation group demonstrated overt fat necrosis, most fields examined showed virtually normal adipose tissue, with scattered mild chronic inflammation and reactive adipocyte changes identified. Tissue from knee replacement patients showed virtually no histological evidence of pathology.
Figure 1.
Representative Histology, Masson stained. Two representative fields (100X magnification) from trichrome stained sections from each cohort are shown. Tissue from amputation patients (panels A and B) showed rare fat necrosis (panel A) amidst relatively normal adipose tissue (panel B). Tissue from control patients (panels C and D) did not show fat necrosis in the sections examined. Scale bar = 200 μm
Protein analysis of adipocyte-related mediators revealed significant differences between groups in IL-6, IL-8, leptin, resistin, and PAI-1 (Table II). Levels of TNF-α, MCP-1, and adiponectin were similar. IL-6 was 200-fold higher in the amputation cohort, and IL-8 was 150-fold higher. Resistin and PAI-1 were also significantly higher in amputees, and leptin was significantly decreased.
Table II.
Comparative subcutaneous adipose protein concentrations
| Amputation (n = 29) | TKA (n = 20) | P | |
|---|---|---|---|
|
| |||
| Mean pg/mg total protein ± SEM | |||
| IL-6 | 293.10 ± 234.10 | 1.50 ± 2.20 | <0.0001 |
| IL-8 | 245.46 ± 234.24 | 1.68 ± 0.15 | 0.0005 |
| Leptin | 1635.66 ± 444.69 | 3434.29 ± 586.24 | 0.0032 |
| TNF-α | 1.08 ± 0.29 | 0.68 ± 0.07 | 0.6545 |
| MCP-1 | 173.46 ± 38.30 | 93.78 ± 14.50 | 0.2147 |
| Adiponectin | 438818.40 ± 85487.62 | 416429.90 ± 33316.05 | 0.0838 |
| Resistin | 2930.30 ± 603.87 | 637.41 ± 116.27 | 0.0001 |
| PAI-1 | 447.37 ± 111.32 | 74.51 ± 9.01 | 0.0008 |
Linear regression analysis was performed to link clinical parameters to the measured adipose-derived mediator levels as summarized in Table III. This analysis demonstrated significant relationships between multiple clinical parameters and the mediators. Age was found to be negatively predictive of IL-6, IL-8, and TNF-α. Male sex was negatively predictive of adiponectin and hypertension was positively predictive of PAI-1. Hyperlipidemia was negatively predictive of leptin, resistin, TNF-α, and adiponectin. Congestive heart failure was positively predictive of resistin and PAI-1. Cerebrovascular disease was positively predictive of resistin. Renal disease was positively predictive of IL-6, IL-8, resistin, and TNF-α. Warfarin was negatively predictive of MCP-1. Statin and insulin use were found to be positively predictive of TNF-α; additionally, insulin negatively predicted adiponectin. Race, diabetes mellitus, hypertension, coronary artery disease, pulmonary disease, smoking history, anti-platelet agent, calcium channel blocker, and beta blocker use, ACE inhibitor/ARB use, oral steroid use, and importantly BMI were not found to be significant predictors of any of the examined markers of adipose phenotype.
Table III.
