Abstract
BALB/c IL-2-deficient (IL-2-KO) mice develop systemic autoimmunity, dying within 3 to 5 weeks from complications of autoimmune hemolytic anemia. Disease in these mice is Th1-mediated, and IFNγ production is required for early autoimmunity. In this study we show that dendritic cells (DC) are required for optimal IFNγ production by T cells in the IL-2-KO mouse. Disease is marked by DC accumulation, activation and elevated production of Th1-inducing cytokines. IL-2-KO DCs induce heightened proliferation and cytokine production by naïve T cells compared to wild-type DCs. The depletion of either conventional or plasmacytoid DCs significantly prolongs the survival of IL-2-KO mice, demonstrating that DCs contribute to the progression of autoimmunity. Elimination of Th1 inducing cytokine signals, type 1 interferon and IL-12 reduce red blood cell-specific antibody production and augments survival, indicating that cytokines derived from both plasmacytoid DCs and conventional DCs contribute to disease severity. DC activation likely precedes T cell activation as DCs are functionally activated even in an environment lacking overt T cell activation. These data indicate that both conventional and plasmacytoid DCs are critical regulators in the development of this systemic antibody-mediated autoimmune disease, in large part through production of IL-12 and type 1 interferons.
Keywords: autoimmune hemolytic anemia, dendritic cell, interleukin-2-deficient, interleukin-12, type 1 interferon
Introduction
Autoimmune hemolytic anemia (AIHA) is an autoimmune disease caused by the production of antibodies to red blood cells (RBCs). Interleukin-2-deficient (IL-2-KO) mice on the BALB/C background develop systemic autoimmune disease, dying between 3 to 5 weeks from complications of AIHA (1). IL-2 was traditionally thought to be a lymphocyte-activating and immune-stimulatory factor; however it is now known that IL-2 is also required for the survival and function of regulatory T cells (Tregs; (2–4)). The major known defect in IL-2-KO mice is a reduction in the percentage and functionality of peripheral Tregs that require IL-2 for their survival and optimal suppressive abilities.
Activation of helper CD4+ T cells is critical to autoimmune pathology in IL-2-KO mice (1, 5). We have previously demonstrated that IFNγ is required for production of the autoantibodies, with IL-2xIFNγ-KO mice surviving significantly longer than their IL-2-KO counterparts (6). IFNγ-producing Th1 cells play a pathogenic role in many autoimmune diseases. In addition to stimulating class switching to IgG isotypes (7), IFNγ stimulates the phagocytic function of macrophages and increases macrophage and dendritic cell (DC) responsiveness to other inflammatory signals, such as enhanced type 1 interferon (T1 IFN) responses (8). IFNγ can also auto-amplify the Th1 response.
Interactions between APCs and T cells are critical to the initiation of an immune response and differentiation of effector T cells. DCs as professional APCs are essential for initiating T-dependent immune responses, and are pivotal to the determination of tolerance versus immune activation (9, 10). These APCs promote inflammation and development of effectors through direct cell-cell interactions involving B7 (CD80/CD86) and CD40 signals, and non-contact mediated secretion of cytokines. DCs produce several cytokines that promote Th1 differentiation and IFNγ responses by CD4+ T cells. DC-derived IL-12, IL-18, IL-27, TNFα and T1 IFNs are all capable of driving Th1 responses, as is IFNγ itself. Conventional DCs (cDC) primarily produce IL-12, while T1 IFNs (α/β) are secreted at very high levels by plasmacytoid DCs (pDC; (11–13)).
Due to their critical role in initiating cell-fate decisions that balance tolerance and immune activation, it is perhaps not surprising that recent studies have highlighted the importance of DCs during abnormal immune responses and autoimmune manifestations. Alterations in antigen presentation, cytokine secretion, maturation, activation state and migration patterns of DCs have been shown to play a role in the development of a variety of autoimmune disease (14–16).
In this study we set out to define the role of DCs in the development of a spontaneous autoantibody-mediated autoimmune disease that is not dependent on immunization. Our results demonstrate that in the IL-2-KO mouse cDCs and pDCs induce IFNγ production by CD4+ T cells and drive the autoimmune pathology. These abnormal DC functions play a role in initiating the autoimmune response, and are not a consequence of aberrant T cell activation. Clarifying the role of DCs in the pathogenesis of autoimmunity will improve our understanding of disease and may allow us to stop disease progression by specifically targeting these aberrant DC populations.
Materials and methods
Mice
All mice were used on the BALB/c background. IL-2-KO mice were backcrossed in our laboratory (backcrossed for >10 generations onto the BALB/c background; The Jackson Laboratory). Transgenic mice expressing the DO11.10 TCR specific for the chicken OVA323–339 peptide mice were obtained from Dr. K. Murphy (Washington University, St. Louis, MO). CD28-KO mice were obtained from Dr. J. Bluestone (University of California, San Francisco, CA). IL-12p40-KO and CD11c-DTR transgenic mice were obtained from The Jackson Laboratory. B7.1/B7.2-deficient mice (B7-KO) were obtained from Dr. A. Sharpe (Harvard Medical School, Boston, MA), B cell-deficient (Jhd) mice were obtained from Dr. D. Huszar (GenPharm International, Mountain View, CA), and IFNAR1-KO mice were obtained from Dr. A. Mellor (Georgia Health Sciences University, Augusta, GA). All mice were bred and maintained in a specific pathogen-free animal barrier facility in accordance with the guidelines of the Laboratory Animal Resource Center of the University of California San Francisco.
Antibody treatment
pDC depletion was performed by i.p. inject of 20 µg PDCA-1 antibody per gram weight three times per week beginning on day 8 (clone mAb927 (17) provided by Dr. M. Colonna, Washington University School of Medicine, St. Louis, MO). cDC depletion was performed by i.p. injection of diphtheria toxin into CD11c-DTR transgenic mice CD11c-DTR transgenic mice at 9, 11 and 13 days of age (3 ng/gram weight on day 9; 4 ng/gram weight on days 11 and 13). Anti-IFNAR1 mAb (clone MAR1-5A3 (18) generously provided by Dr. R. Schreiber, Washington University School of Medicine, St. Louis, MO) was administered by i.p injection at 20 µg per gram weight three times per week beginning on day 8.
Staining and Flow cytometry
Splenocytes and lymphocytes were stained with FITC, PE, PerCp, APC conjugated antibodies or tandem fluorochrome antibodies following Fc-block (anti-CD16/CD32). For intracellular cytokine staining, cells were stimulated for 4–6 h (at 37 degrees C), treated with Brefeldin A (10 µg/ml) for the final 2 h, then fixed and permeabilized as previously described (19). All antibodies were purchased from BD Biosciences or eBioscience. Flow cytometry was performed on a FACSCalibur or LSRFortessa (BD, San Jose, CA) and data analyzed using FCS Express with Diva (DeNovo Software, Los Angeles, CA).
Cell preparations, purifications, stimulations and adoptive transfers
After injection with 4000 U/ml collagenase D, spleens were teased apart, incubated for 30 min at 37 degrees C, then pressed through a nylon mesh filter and subjected to hypotonic RBC lysis. DCs were enriched using the EasySep CD11c-PE positive selection kit (Stem Cell Technologies, Vancouver, B.C., Canada) according to product insert. CD11chi (cDC) or CD11clo/PDCA-1+ (pDC) cells were then isolated to 95% purity using a MoFlo cell sorter (DakoCytomation, Carpinteria, CA). For in vitro proliferation assays, DO11.10 CD4+ T cells were purified from spleen and lymph nodes using the Mouse CD4+ T cell enrichment kit (Stem Cell Technologies). 1×105 CD4+ T cells were co-cultured with purified DCs at 5:1, 10:1 or 20:1 (T cell : DC ratio). T cells were stimulated with 0.01 µg/ml, 0.1 µg/ml and 1 µg/ml OVA peptide (323–339) for 3 to 5 days and proliferation measured by CFSE dilution. For cytokine measurements, DCs were stimulated with 1 µg/mL LPS (cDC) or 5 µg/mL CpG (pDC) for 24 h. For in vivo stimulations, 5×105 pDCs or 1×106 cDCs were transferred intravenously into recipient mice.
