Abstract
Immunization of BALB/c mice with irradiated sporozoites (IrSp) of Plasmodium yoelii can lead to sterile immunity. The circumsporozoite protein (CSP) plays a dominant role in protection. Nevertheless after hyper-immunization with IrSp, complete protection is obtained in CSP- transgenic BALB/c mice that are T- cell tolerant to the CSP and cannot produce antibodies [CSP-Tg/JhT(−/−)]. This protection is mediated exclusively by CD8+ T cells [1]. To identify the non-CSP protective T cell antigens, we studied the properties of 34 P. yoelii sporozoite antigens that are predicted to be secreted and to contain strong Kd-restricted CD8+ T cell epitopes. The synthetic peptides corresponding to the epitopes were used to screen for the presence of peptide-specific CD8+ T cells secreting interferon-γ (IFN-γ) in splenocytes from CSP-Tg/JhT(−/−) BALB/c mice hyper immunized with IrSp. However, the numbers of IFN-γ-secreting splenocytes specific for the non-CSP antigen-derived peptides were 20 to 100 times lower than those specific for the CSP-specific peptide. When mice were immunized with recombinant adenoviruses expressing selected non-CSP antigens, the animals were not protected against challenge with P. yoelii sporozoites although large numbers of CD8+ specific T cells were generated.
Keywords: plasmodium, malaria vaccine, circumsporozoite protein (CSP), non-CSP antigens
1. Introduction
Multiple immunizations of rodents, monkeys and humans with irradiated mosquitoes infected with sporozoites (IrSp) lead to sterile immunity to sporozoite challenge [2–4]. Immunization of mice with genetically-attenuated sporozoites (GaSp) is also effective [5,6]. The mechanisms of protection elicited by IrSp and the uis3/uis4 GaSp are similar [7], and involve both humoral and cellular immune responses that target the invading sporozoite and the infected hepatocyte respectively. The antibody responses are predominantly directed against the repetitive central domain of CSP [8]. The adoptive transfer of CSP-specific CD8+or CD4+ T cell clones, albeit in large numbers, leads to full protection against sporozoite challenge [9–11]. These observations indicated that CSP plays an important role in protection elicited by IrSp. This view was greatly strengthened by IrSp immunization of T-cell tolerant, CSP-transgenic BALB/c or C57BL/6 mice that could not make antibodies (CSP-Tg/JhT mice). After priming and boosting the transgenic mice and controls with 105 IrSp, protection was reversed by more than 90% in the BALB/c mice and about 50% in C57BL/6 [1,7]. Nevertheless, sterile immunity was obtained in the CSP-Tg/JhT(−/−) BALB/c mice after a second booster injection with 105 IrSp. The protection in the absence of CSP-specific immunity was mediated exclusively by CD8+ T cells [1].
The main objective of the present study is to identify the non-CSP antigens that elicit those potent protective CD8+ T cell responses in the CSP-Tg/JhT(−/−) BALB/c mice. We hoped to find new dominant or sub-dominant sporozoite antigens that might be used to prevent malaria infection in humans. The current lead malaria vaccine, GlaxoSmithKline’s RTS,S, contains a portion of CSP that includes its C-terminus and the dominant repeat B cell epitope [12–13]. RTS,S has a favorable safety profile, and in many human trials, including in children and infants, consistently protected 30–50% of the vaccinees against infection. In one field study, the vaccine was capable of providing a sustained clinical benefit for up to 42 months following vaccination [14,15]. Although the results from RTS,S vaccination are impressive, there remains the goal to increase the level of efficacy of this first generation malaria vaccine candidate. One possible approach to achieve this goal is to add protective non-CSP antigens to RTS,S, or use non-CSP antigens instead of RTS,S. Unfortunately, to date few non-CSP antigens have been used in humans. The best studied antigen is ME-TRAP. Prime-boost regimens of ME-TRAP with simian adenovirus and modified vaccinia Ankara (MVA) vectors protected mice against challenge, and generated strong T cell responses in non-human primates. Nevertheless the delivery of ME-TRAP using DNA and MVA vaccines failed to protect adults in Gambia [16,17]. The IL-10 responses to Liver Stage Antigen 1 (LSA1) are associated with resistance to P. falciparum infection in endemic areas [18]. Nevertheless the LSA1 vaccine was not protective in humans [19]. Genetic immunization with LSA3 protected chimpanzees against falciparum malaria [20], but we found no information of its use in humans. Recently it has been reported that CelTOS is a protective antigen both in BALB/c and outbred mice [21]. CelTOS is a micronemal protein secreted by sporozoites during their migration from the skin to the liver [22]. Antibodies generated by CelTOS immunization immobilize sporozoites and inhibit infection of hepatocytes in vitro, suggesting that protection is at least in part antibody mediated [23].