Association of clinical factors with key mediators
| IL-6
|
IL-8
|
Leptin
|
TNF-α
|
MCP-1
|
Adiponectin
|
Resistin
|
PAI-1
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | |
| Age | −0.5493 | 0.026 | −0.5352 | 0.035 | −0.2006 | 0.372 | −0.6250 | 0.005 | −0.3297 | 0.183 | −0.3731 | 0.125 | −0.1896 | 0.332 | −0.2620 | 0.253 |
| Sex | 0.0690 | 0.707 | 0.0924 | 0.626 | −0.2169 | 0.220 | −0.1052 | 0.515 | −0.2822 | 0.146 | −0.4319 | 0.027 | −0.1623 | 0.289 | −0.0589 | 0.739 |
| Race | 0.0289 | 0.875 | 0.0450 | 0.812 | 0.1473 | 0.402 | 0.1710 | 0.295 | 0.1210 | 0.527 | 0.1535 | 0.413 | 0.0379 | 0.803 | −0.0216 | 0.903 |
| BMI | −0.3552 | 0.173 | −0.3481 | 0.194 | 0.0339 | 0.889 | −0.2535 | 0.265 | 0.2011 | 0.450 | −0.1887 | 0.468 | −0.0486 | 0.818 | −0.2070 | 0.404 |
| DM | 0.2352 | 0.574 | 0.1865 | 0.664 | 0.3793 | 0.342 | 0.4276 | 0.249 | 0.3745 | 0.389 | 0.7117 | 0.101 | 0.4364 | 0.211 | 0.3907 | 0.335 |
| HTN | 0.2330 | 0.308 | 0.2233 | 0.342 | 0.0110 | 0.959 | 0.2196 | 0.274 | 0.1506 | 0.521 | 0.1023 | 0.655 | 0.1061 | 0.570 | 0.4666 | 0.040 |
| HLP | −0.2685 | 0.228 | −0.2693 | 0.240 | −0.5246 | 0.018 | −0.5273 | 0.011 | −0.1352 | 0.553 | −0.5118 | 0.028 | −0.3929 | 0.038 | −0.2263 | 0.290 |
| CAD | 0.3750 | 0.188 | 0.3240 | 0.267 | 0.3074 | 0.253 | 0.3134 | 0.209 | 0.1522 | 0.600 | −0.0232 | 0.935 | 0.2856 | 0.222 | 0.4660 | 0.093 |
| CHF | 0.1901 | 0.320 | 0.1684 | 0.391 | −0.0891 | 0.621 | 0.3355 | 0.052 | 0.2423 | 0.223 | 0.2862 | 0.143 | 0.4042 | 0.015 | 0.3916 | 0.040 |
| CVA | 0.1534 | 0.514 | 0.0996 | 0.680 | −0.0317 | 0.886 | 0.2485 | 0.233 | 0.3736 | 0.132 | 0.2975 | 0.216 | 0.4245 | 0.035 | 0.2936 | 0.200 |
| Renal Disease | 0.6544 | 0.012 | 0.6397 | 0.016 | −0.1370 | 0.554 | 0.5954 | 0.009 | 0.3900 | 0.130 | −0.2392 | 0.335 | 0.4185 | 0.045 | 0.4599 | 0.058 |
| Pulm Disease | 0.2209 | 0.268 | 0.2511 | 0.223 | −0.0043 | 0.982 | 0.1392 | 0.424 | −0.1173 | 0.566 | −0.0880 | 0.659 | 0.0474 | 0.770 | −0.2469 | 0.200 |
| Smoking | −0.0982 | 0.624 | −0.0901 | 0.662 | 0.1484 | 0.436 | 0.0745 | 0.671 | 0.0324 | 0.875 | 0.3241 | 0.117 | −0.1225 | 0.459 | −0.0448 | 0.816 |
| Anti-platelet | 0.3378 | 0.340 | 0.3912 | 0.284 | 0.5417 | 0.113 | 0.3686 | 0.238 | −0.4758 | 0.198 | 0.2229 | 0.531 | −0.0453 | 0.875 | −0.2551 | 0.453 |
| Warfarin | 0.2766 | 0.329 | 0.3456 | 0.238 | 0.1741 | 0.515 | 0.1569 | 0.526 | −0.5937 | 0.049 | −0.2723 | 0.342 | −0.3393 | 0.151 | −0.2787 | 0.307 |
| CaCh Blocker | −0.2986 | 0.176 | −0.2977 | 0.190 | −0.1556 | 0.452 | −0.3845 | 0.052 | −0.4506 | 0.053 | 0.2417 | 0.276 | −0.3079 | 0.094 | −0.3102 | 0.146 |
| Beta Blocker | −0.1512 | 0.674 | −0.2131 | 0.565 | 0.4604 | 0.184 | 0.3163 | 0.320 | 0.7397 | 0.055 | 0.6435 | 0.085 | 0.4028 | 0.180 | 0.1861 | 0.591 |
| ACEI/ARB | −0.2358 | 0.312 | −0.2417 | 0.314 | 0.1683 | 0.444 | −0.2268 | 0.269 | −0.2520 | 0.296 | −0.2112 | 0.369 | −0.0236 | 0.901 | −0.4384 | 0.057 |