Complete blood counts
Cardiac punctures were performed immediately following cervical dislocation, and blood drawn into heparinized microhematocrit tubes (Terumo, Elkton, MD). For survival studies, blood was collected from the tail vein. Complete blood counts (including erythrocyte and white blood cell counts, hematocrit percentages and hemoglobin values) were then evaluated using a Hemavet 950FS or Hemavet 850 (Drew Scientific, Dallas, TX).
RBC antibody detection
Serum erythrocyte antibody levels were detected using flow cytometry as previously described (20). Erythrocytes were freshly isolated from young wild-type or IL-2-KO mice by tail vein or terminal bleed and washed three times in cold PBS. Cells were then incubated with anti-murine IgM-FITC (1:150 dilution; 4°C) or IgG-FITC (1:50 dilution; at 37°C; Jackson ImmunoResearch, West Grove, PA). The percentage of erythrocytes bound by antibody was determined by flow cytometry.
Real-time PCR
Total RNA was isolated from sorted DCs following stimulation using RNeasy micro kits (Invitrogen, Carlsbad, CA), then reverse transcribed to cDNA using Superscript III First-Strand Synthesis kit (QIAGEN, Valencia, CA). cDNA was quantified on a NanoDrop (Thermo Scientific, Wilmington, DE) and amplified by real-time PCR using SYBR-green PCR Master Mix (Applied Biosystems, Carlsbad, CA) on an iQ5 Real-Time PCR Thermal Cycler (Bio-Rad, Hercules, CA). Ct values were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) levels and fold induction shown relative to controls isolated from WT BALB/c mice. Primer sequences: IL-10 (fwd 5’AGTGGAGCAGGTGAAGAGTGA; rev 5’CGAGGTTTTCCAAGGAGTTG), TNFα (fwd 5’TGGCCTCCCTCTCATCAGTT; rev 5’- TCCTCCACTTGGTGGTTTGC), IL-27p28 (fwd 5’CAGGTGACAGGAGACCTTGG; rev 5’TGGCAAGGTACAGGCTGACT), BAFF (fwd 5’GCCGCCATTCTCAACATGAT; rev 5’TTAGGGCACCAAAGAAGGTG), IL-6 (fwd 5’TAGTCCTTCCTACCCCAATTTCC; rev 5’TTGGTCCTTAGCCACTCCTTC), IL-12p35 (fwd 5’GCCAGGTGTCTTAGCCAGTC; rev 5’GCTCCCTCTTGTTGTGGAAG), IL-12p40 (fwd 5’TCTGAGCCACTCACATCTGC; rev 5’TCAGGGGAACTGCTACTGCT), IL-18 (fwd 5’ACTTTGGCCGACTTCACTGT; rev 5’CAGAGAGGGTCACAGCCAGT). Primer sequences are described elsewhere for HPRT (21).
ELISA
IFNα production following stimulation was measured using the Verikine Mouse Interferon-alpha ELISA kit according to the manufacturer’s instructions (pbl interferon source, Piscataway, NJ). Immunoglobulin ELISAs were performed by standard methods. Briefly, 96-well microtiter plates were incubated overnight at 4°C with 2 µg/ml anti-mouse Ig (H&L) Ab (Pharmingen, San Diego, CA) in PBS. The plates were washed three times with PBS/0.5% BSA/0.1% Tween 20 and blocked for 1 h, and then samples of mouse serum were added in duplicate at increasing serial dilutions. After 2 h, the plates were washed, and AP-linked anti-IgM or anti-IgG2a Ab was added for 1 h. Finally, wells were washed and incubated with p-nitrophenol phosphate substrate (Sigma-Aldrich, St. Louis, MO), and absorbance was determined with an ELISA plate reader (Molecular Devices, Sunnyvale, CA) at 405 nm. The immunoglobulin concentrations were calculated by comparison against a standard curve of serially diluted IgM or IgG2a.
Ex vivo expansion of Tregs
CD4+CD25+Foxp3+ Tregs were purified to 99% purity from spleen and peripheral LN cells of Foxp3GFP+ mice by flow-cytometric cell sorting. Purified Tregs were cultured for 8–12 days in the presence of anti-CD3 and anti-CD28-coated 4.5-µm paramagnetic microbeads (Xcyte Therapies, Carlsbad, CA) and recombinant human IL-2 (2000 U/ml; Chiron Corp., Emeryville, CA) for six days as previously described (22). Expanded Tregs were depleted of Xcyte beads using a EasySep magnet and washed three times in PBS immediately prior to in vivo injection.
Statistical analysis
Statistical differences between experimental groups were determined by paired t test using Prism software (GraphPad, La Jolla, CA). Bar graphs indicate means; Error bars indicate standard deviation.
Results
Antigen presenting cells, but not B cells, are required for T cell activation during autoimmunity
We have previously demonstrated that IFNγ is required for the early autoimmunity that develops in IL-2-deficient mice (6). Therefore, it is important to determine what is driving IFNγ production in the absence of IL-2. Since APCs are a critical component in the generation of Th1 cells, we first asked if APCs are integral in promoting IFNγ production by CD4+ T cells in the IL-2-KO mouse. To address this we utilized B7-KO mice to evaluate the importance of APC costimulation to the activation of CD4+ T cells and IFNγ production during disease. T cell activation in B7-KO mice is defective due to the inability of the APCs to costimulate the T cells through CD28 (23, 24)), and lymphocyte numbers are normal in these animals (25). IL-2xB7-KO mice also have normal B and T cell numbers and do not develop early lethal AIHA (data not shown).
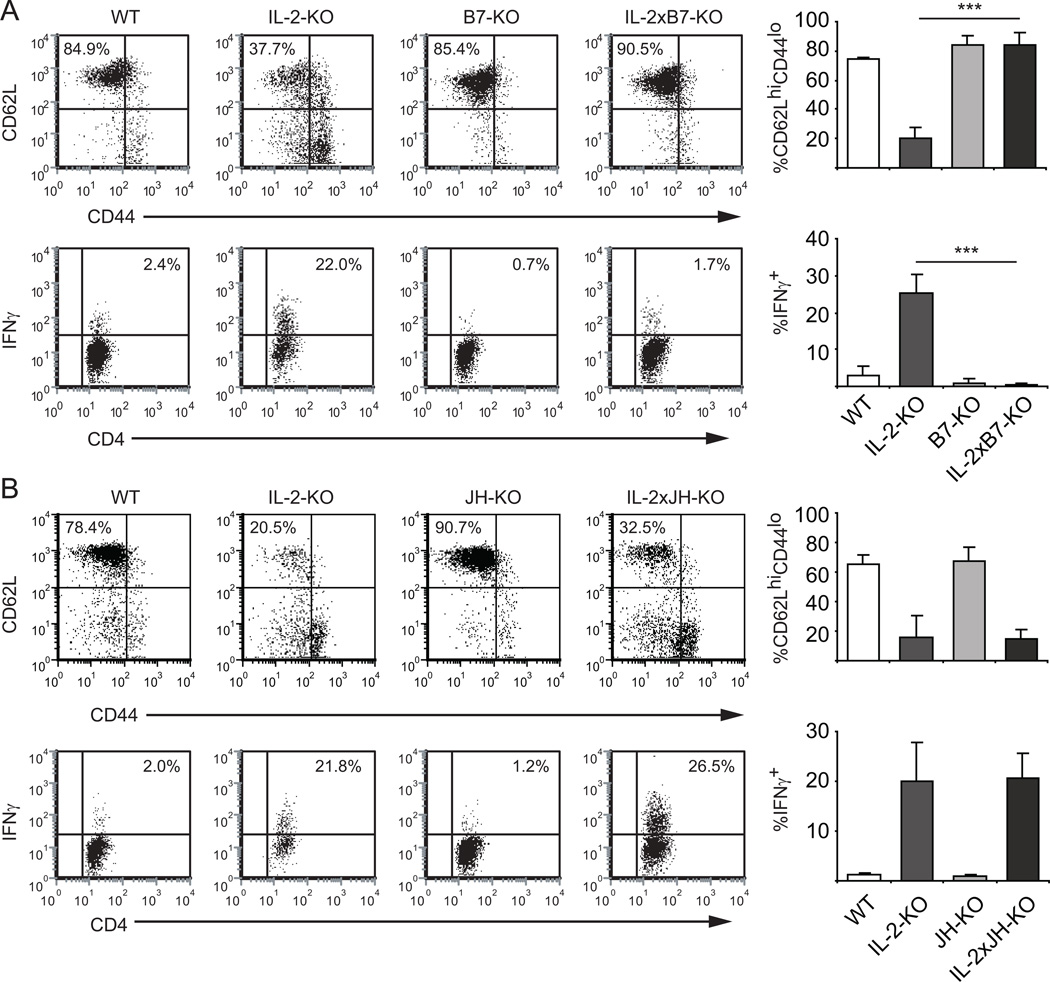
Comparing IL-2-KO to IL-2xB7-KO mice, we found that IL-2xB7-KO CD4+ T cells remained in a naïve state (CD62LhiCD44lo), while IL-2-KO CD4+ T cells expressed decreased CD62L and elevated CD44 indicating activation (Figure 1A). To assess the ability of APCs from these mice to induce Th1 differentiation, CD4+ T cells were restimulated ex vivo and stained for IFNγ. IL-2-KO CD4+ T cells exhibited increased IFNγ production relative to WT CD4+ T cells, while the IL-2xB7-KO CD4+ T cells did not produce elevated IFNγ. Thus, in the absence of costimulation by APCs, IL-2-KO CD4+ T cells are not activated and do not produce IFNγ, indicating that APC:T cell interactions through B7 are necessary for Th1 differentiation and IFNγ production in the absence of IL-2.