In addition to looking for non-CSP protective antigens elicited by hyper-immunization of BALB/c mice with IrSp, we performed some experiments to evaluate the protective potential of selected non-CSP malaria antigens. For this purpose we used adenovirus vectors that are known to induce potent CD8+ T cell-mediated protective immunity and also antibody responses against pathogens [24–26].
2. Materials and methods
2.1. Selection of non-CSP antigens and epitopes
We preferentially selected non-CSP antigens with predicted signal sequences from sporozoite and liver stage libraries [27,28]. This is because, in order for the antigens to be recognized by effectors T cells, the antigens have to be secreted into the cytoplasm of the infected liver cells [or of neighboring cells] and then processed and presented on the plasma membrane in association of MHC class I [1]. Because the liver stages develop inside a parasitophorous vacuole (PV) and need to traverse the PV membrane to enter the cytoplasm, a higher priority was given to antigens bearing Pexel motifs [29,30]. The effectiveness of T cell recognition should increase when large numbers of peptides derived from non-CSP antigens are present on the surface of the target cells. Thus we selected preferentially highly transcribed P. yoelii genes [27,28] assuming that the corresponding protein would also be highly represented in the parasite. We made sure that all of the selected genes have P. falciparum orthologs. A total of 34 non-CSP candidate antigens were selected, including a previously reported protective antigen CelTOS, also named antigen 2 [21,23,31]. From each candidate we chose CD8+ T cell epitopes based on three independent computer algorithms that predict binding affinity to MHC class I molecule:
http://www-bimas.cit.nih.gov/cgi-bin/molbio/ken_parker_comboform (NIH)
http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm (SYFPEITHI)
http://tools.immuneepitope.org/analyze/html/mhc_binding.html (IEDB)
All selected molecules, epitopes and predicted binding to Kd are shown in the supplementary Table 1.
2.2. Mice
Six- to eight-week-old female BALB/c (H2-Kd) mice were purchased from Taconic (Germantown, NY). BALB/c mice transgenic for CS that were also unable to make antibodies [CSP-Tg/JhT(−/−)] were bred and maintained at the NYU School of Medicine. All mice were housed and maintained at NYU School of Medicine Animal Facility in a pathogen free mouse facility according to institutional guidelines. All animal studies were approved by the institution’s Institutional Animal Care and Use Committee.
2.3. Mosquitoes and IrSp immunization
Anopheles stephensi mosquitoes were reared at 27°C and 80% humidity under a 12/12 h light/dark cycle, and adults were fed on 10% sucrose solution. P. yoelii -infected mosquitoes were maintained at 25°C. Live salivary gland sporozoites were obtained 14 days after the infective meal. The mosquitoes were rinsed in 70% ethanol, washed in DMEM, and the salivary glands removed. The glands were gently ground in a tissue homogenizer, centrifuged at 800 rpm for 4 min to remove mosquito debris, and sporozoites were counted in a haemocytometer and kept on ice until use. For challenge, 104 sporozoites were inoculated into mice intravenously. Prior to immunization, the sporozoites were irradiated (15,000 rads). Mice were immunized intravenously three times with 105 IrSp at 2 weeks intervals.
2.4. ELISPOT assay
Multiscreen plates (Millipore, Bedford MA) were coated with 10 μg/ml of anti-mouse IFN-γ (BD Pharmingen). After overnight incubation, the wells were washed twice with PBS and then twice with wash Medium (DMEM containing 10% FCS). After blocking the wells with wash medium for 6 hours at 37°C, splenocytes obtained from immunized mice and synthetic peptides corresponding to the CD8+ T cell epitopes were added to the wells. After incubation at 37°C and 5% CO2, for 24–28 h, the plates were extensively first washed with PBS, and then with PBS containing 0.05% of Tween 20 (PBST). The wells were then incubated with a solution of 2μg/mL of biotinylated anti-mouse IFN-γ (BD Pharmingen) overnight. Finally, the plates were incubated with peroxidase-labeled streptavidin (Kirkegaard and Perry Laboratories, KPL, Gaithesburg, MD), and the spots were developed by adding AEC substrate set (BD bioscience) [32] according to the manufacture’s instruction.