| Statin | 0.3828 | 0.165 | 0.4015 | 0.157 | 0.1987 | 0.440 | 0.4878 | 0.048 | 0.2543 | 0.366 | 0.2667 | 0.333 | −0.0132 | 0.953 | 0.1342 | 0.606 |
| Insulin | −0.5661 | 0.278 | −0.4999 | 0.350 | −0.9100 | 0.072 | −1.1788 | 0.014 | −0.8871 | 0.105 | −1.1738 | 0.032 | −0.6214 | 0.152 | −0.8692 | 0.089 |
| Steroid | −0.1145 | 0.553 | −0.1072 | 0.589 | 0.0584 | 0.749 | 0.0183 | 0.914 | 0.1123 | 0.574 | 0.1713 | 0.382 | 0.0823 | 0.604 | 0.0234 | 0.899 |
| Amputation | −0.2700 | 0.477 | −0.2247 | 0.564 | −0.2757 | 0.444 | −0.3099 | 0.354 | −0.2957 | 0.452 | −0.5031 | 0.195 | −0.2078 | 0.506 | −0.2015 | 0.580 |
| NSAID | 0.1562 | 0.582 | 0.1114 | 0.703 | −0.5404 | 0.053 | 0.0489 | 0.844 | 0.3257 | 0.272 | −0.0756 | 0.792 | 0.1797 | 0.443 | 0.3785 | 0.173 |
Discussion
Utilizing fresh adipose samples, we describe dramatic phenotypic differences between two similarly aged real-world patient cohorts undergoing surgery. The two groups did display substantially different characteristics prior to surgery. In general, the amputation cohort had more chronic disease processes, was more likely to be on medications such as calcium channel blockers, beta blockers, statins, and insulin, and they were leaner (lower BMI). This was expected given that the amputation patients are recognized to be a very ill population with a high incidence of significant comorbidities and corresponding use of medications.13
Evidence assembled in recent years unequivocally links inflammation with metabolic diseases and implicates molecular mediators as active participants in this process.14 Adipocytes appear to play a central role in the dyslipidemia, hypertension, insulin resistance, and prothrombotic/proinflammatory states of the metabolic syndrome.15–18 Adiposopathy has also been directly linked to endothelial dysfunction and promotion of atherosclerosis.4 Bariatric surgery may ameliorate cardiovascular disease risk through a partial recovery from adiposopathy (improved adiponectin and leptin/adiponectin ratio).19 Much of the prior knowledge relating these mediators to clinical parameters is based on serum samples.20–22 Our findings add to the growing pool of knowledge about adipose as a distinct tissue type and specifically to adipose in patients with advanced medical conditions such as diabetes and peripheral vascular disease necessitating major amputation.
Subcutaneous adipose is negatively correlated with atherogenic metabolic risk factors, but little is understood about differences between subcutaneous depots across different patient groups when stratified by disease profile.23 Our study is among the first to define the differences in this regard and to establish the pro-inflammatory nature of subcutaneous adipose tissue in a patient cohort with advanced limb ischemia.