Figure 1. Stimulation with APCs, but not B cells, is necessary for IFNγ production by CD4+ T cells.
(A) Elimination of B7 costimulatory proteins. (B) Elimination of peripheral B cells. Top panels: Surface expression of CD44 and CD62L on LN cells from 3–4 week old mice was analyzed by flow cytometry. Lower Panels: LN cells were stimulated with 70 ng/ml PMA and 700 ng/ml ionomycin for 5 hours, and cytokine production by CD4+ T cells was measured by intracellular cytokine staining. n=3 mice from three experiments. Bar graphs indicate cumulative data from three experiments. ***, p<0.001.
Since either DCs or B cells could be the APC population promoting IFNγ production through B7, we next asked whether B cells are required for the generation of IFNγ producing CD4+ T cells using the IL-2xJH-KO mouse, which does not develop mature peripheral B cells (26). IL-2-KO CD4+ T cells from these B cell-deficient mice still become activated, as assessed by upregulation of CD44 and downregulation of CD62L. Both IL-2-KO and IL-2xJH-KO CD4+ T cells exhibited similar elevated production of IFNγ following ex vivo restimulation, suggesting that B cells are not required for IFNγ production (Figure 1B). Although the IL-2xJH-KO CD4+ T cells are activated, expanded and produce elevated IFNγ, these mice do not succumb to the early, lethal antibody-mediated autoimmunity (i.e. AIHA) due to the absence of B cells and thus the absence of autoantibodies (data not shown). As further evidence that B cells are not required for Th1-differentiation and cytokine production during this autoimmune disease, adoptive transfer of IL-2-KO B cells into IL-2xB7-KO recipients did not drive IFNγ production by endogenous T cells (data not shown). These data indicate that APC interactions are necessary for T cell activation and Th1 differentiation during autoimmune disease development, but B cells are not necessary for these effects. These data further indicate that other APC populations, presumably DCs, are essential in driving Th1 differentiation and T cell activation in the IL-2-KO setting of autoimmunity.
In the absence of IL-2, DCs are expanded, activated and exhibit a mature phenotype
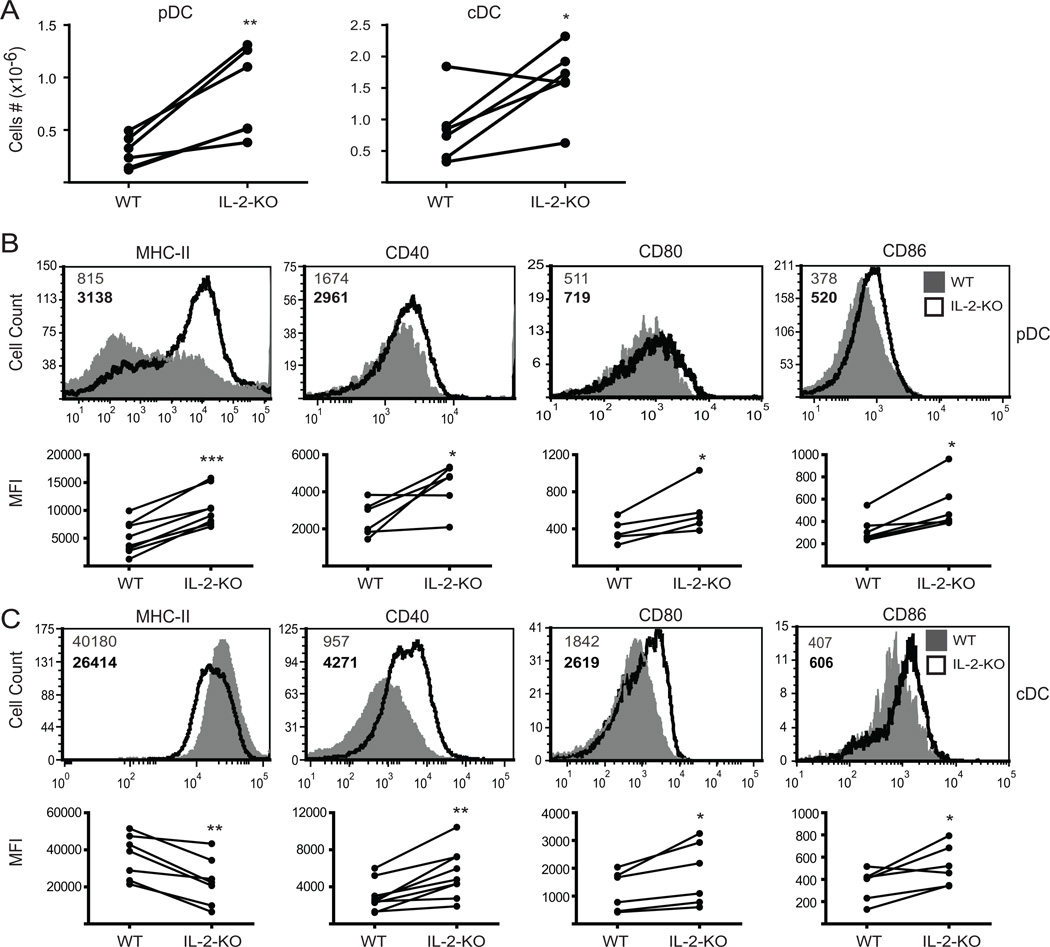
Since B cells are not the source of the APC-derived B7 signals important in priming IL-2-KO CD4+ T cells during autoimmunity, we next evaluated DC numbers and phenotype during disease development to determine whether these cells are dysregulated. Splenic plasmacytoid DC (pDC; Thy1.2−CDllclow B220+PDCA-1+) numbers were increased by 3-fold, and conventional DC (cDC; Thy1.2−CD11chiB220−) numbers by about 2-fold in the absence of IL-2 at 3 weeks of age compared to WT DCs (Figure 2A). IL-2-KO pDCs expressed increased levels of surface MHC-II, CD40, CD80 and CD86, indicating a more mature, activated phenotype compared to WT. cDCs had decreased MHC-II expression, but increased CD40, CD80 and CD86 expression as compared to WT. This suggests that IL-2-KO cDCs may be better at T cell activation compared to WT cDCs, while still efficiently taking up antigen. Thus, in the absence of IL-2, the two major splenic DC populations are expanded and activated.