2.5. Recombinant adenovirus
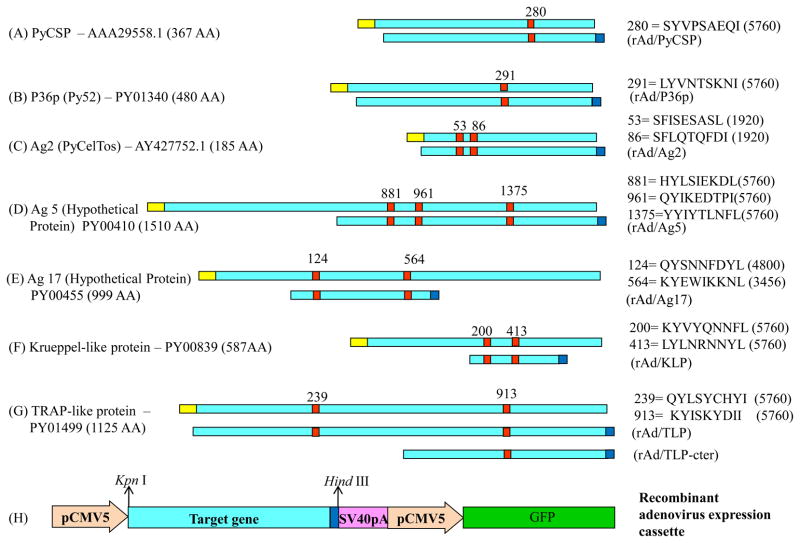
The general strategy for the construction of recombinant adenoviruses expressing non-CS pre-erythrocytic antigens is presented in Fig. 3. Selected non-CSP candidate antigens were codon optimized for the expression into mammalian cells. Signal peptide and transmembrane domain were removed from the codon optimized gene and c-Myc-tag was placed at the end of the gene. Partial or full length of the gene was cloned into modified pShuttle-CMV5 vector (Stratagene) at Kpn I and Hind III site. The GFP expression cassette was introduced into the pShuttle-CMV5 vector (pShuttle-CMV5-GFP) to produce the fluorescent virus. The rest of the procedure for the generation of recombinant adenovirus was performed as described in AdEasy™ Adenoviral Vector System (Stratagene).
Fig 3.
Construction of recombinant adenoviruses. (A–G) Schematic representation of the candidates used to generate recombinant adenoviruses. (H) pShuttle-CMV5-antigen-GFP plasmid. Symbols: yellow box, signal sequence; Aqua box, target gene; red box, peptide; blue box, c-Myc tag.
2.6. Western blot analysis
For the detection of antigen expressed by recombinant adenoviruses, Western blot analysis was performed. Briefly, AD-293 cells (Stratagene) were infected with a recombinant adenovirus expressing each of the antigens and then resuspended in SDS sample buffer and boiled for 5 min to make cell extracts. The cell extracts were run on SDS-PAGE and transferred to Sequi-Blot™ polyvinylidene difluoride membranes (Bio-Rad). After the membrane was incubated with a mouse anti-human c-Myc monoclonal antibody (BD Pharmingen), bound antibody was detected with horse radish peroxidase (HRP)-coupled anti-mouse IgG. Finally, blot was developed using NovexR ECL HRP chemiluminescent substrate reagent kit (Invitrogen).
2.7. Recombinant adenovirus immunization, and challenge
BALB/c mice were immunized i.m. with 1010 virus particle of the recombinant adenovirus expressing the plasmodial sequence. We made recombinant adenoviruses of P36p (PY01340) [33,34], TRAP-like protein (PY01499) [35,36], Krueppel-like Protein (PY00839), and to a group of proteins that were highly immunogenic in human volunteers vaccinated and protected against sporozoite challenge after immunization with falciparum IrSp, ie, Ag2 or CelTOS (AY427752.1), Ag5 (PY00410) and Ag17 (PY00455) [31]. Two weeks after immunization, splenocytes were isolated and IFN-γ ELISPOT was performed. Another group of immunized mice was challenged by the i.v. administration of 1 × 104 live P. yoelii sporozoites and the level of protection measured as described below.
2.8. Evaluation of protection by RT-PCR
The amounts of Plasmodial rRNA in the livers of immunized mice were measured 42 hours after sporozoite challenge. The liver was washed twice in PBS and homogenized in 10ml Trizol (Invitrogen). 200μl of the homogenized liver was transferred into fresh tubes containing 600μl trizol. RNA was isolated according to the manufacturer’s instructions. 400 ng of total RNA was reverse-transcribed from each of the samples using RT-PCR kit (PE Applied Biosystems) with random hexamers. The real time PCR was performed as described earlier [37].
2.9. Statistical Analysis
All results are presented as mean values ± SD. Student’s t tests were used to determine the statistical significance.