Major amputation patients displayed upregulation of proinflammatory cytokines IL-6, IL-8, PAI-1, and resistin, and downregulation of the adipose-derived hormone leptin. Our findings are consistent with a previous study showing higher PAI-1, IL-6, and IL-8 levels in subcutaneous adipose tissue of patients with metabolic syndrome as compared with controls.7 IL-6 plasma levels drawn from the portal vein have been demonstrated to be higher than radial artery levels in obese individuals undergoing open gastric bypass surgery, suggesting increased secretion by visceral fat depots which drain into the portal circulation of the liver24, and increased circulating concentrations of IL-6 have been shown to correlate with increased incidence of diabetes mellitus and cardiovascular disease.25 Plasma IL-8 has been shown to be an independent predictor of cardiovascular events in patients with stable coronary artery disease26, and subcutaneous adipose tissue expression has been demonstrated to decrease significantly after gastric bypass surgery.27 Additionally, PAI-1 shows evidence of higher expression in subcutaneous adipose tissue in patients with cardiovascular disease than in patients without.28
Analysis of adipocyte-derived mediators in adipose tissue and comparison with clinical data yielded a number of interesting insights. We discovered that a number of clinical parameters, including age, male sex, hyperlipidemia, congestive heart failure, cerebrovascular disease, renal disease, antiplatelet therapy, warfarin, calcium channel blocker, beta blocker, statins, and insulin use are predictive of the adipose associated mediators. Other factors including obesity, race, diabetes mellitus, hypertension, coronary artery disease, pulmonary disease, smoking history, ARB/ACE inhibitors, and steroid use did not predict adipose phenotype under the conditions of this cross-sectional study. The findings reported reflect the single time point end-product of a number of adipose phenotype determinants, and further work is required to individually isolate the impact of these factors. Although the relationship between visceral adipose mass and metabolic syndrome has been well established, it has also been demonstrated that adipose phenotype is not necessarily predicted by adipose volume alone, which is in our agreement with our finding that BMI was not predictive.29 Prior research has shown that obese individuals without an inflamed adipose phenotype display a more favorable clinical profile; we did not stratify our groups by inflamed/non-inflamed phenotype, but rather looked at each cohort as a whole.30
Clear limitations to the dataset and research strategy are acknowledged. We hypothesized that certain clinical conditions would independently drive elevated adipose inflammation. While we define clear associations, it is possible that the adiposopathy may be an underlying cause of some diseases. Our study included a limited number of patients, and the amputation and control cohorts were not perfectly matched for a number of clinical characteristics. Certainly some of the amputation cohort specimens may have had confounding tissue-level ischemia, but we strived to harvest adipose from the proximal, better perfused portion of the limb at which normal wound healing was expected to occur. Furthermore, the adipose tissue analysis employed here does not indicate whether the phenotypic signatures observed were due to, causative, or merely associated with of the numerous medical conditions present in the patient cohorts. The results are essentially the net effect of a number of factors, some of which may be competing. Similarly, the correlations that we found between clinical parameters and tissue biomarker levels also do not necessarily imply a cause-and-effect relationship. Finally, we did not assay serum biomarkers, which are known to mediate biologic events, and cannot comment on the exact relationship between tissue and circulating levels. However, the relationship of circulating mediator levels with clinical parameters has been previously reported.21, 22, 31, 32
Conclusion
In conclusion, this work establishes important BMI-independent inflammatory phenotypic differences between subcutaneous adipose tissue in patients with end-stage peripheral vascular disease undergoing major amputation and a control patient cohort. As adipose biology is increasing linked to surgical diseases and outcomes, adipose phenotypic differentiation represents a fertile field for accelerated exploration.
Acknowledgments
Sources of funding: Supported by the National Heart, Lung, and Blood Institute (R01HL079135, 1R01HL079135-06S1, T32HL007734), American Heart Association (12GRNT9510001), and the Carl and Ruth Shapiro Family Foundation
Footnotes
Disclosures: None of the authors hold any commercial affiliations (including consultancies, stock or equity interests, and patent-licensing arrangements) that are a conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaldakov GN, Stankulov IS, Hristova M, et al. Adipobiology of disease: adipokines and adipokine-targeted pharmacology. Curr Pharm Des. 2003;9:1023–31. doi: 10.2174/1381612033455152. [DOI] [PubMed] [Google Scholar]