Figure 2. DCs are activated and expanded in the absence of IL-2.
Collagenase-treated splenocytes from 18–24 day old WT or IL-2-KO mice were stained and analyzed by flow cytometry. (A) Absolute splenic DC numbers shown as column graphs of 6 individual mice from at least four experiments. (B) Top: FACS profiles of pDCs gated on Thy1.2−CD11clo B220+PDCA-1+ cells. Bottom: Mean fluorescent intensity (MFI) from at least five individual mice. (C) Top: FACS profiles of cDCs gated on Thy1.2−CD11chiB220− cells. Bottom: MFI from at least five individual mice. Numbers in B and C represent the MFI of WT cells (grey upper number) and IL-2-KO cells (bold lower number). Representative FACS data are from 6 individual mice and at least four experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
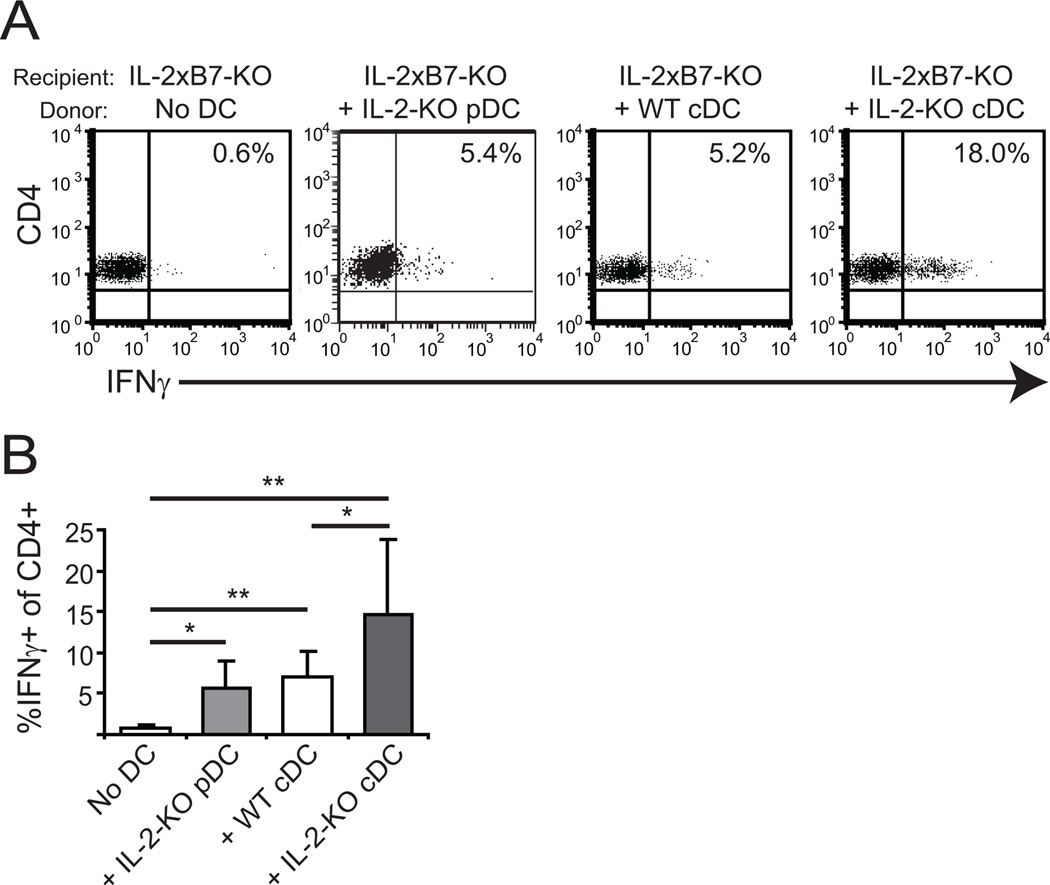
Since the DCs are phenotypically activated in the absence of IL-2, we next tested the functional capacity of the DCs in vivo. To do this, 5×105 pDCs or 1×106 cDCs were transferred intravenously into IL-2xB7-deficient mice and cytokine production by endogenous CD4+ T cells was assessed. We have previously shown that in such an experimental system, the APC function is provided entirely by the transferred DCs since endogenous APCs lack B7 (24). IL-2-KO cDCs were more efficient at driving IFNγ production than their WT counterparts (Figure 3). IL-2-KO pDCs also promoted IFNγ production, but less efficiently than cDCs. Neither DC population initiated anti-RBC antibody production or autoimmunity even after three injections of DCs (data not shown). This is likely because ongoing DC:T cell interactions are required for disease induction. These in vivo data suggest a role for IL-2-KO DCs in initiating production of IFNγ by CD4+ T cells during early activation leading to autoimmune disease.
Figure 3. IL-2-KO DCs are functionally activated and induce IFNγ production by CD4+ T cells.
1×106 WT or IL-2-KO cDCs or 5×105 pDCs were adoptively transferred into IL-2xB7-KO recipients by tail vein injection. Injected DCs were pooled from at least three mice. Six days later, splenocytes were isolated and stimulated ex vivo with 70 ng/ml PMA and 700 ng/ml ionomycin for 6 hours with BFA added during the final 2 hours. (A) Cells were gated on CD4 and analyzed for IFNγ production by flow cytometry. (B) Bar graphs show cumulative data from three individual recipient mice and at least two experiments. *, p<0.05; **, p<0.01.
Conventional and plasmacytoid DCs drive early spontaneous autoimmunity
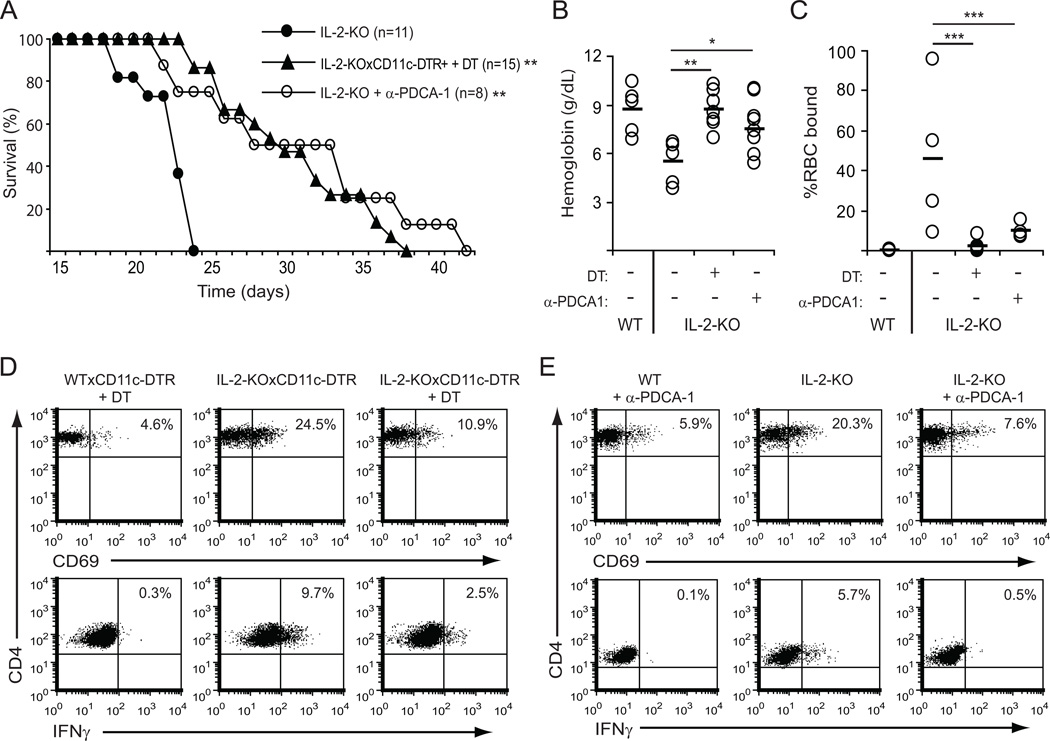
To directly address whether DCs are necessary for Th1 induction and autoimmunity in the IL-2-KO mouse, we specifically depleted cDC and/or pDC populations. cDCs were depleted using the CD11c-human diphtheria toxin receptor mouse (CD11c-DTR; (27)) crossed to the IL-2-KO mouse. Mice were injected with diphtheria toxin at 9, 11 and 13 days of age, allowing selective reduction of CD11chigh cells (cDCs) for 6 days. Despite such a short-term depletion of cDCs, lethal autoimmunity was delayed in IL-2-KO mice by up to two weeks. To deplete pDCs, IL-2-KO mice were treated with a monoclonal antibody to PDCA-1 (17). Elimination of pDCs also delayed the onset of autoimmunity by up to two weeks (Figure 4A). Depletion of either DC population in the IL-2-KO mice resulted in a significant increase in hemoglobin levels (Figure 4B) and a reduction in the percentage of RBCs bound by antibody relative to untreated IL-2-KO mice (Figure 4C). Depletion of both DC populations together had no additive effect on survival, anti-RBC antibody production or hemoglobin levels (data not shown). Finally, depletion of either cDCs or pDCs resulted in a significant reduction in CD4+ T cell activation (CD69 expression) and IFNγ production on day 15 (Figure 4D and 4E). Together, these data indicate that DCs are important for the initiation of early lethal autoimmunity in the absence of IL-2.