3. Results
3.1. T Cell Responses to non-CSP antigens
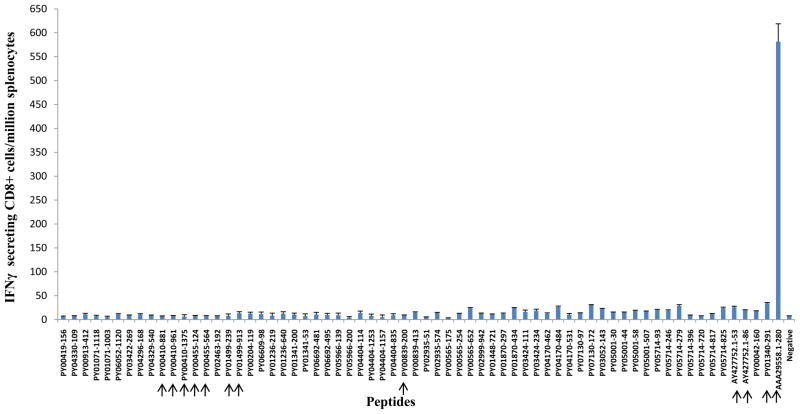
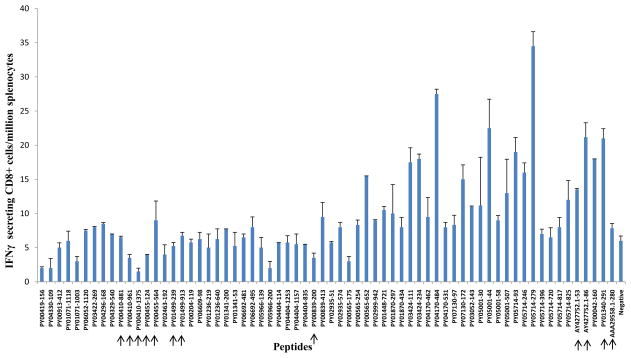
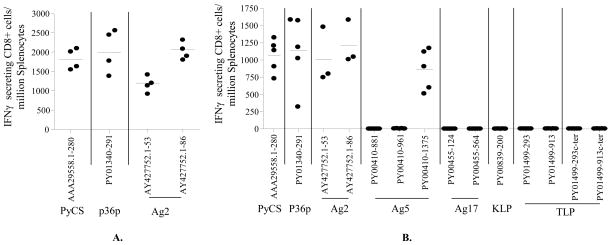
Groups of wt BALB/c mice and [CSP-Tg/JhT(−/−)] mice with the BALB/c background were immunized with three doses of 105 P. yoelii IrSp with intervals of 2 weeks. The level of CD8+ T cell responses were evaluated by IFN-γ ELISPOT assay, in which splenocytes from immunized mice were incubated with peptides corresponding to the CD8+ T cell epitopes of different antigens. The CSP-Tg/JhT(−/−) mice were used to exclude the possibility that the high level of immune responses to CSP generated by the IrSp immunization would interfere with the generation of T cells specific to non-CSP antigens in wt mice. A total of 34 non-CSP candidate antigens were studied (Table 1). The results are shown for wt BALB/c mice in Fig. 1 and for the [CSP-Tg/JhT(−/−)] mice in Fig. 2. The immunodominant CD8+ T cell epitope - SYVPSAEQI (H-2Kd restricted)-derived from P. yoelii CSP (AAA29558.1) was used as positive control. As shown, the level of CD8+ T cell responses to non-CSP antigens were 20 to 100 times lower than that to CSP (Fig. 1). Although unlikely it is possible that larger number of CD8+ T cells were present in the liver or elsewhere. As expected, the response to the CD8+ T cell epitope of CSP in [CSP-Tg/JhT(−/−)] mice was negligible, because T cells to CSP were tolerant in these mice. The overall responses to non-CSP antigens in the transgenic mice were still very low (Fig. 2).
Table 1.
All the antigens and peptides used in this study.