- 2.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 4.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Roubicek T, Bartlova M, Krajickova J, et al. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–8. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Ikeoka DT, Pachler C, Mader JK, et al. Lipid-Heparin Infusion Suppresses the IL-10 Response to Trauma in Subcutaneous Adipose Tissue in Humans. Obesity (Silver Spring) 2011;19:715–21. doi: 10.1038/oby.2010.227. [DOI] [PubMed] [Google Scholar]
- 7.Bremer AA, Devaraj S, Afify A, et al. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1782–8. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witasp A, Carrero JJ, Heimburger O, et al. Increased expression of pro-inflammatory genes in abdominal subcutaneous fat in advanced chronic kidney disease patients. J Intern Med. 2011;269:410–9. doi: 10.1111/j.1365-2796.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 9.Teplan V, Jr, Vyhnanek F, Gurlich R, et al. Increased proinflammatory cytokine production in adipose tissue of obese patients with chronic kidney disease. Wien Klin Wochenschr. 2010;122:466–73. doi: 10.1007/s00508-010-1409-y. [DOI] [PubMed] [Google Scholar]
- 10.Lim TS, Finlayson A, Thorpe JM, et al. Outcomes of a contemporary amputation series. ANZJ Surg. 2006;76:300–5. doi: 10.1111/j.1445-2197.2006.03715.x. [DOI] [PubMed] [Google Scholar]
- 11.Keagy BA, Schwartz JA, Kotb M, et al. Lower extremity amputation: the control series. J VascSurg. 1986;4:321–6. [PubMed] [Google Scholar]
- 12.Cruz CP, Eidt JF, Capps C, et al. Major lower extremity amputations at a Veterans Affairs hospital. AmJ Surg. 2003;186:449–54. doi: 10.1016/j.amjsurg.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Hasanadka R, McLafferty RB, Moore CJ, et al. Predictors of wound complications following major amputation for critical limb ischemia. J Vasc Surg. 2011;54:1374–82. doi: 10.1016/j.jvs.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 15.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54:819–31. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bays H. Adiposopathy, metabolic syndrome, quantum physics, general relativity, chaos and the Theory of Everything. Expert Rev Cardiovasc Ther. 2005;3:393–404. doi: 10.1586/14779072.3.3.393. [DOI] [PubMed] [Google Scholar]
- 17.Bays H. Adiposopathy: role of adipocyte factors in a new paradigm. Expert Rev Cardiovasc Ther. 2005;3:187–9. doi: 10.1586/14779072.3.2.187. [DOI] [PubMed] [Google Scholar]
- 18.Bays H, Abate N, Chandalia M. Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future Cardiol. 2005;1:39–59. doi: 10.1517/14796678.1.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Appachi S, Kelly KR, Schauer PR, et al. Reduced cardiovascular risk following bariatric surgeries is related to a partial recovery from “adiposopathy”. Obes Surg. 2011;21:1928–36. doi: 10.1007/s11695-011-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Luis DA, Aller R, Izaola O, et al. The serum profile of adipokines in naive patients with diabetes mellitus type 2 and obesity. J Clin Lab Anal. 2011;25:409–13. doi: 10.1002/jcla.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faber DR, Moll FL, Vink A, et al. Adipose tissue quantity and composition contribute to adipokine concentrations in the subclavian vein and the inferior mesenteric vein. Int J Obes (Lond) doi: 10.1038/ijo.2011.214. [DOI] [PubMed] [Google Scholar]
- 22.Stepien M, Rosniak-Bak K, Paradowski M, et al. Waist circumference, ghrelin and selected adipose tissue-derived adipokines as predictors of insulin resistance in obese patients: Preliminary results. Med Sci Monit. 17:PR13–8. doi: 10.12659/MSM.882030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–73. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 24.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 25.Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Komoda H, Nonaka M, et al. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol. 2008;124:319–25. doi: 10.1016/j.ijcard.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Alvehus M, Simonyte K, Andersson T, et al. Adipose tissue IL-8 is increased in normal weight women after menopause and reduced after gastric bypass surgery in obese women. Clin Endocrinol (Oxf) 2012 doi: 10.1111/j.1365-2265.2011.04322.x. [DOI] [PubMed] [Google Scholar]
- 28.Weiss TW, Seljeflot I, Hjerkinn EM, et al. Adipose tissue pro-inflammatory gene expression is associated with cardiovascular disease. Int J Clin Pract. 2011;65:939–44. doi: 10.1111/j.1742-1241.2011.02717.x. [DOI] [PubMed] [Google Scholar]
- 29.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 30.Farb MG, Bigornia S, Mott M, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 58:232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens CD, Kim JM, Hevelone ND, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. J Vasc Surg. 2010;51:1152–9. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlowska L, Rydzewski A, Fiderkiewicz B, et al. Adiponectin, resistin and leptin response to dietary intervention in diabetic nephropathy. J Ren Nutr. 2010;20:255–62. doi: 10.1053/j.jrn.2010.01.009. [DOI] [PubMed] [Google Scholar]