Figure 4. DC depletion reduces antibody production and prolongs survival of IL-2-KO mice.
Mice were treated with diphtheria toxin (DT; 3–4 ng/g weight) on day 9, 11 and 13, or with anti-PDCA-1 (20 ng/g weight) 3 times per week beginning on day 8. (A) Kaplan-Meier survival plots. (B) Hemoglobin levels (g/dL) were measured from peripheral blood on day 18. (C) RBCs were stained with anti-mouse IgM-FITC and analyzed by flow cytometry to detect bound anti-RBC antibodies. (D/E) Surface expression of CD69 on CD4+ splenocytes from 15-day-old mice treated with DT (D) or anti-PDCA-1 (E) was analyzed by flow cytometry, or splenocytes were stimulated with 70 ng/ml PMA and 700 ng/ml ionomycin for 5 hours, and IFNγ production by CD4+ T cells was measured by intracellular cytokine staining. Data in A, B, C are from at least four individual mice and four experiments. Each dot in B and C represents one mouse; horizontal bars indicate the mean. Representative FACS data in D and E are from 3 individual mice and three experiments. Calculated statistics are relative to IL-2-KO; *, p<0.05; **, p<0.01; ***, p<0.001.
IL-12 and type 1 interferon signaling contributes to autoimmune disease
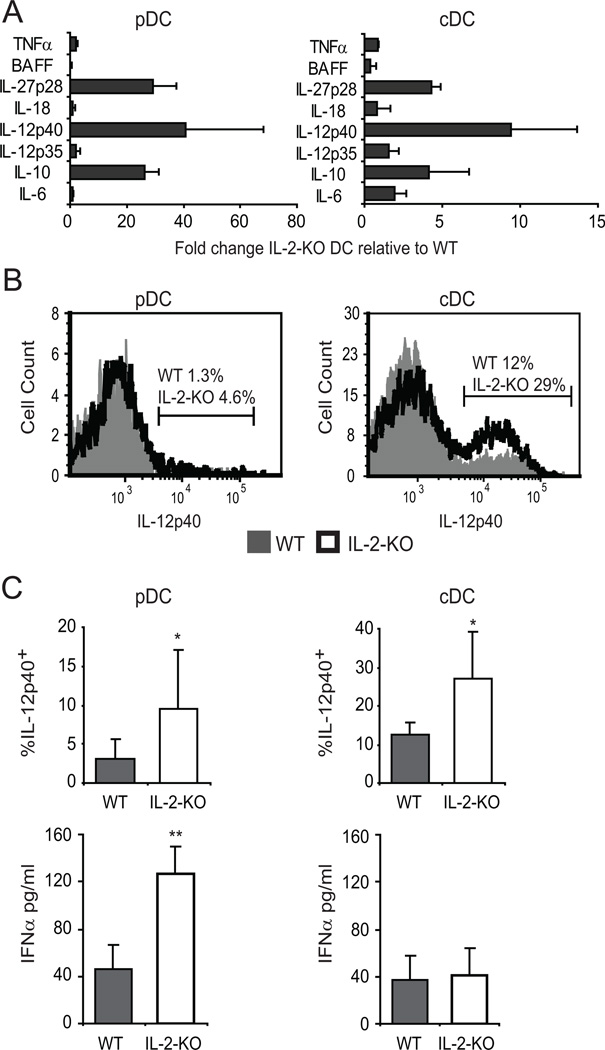
Since early autoimmunity in the IL-2-KO mouse is Th1-mediated and the Th1 response requires DC activation, we measured production of Th1-inducing cytokines by DCs. Sorted DCs were stimulated for 24 hours, and cytokine mRNA was analyzed by real-time PCR (Figure 5A). Cytokine mRNA for several Th1-inducing cytokines, including IL-12 and T1 IFN, was elevated in IL-2-KO DCs as compared to WT. IL-12 signaling is a strong inducer of IFNγ production, thus we sought to verify that production of IL-12 protein was also elevated. Both pDCs and cDCs from IL-2-KO mice produced higher levels of p40 (the cytokine subunit found in both IL-12 and IL-23) in response to LPS than did WT cDCs (Figure 5B). T1 IFNs are the principal cytokines produced by pDCs, and function in part to drive Th1-effector differentiation. IL-2-KO pDCs stimulated with CpG for 24 hours secreted three times more IFNα than did WT pDCs (Figure 5C). Thus, in the absence of IL-2, and upon stimulation, DCs produce elevated levels of cytokines known to induce Th1 differentiation of CD4+ T cells.
Figure 5. IL-2-KO DCs produce elevated levels of Th1-inducing cytokines.
DCs were sorted on a MoFlo from 18–23 day old mice, stimulated with 5 µg/ml CpG (pDC) or 1 µg/ml LPS (cDC) for 24 hours. (A) mRNA was isolated from the DCs and cytokine mRNA analyzed by real-time PCR. Data are from at least four experiments with DCs pooled from at least three mice per experiment. (B) IL-12/23p40 production was measured by intracellular staining. Bar graphs show cumulative data from three individual mice and three experiments (C) IFNα secretion in culture supernatants was measured by ELISA. Data are from three experiments with DCs sorted from at least three pooled mice per experiment. *, p<0.05; **, p<0.01.
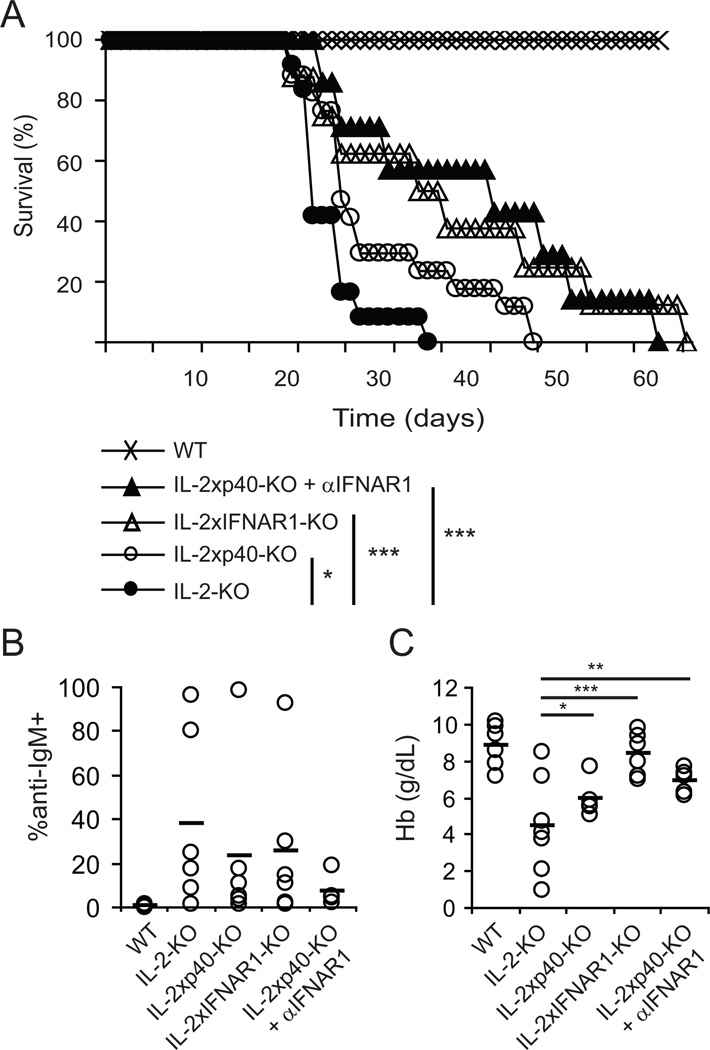
To determine whether these Th1-inducing cytokine signals from activated DC populations are important for early autoimmunity and elevated IFNγ production in the absence of IL-2, we eliminated IL-12 and T1 IFN signals. First, IL-2-KO mice were crossed with mice lacking IL-12p35 (IL-2xp35-KO) or IL-12p40 (IL-2xp40-KO; p40 is also a subunit of IL-23). Kaplan-Meier survival curves of IL-2-KO, IL-2xp35-KO and IL-2xp40-KO mice showed that elimination of IL-12 from IL-2-KO mice had a significant impact on autoimmune development and survival (Figure 6A and data not shown (IL-2xp35)). The mean percentage of RBCs coated with antibodies was decreased in the absence of IL-12/23 (36.8% for IL-2-KO RBCs and 23.0% for IL-2xp40-KO RBC), although this change was not significant (Figure 6B). In contrast, hemoglobin levels in IL-2-KO mice significantly increased with the elimination of IL-12 signaling, indicating a decrease in anemia (Figure 6C). This difference between RBC-directed antibodies and hemoglobin levels may indicate that, although self-antibodies had begun to form, RBC destruction by the immune system had not yet resulted in the loss of hemoglobin. Thus, IL-12 plays a role in the induction of autoimmunity in the absence of IL-2, as its elimination augments survival of a portion of IL-2-KO mice and delays RBC destruction.
Figure 6. Th1-inducing cytokines drive early autoimmunity and antibody production.
IL-2-KO, IL-2xp40-KO, IL-2xIFNAR1-KO, and IL-2xp40-KO mice treated with anti-IFNAR1 (20 µg/g weight from 8 to 24 days of age) were monitored for disease. (A) Kaplan-Meier survival curve. Individual mice from at least three experiments: WT=10, IL-2-KO=11, IL-2xp40=17, IL-2xIFNAR1=9, IL-2xp40 + anti-IFNARI=7. (B) RBCs from 18-day old mice were stained with anti-mouse IgM-FITC and analyzed by flow cytometry to detect bound anti-RBC antibodies. (C) Hemoglobin levels (g/dL) were measured from peripheral blood at 18 days of age. Data in B and C are from at least four individual mice and three experiments. Each dot represents one mouse; horizontal bars indicate the mean. Calculated statistics are relative to IL-2-KO; *, p<0.05; **, p<0.01; ***, p<0.001.
We next asked if T1 IFN signaling is important for the development of early autoimmunity in the absence of IL-2. To address this question, IL-2-KO mice were crossed with IFNAR1-KO mice and IL-2xp40-KO mice were treated with an anti-IFNAR1 antibody. Elimination of T1 IFN signals significantly delayed anemia and prolonged survival of IL-2-KO mice, even more than elimination of IL-12. Blockade of both IL-12 and T1 IFN signals had a small additional effect on the onset of autoimmunity compared to elimination of IL-12 alone (Figure 6A). Elimination of IL-12 and IFN signals reduced the percentage of RBCs bound by antibody (Figure 6B) and increased the hemoglobin levels (Figure 6C) compared to IL-2-KO mice. These data suggest that Th1-mediated induction of autoantibodies resulting in anemia and lethal autoimmunity is promoted in part by IL-12 and T1 IFN signals. Further, T1 IFN signals have a stronger influence on disease than do IL-12 signals. The incomplete protection provided by removing these signals suggests that other mechanisms may contribute to the genesis and/or progression of autoimmunity.
Abnormal DC function is partially independent of T cell activation
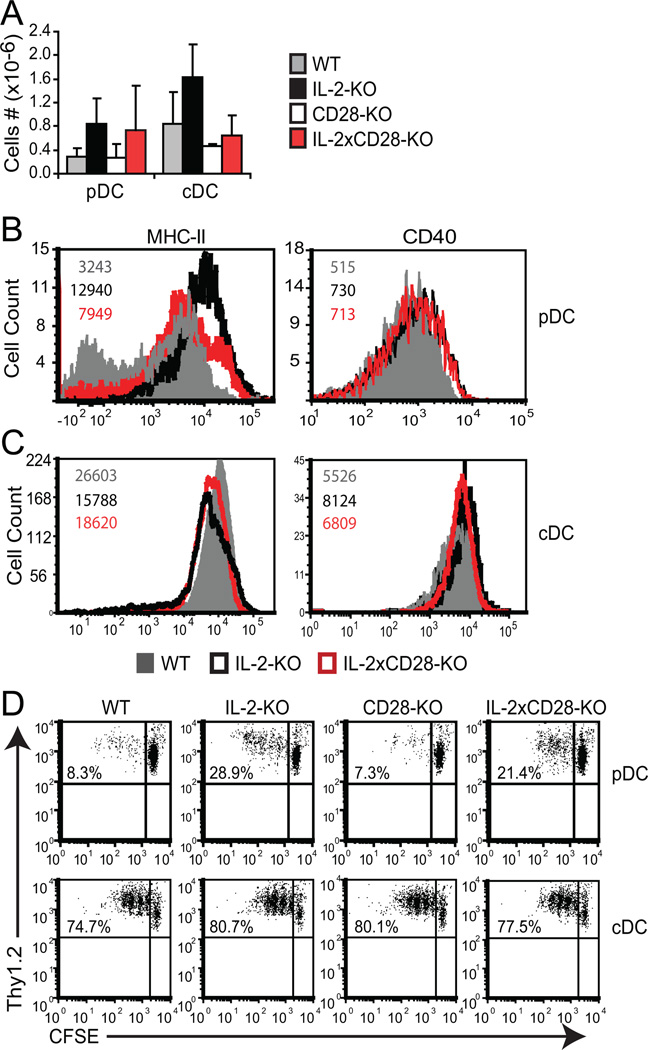
It is possible that activation of DCs is a consequence of uncontrolled T cell responses in IL-2-KO mice. To determine if T cell activation is required for the altered DC phenotype, we utilized IL-2xCD28-KO mice, which demonstrate no overt T cell expansion or activation at 3–4 weeks of age and do not develop AIHA (5). pDC numbers in IL-2xCD28-KO mice were expanded by 3 fold relative to WT pDC, similar to abnormally high IL-2-KO pDC numbers (Figure 7A). cDC numbers from a couple of IL-2xCD28-KO mice were mildly elevated, but combined, the data was not significant. Phenotypic analyses showed that CD40 and MHC-II expression on IL-2xCD28-KO DCs was altered compared to WT mice, but generally less so than in the IL-2-KO mice (Figure 7B, 7C). Thus, in the absence of T cell activation (CD28-KO), IL-2-KO pDCs and cDCs demonstrate an intermediate activation state between that of WT and IL-2-KO DCs. These data indicate that the initial activation and maturation of DCs in IL-2-KO mice is partially independent of T cell activation and effector function.
Figure 7. DC dysregulation is partially independent of T cell activation.
Collagenase-treated splenocytes were isolated from 18–24 day old WT, IL-2-KO, CD28-KO and IL-2xCD28-KO mice, stained and analyzed by flow cytometry. (A) Absolute splenic DC numbers shown as bar graphs representing the mean +/− SD of at least 4 individual mice from four experiments. (B) FACS profiles of pDCs gated on Thy1.2−CD11cloB220+PDCA-1+ cells. (C) FACS profiles of cDCs gated on Thy1.2−CD11chiB220− cells. Numbers in B and C represent the MFI of WT cells (grey upper number), IL-2-KO cells (bold middle number) and IL-2xCD28-KO cells (lower red number). Representative FACS data are from three independent experiments and at least three individual mice. (D) 1×105 sorted naïve DO11.10 (KJ+Thy1.2+) CD4+ T cells were co-cultured with 1×104 sorted, pooled DCs in the presence of 0.1 µg/ml OVA peptide (323–339) for 3 (cDC) or 5 (pDC) days and proliferation measured by CFSE dilution. Representative FACS data are from three independent experiments using pooled DCs from at least three mice per experiment.
To evaluate DC functionality in the absence of overt T cell activation, DCs from IL-2-KO and IL-2xCD28-KO mice were compared for their ability to stimulate proliferation of naïve T cells. DO11.10 CD4+ T cells were co-cultured with sorted DCs (at a 5:1, 10:1 or 20:1 ratio) in the presence of OVA peptide for 3 days (cDC) or 5 days (pDC) and proliferation measured by CFSE dilution. DO11.10 CD4+ T cells displayed similar levels of proliferation in the presence of each cDC population (Figure 7D). We have also assessed proliferation of WT and IL-2-KO cDCs at various doses of AVA peptide with similar proliferation results (Supplemental Figure 1). In contrast, DO11.10 CD4+ T cells proliferated to a greater extent when stimulated by IL-2-KO or IL-2xCD28-KO pDCs than when stimulated by WT or CD28-KO pDCs. These data indicate that the functional state of pDCs is independent of aberrant T cell activity, while that of cDCs appears to be dependent on T cell activation. These data further suggest that increased pDC maturation and function in IL-2-KO mice occurs prior to and largely independent of T cell alterations.
Regulatory T cells can control plasmacytoid DC activation
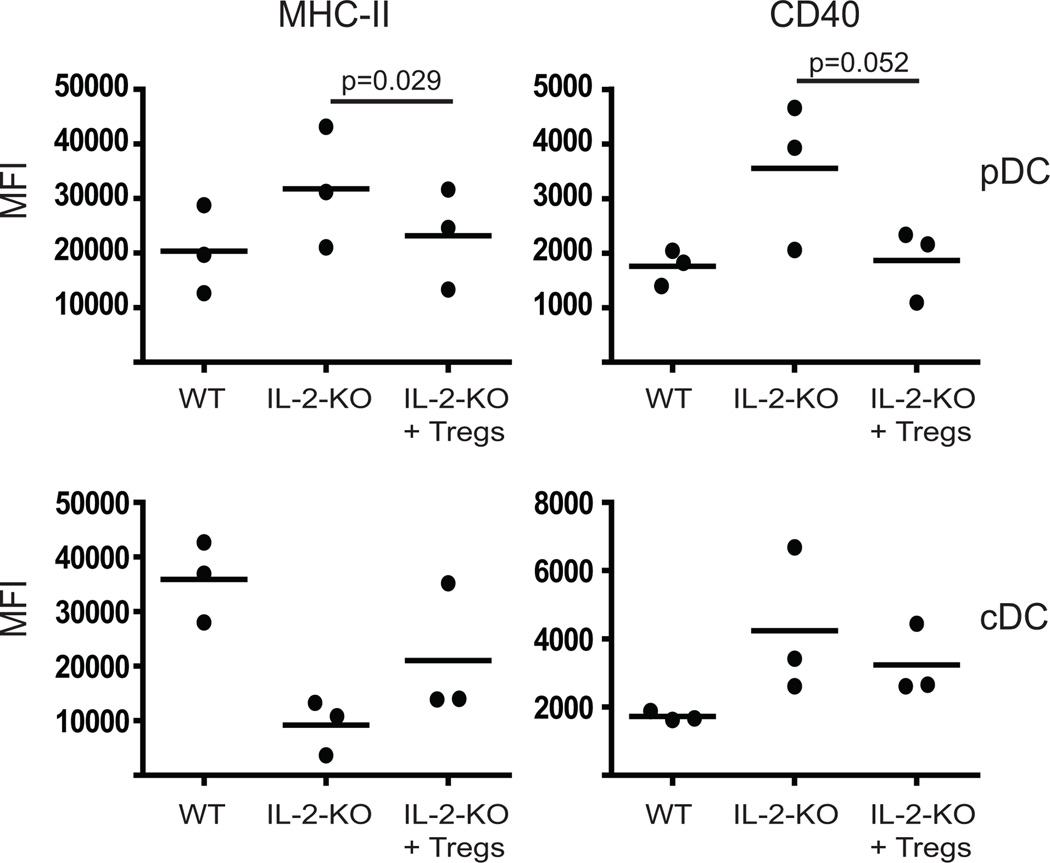
Recent studies have indicated that Tregs can inhibit DC expansion and activation (28–30). As decreased Tregs are the major defect in the IL-2-KO mouse, it is possible that the absence of Tregs allows the activation of DCs. To test whether WT Tregs are capable of controlling IL-2-KO DCs, we adoptively transferred in-vitro expanded Tregs into these mice and analyzed DC activation markers. Tregs were sorted from Foxp3GFP+ mice and expanded in-vitro. 2–4×106 expanded Tregs were administered by i.p. injection at day 8 and 12 into recipient mice and DC activation markers were evaluated on day 16. Adoptive transfer of Tregs reduced the expression of MHC-II and CD40 on the surface of IL-2-KO pDCs to at or near WT levels (Figure 8). IL-2-KO cDCs expressed slightly higher MHC-II and lower CD40 following the addition of Tregs, trending towards WT levels, but not reaching significance. These data indicate that pDCs may be a primary target of Tregs, while in our model, cDC activation is not fully controlled by Tregs. Our data further suggest that pDC activation can act as an initiating event in the development of autoimmunity.
Figure 8. Tregs can control pDC activation.
Tregs were tested for their ability to control DC activation. 2×106 in-vitro expanded Tregs were injected (i.p) on day 8 and 12 into recipient mice. Collagenase-treated splenocytes from 16-day old WT and IL-2-KO mice were stained and analyzed by flow cytometry. FACS profiles of pDCs (top panel) were gated on Thy1.2−CD11clo B220+PDCA-1+ cells and cDCs (bottom panel) were gated on Thy1.2−CD11chiB220− cells then surface marker expression analyzed by mean fluorescent intensity (MFI). Dots represent the MFI from three individual mice in three experiments.
Discussion
In this study we have shown that DC expansion and activation is a critical step in the development of a systemic autoimmune disease. When DC numbers are reduced, antibody development and lethal disease are significantly delayed in the IL-2-KO mouse. Our data suggests that in the absence of control by Tregs, dysregulated DCs initiate early autoimmune events and once autoimmunity is initiated, it is further propagated by these DCs, establishing an amplification loop between DCs and T cells that exacerbates disease.
It is striking that depletion of cDCs for a short duration significantly prolonged survival of IL-2-KO mice. At 10 days of age, the first signs of T cell activation can already be observed in IL-2-KO mice, thus elimination of cDCs at this stage removes their influence on the propagation of autoimmunity. Our evaluation of cDC phenotype and functionality in the absence of T cell activation (IL-2xCD28-KO mice) indicates that cDC alterations are less severe in these mice, suggesting that activation of cDCs is partially dependent on T cells.
In contrast, the activation and function of IL-2-KO pDCs appears to be largely independent of T cell activation. IL-2-KO pDCs are functionally activated even in the absence of endogenous T cell activation, and these pDCs induce elevated proliferation of naïve T cells relative to WT pDCs. Depletion of pDCs or inhibition of T1 IFN signaling impaired the development of autoantibodies and lethal autoimmunity. One outcome of pDC depletion was the decrease in IFNγ production, which is known to promote early autoimmunity in these mice (6). IL-2-KO pDCs produced elevated IFNα and contributed to the elevated expression of IL-12, cytokines which stimulate Th1 differentiation and IFNγ production. Since disease in the IL-2-KO mouse is Th1-mediated, it is tempting to hypothesize that pDCs preferentially drive Th1 responses. There is some data to support this idea. For example, Flt3L-induced pDCs augment Th1 cytokine production following RSV infection (31). Together, our data indicate that pDCs and cDCs may have a central role in regulating autoreactive Th1 responses and autoantibody production, thereby inducing antibody-mediated autoimmunity.
Peripheral tolerance and maintenance of immune homeostasis require a careful balance of effector T cells and Tregs. Due to their pivotal role in regulating immune responses, Tregs might be expected to impact the functional maturation state of DCs. Indeed, several recent studies have identified a role for Tregs in controlling tolerance through the maintenance of cDC and pDC activation (28, 29, 32). It has been proposed that during homeostasis an increase in DC numbers results in a subsequent increase in Tregs through MHC-II interactions (33). In the absence of IL-2, when Treg percentages are low, pDCs upregulate MHC-II, perhaps as a part of the feedback loop that would expand Treg numbers. As IL-2 is necessary for the survival of peripheral Tregs, the high MHC-II expressed on IL-2-KO pDCs would not alter Treg numbers. Instead, high MCH-II expression on IL-2-KO pDCs may drive the expansion and activation of effector T cells. We found that addition of WT Tregs reduced MHC-II and CD40 expression on IL-2-KO pDCs to near WT levels, but only slightly altered these markers on cDCs. Tregs have been shown in some models to prevent induction by antigen-specific T effector cells of CD80 and CD86 (29, 30), and MHC-II or CD40 on mature DCs ((28). Our data in combination with published studies suggests that DCs can be a primary target of Tregs and that DC activation can act as an initiating event in the development of autoimmunity.
In this systemic autoimmune model, elimination of IL-2 (and thus reduced Treg numbers and functionality) is the likely cause of DC dysregulation. Our data support the idea that Treg-mediated suppression of DCs normally acts as one mechanism to control an autoreactive response. In the absence of IL-2, when Tregs are reduced or their function is impaired, DCs upregulate activation and maturation markers, become functionally altered and can initiate autoimmunity.
An alternative mechanism for altered DC function involves a direct effect of IL-2 on DC development. Recent work by Lau-Kilby et al. demonstrated that IL-2 inhibits the flt3L-dependent generation of pDCs and cDCs (34). As the feedback loop between DCs and Tregs proposed by Darasse-Jeze et al. also required flt3L, these two mechanisms for DC activation may not be mutually exclusive. Lau-Kilby observed a reduced capacity of both pDCs and cDCs to stimulate T cell proliferation when grown in the presence of IL-2. We found that IL-2-KO pDCs, but not cDCs promoted increased T cell proliferation (Figure 7D and Supplemental Figure 1). The difference in cDC capacity to drive T cell division between these studies may reflect the source of the cDCs (in vivo-derived splenic DCs versus in vitro BM-derived DCs), other factors in vivo that alter the functionality of IL-2-KO cDC, or the genetic background and TCR transgene utilized. These and other recent studies have clearly demonstrated the importance of DCs to the dysregulated environment of autoimmune disease, and are beginning to clarify the role of IL-2 and Tregs to DC maturation and function.
Our data supports the existence of an amplification loop between DCs and CD4+ T cells that is normally controlled, in part, by Tregs. When IL-2 is absent or limiting, Treg numbers are reduced and pDCs become dysregulated, upregulating MHC-II and CD40. Mature, activated DCs interact with naïve T cells through CD80/CD86 and secretion of IFNα or IL-12 to promote the expansion of Th1 effector cells. The activated effector cells produce IFNγ that acts on both pDCs and cDCs to further promote their activation, driving the upregulation of CD80/CD86 and expanding DC numbers. These DCs propagate the differentiation and expansion of Th1-effector cells likely through IL-12 and IFNα secretion, ultimately resulting in autoimmune disease. We conclude that autoimmune disease in this model is initiated by pDCs, whose activation and expansion are not properly controlled by Tregs.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health awards P01AI35297, AI73656, K99HL090706 (to K.K.H) and F32AI077199 (to S.D.K).
We thank Dr. Abul Abbas for his support and mentorship, Dr. Robert Schreiber (Washington University School of Medicine, St. Louis, MO) for generously providing the anti-IFNAR1 antibody, Drs. Qizhi Tang and Michelle Hermiston for helpful discussions, Dr. Shu-wei Jiang for cell sorting, Carlos Benitez for mouse typing, and the UCSF Mouse Pathology Core for assistance with complete blood counts.
Abbreviations used in this article
- AIHA
autoimmune hemolytic anemia
- DC
dendritic cell
- IFNAR-KO
IFN alpha receptor deficient
- IL-2-KO
interleukin-2-deficient
- MFI
mean fluorescence intensity
- RBC
red blood cell
- Treg
regulatory T cell
- WT
wild-type
Footnotes
The authors have no competing financial interests.
References
- 1.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 2.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164:2905–2914. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer KK, Wolslegel K, Dooms H, Abbas AK. Targeting T cell-specific costimulators and growth factors in a model of autoimmune hemolytic anemia. J Immunol. 2007;179:2844–2850. doi: 10.4049/jimmunol.179.5.2844. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer KK, Kuswanto WF, Gallo E, Abbas AK. Distinct roles of helper T-cell subsets in a systemic autoimmune disease. Blood. 2009;113:389–395. doi: 10.1182/blood-2008-04-153346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossie A, Vitetta ES. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell Immunol. 1991;135:95–104. doi: 10.1016/0008-8749(91)90257-c. [DOI] [PubMed] [Google Scholar]
- 8.Wirth JJ, Kierszenbaum F, Sonnenfeld G, Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985;49:61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogge L, D'Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, Adorini L, Sinigaglia F. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567–6574. [PubMed] [Google Scholar]
- 12.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 14.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 16.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 19.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee NJ, Rigby RJ, Gill H, Boyle JJ, Fossati-Jimack L, Morley BJ, Vyse TJ. Multiple loci are linked with anti-red blood cell antibody production in NZB mice -- comparison with other phenotypes implies complex modes of action. Clin Exp Immunol. 2004;138:39–46. doi: 10.1111/j.1365-2249.2004.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell JB, Crane CA, Smith MF, Jr, Roberts MR. Differential ex vivo nitric oxide production by acutely isolated neonatal and adult microglia. J Neuroimmunol. 2007;189:75–87. doi: 10.1016/j.jneuroim.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–669. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- 25.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 27.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 29.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 31.Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau-Kilby AW, Kretz CC, Pechhold S, Price JD, Dorta S, Ramos H, Trinchieri G, Tarbell KV. Interleukin-2 inhibits FMS-like tyrosine kinase 3 receptor ligand (flt3L)-dependent development and function of conventional and plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2011;108:2408–2413. doi: 10.1073/pnas.1009738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.