| P. yoelii gene ID | Annotation | Peptide position | Peptide sequence |
|---|---|---|---|
| * PY00419 | Hypothetical Protein | 156 | IYLLAGGFI |
| * PY04330 | PfATPase3 | 109 | FYGNKKNRI |
| * PY00913 | CAAT-box DNA binding protein subunit B | 412 | KYNNNLNEI |
| * PY01071 | Multidomain scavenger receptor protein PbSR precursor | 1118 | NYYTPDTSI |
| 1003 | KYILTEHEI | ||
| PY06052 | Erythrocyte membrane protein PFEMP3 | 1120 | KYIPTNKEI |
| PY03422 | Hypothetical Protein | 269 | FYLFENTRI |
| * PY04296 | Protein disulfide isomerase precursor | 168 | KYYNMINDL |
| PY04329 | PfSUB-1 | 540 | TYKQVVSIL |
| PY00410 | Ag5 | 881 | HYLSIEKDL |
| 961 | QYIKEDTPI | ||
| 1375 | YYIYTLNFL | ||
| PY00455 | Ag17 | 124 | QYSNNFDYL |
| 564 | KYEWIKKNL | ||
| * PY02463 | Hypothetical Protein | 192 | FYMPSYTEI |
| PY01499 | TRAP-like protein | 239 | QYLSYCHYI |
| 913 | KYISKYDII | ||
| * PY00204 | Hypothetical Protein | 119 | DYQEQYPEI |
| * PY06609 | Hypothetical Protein | 98 | NYIDKKNII |
| * PY01236 | Hypothetical Protein | 219 | SYLYLLNKL |
| 640 | AYLITVISI | ||
| PY01341 | Pbs36 | 200 | AYPGDVVGI |
| 53 | DYNKTIKLL | ||
| * PY06692 | Eukaryotic aspartyl protease, putative | 481 | KYVFGKLVI |
| 495 | EYMIVNDDL | ||
| * PY05966 | Hypothetical Protein | 139 | LYKKHKDKI |
| 200 | IYDKFGEKI | ||
| * PY04404 | Ser/Thr protein phosphatase, putative | 114 | IYTHIFNEL |
| 1253 | TYDEMKYSL | ||
| 1157 | EYEENEENI | ||
| 835 | GYFSAHDNL | ||
| PY00839 | Krueppel-like protein | 200 | KYVYQNNFL |
| 413 | LYLNRNNYL | ||
| * PY02935 | Hypothetical Protein | 511 | KYLKLHEYI |
| 574 | RYLKERNEI | ||
| * PY00565 | ClpB protein | 175 | AYVEAEMLL |
| 254 | EYISIEHLL | ||
| 652 | MYVDNIRAI | ||
| PY02999 | Guanylyl cyclase enzyme-related | 942 | IYLFTITFI |
| PY01448 | Hypothetical Protein | 721 | SYMNLIPHI |
| PY01870 | ATP-dependent RNA Helicase | 297 | IYLSDAHEI |
| 434 | RYGHLGLAI | ||
| PY03424 | Falstatin | 111 | NFSDNNEEI |
| 234 | GYIWALLGV | ||
| PY04170 | Lecithin:cholesterol acyltransferase, putative | 462 | GYIDGKDIL |
| 484 | KYEVLKSHI | ||
| 531 | KYINIVMHI | ||
| PY07130 | Hypothetical Protein | 97 | IYEKDKTPL |
| 172 | IYKESISLL | ||
| PY03052 | Sporozoite surface protein 2 precursor | 143 | MYRPDAIQL |
| * PY05001 | Heat shock protein | 30 | IYIWCGFGL |
| 44 | IYMCKYVFL | ||
| 58 | TYFSFSYFL | ||
| 507 | TYQDNQPAV | ||
| PY05714 | Hypothetical Protein | 93 | KYFFIFRSI |
| 246 | IYDYIITFI | ||
| 279 | KYIFKKEQL | ||
| 396 | NYRKNNNDI | ||
| 720 | SYNKLKKEI | ||
| 817 | IYAPYISLI | ||
| 825 | IYISYIVAI | ||
| AY427752.1 | Ag2 or CelTOS | 53 | SFISESASL |
| 86 | SFLQTQFDI | ||
| PY00042 | Unknown protein | 160 | FYIFVAIFL |
| * PY01340 | Py52 (P36p) | 291 | LYVNTSKNI |
| * AAA29558.1 | Circumsporozoite protein | 280 | SYVPSAEQI |
PEXEL/VTS motif present.
Fig 1.
T- Cell Responses to non-CSP antigens in BALB/c mice induced by immunization with irradiated sporozoites. Mice were immunized 3 times with 105 IrSp with interval of two weeks. The frequency of epitope-specific CD8+ T cells in spleens was assessed 10 days after the last immunization by IFN-γ ELISPOT using peptides representing CD8+ T cell epitope. Results are expressed as the mean±SD of epitope–specific T cells. A total of 34 non-CSP candidates (65 CD8+ epitopes) were studied. The arrows point to candidates used to generate recombinant adenoviruses.
Fig 2.
T- Cell Responses to non-CSP antigens in [CSP-Tg/JhT(−/−)] BALB/c mice. The experiments were done precisely as described in the legend of fig. 1.
3.2 Immunization with recombinant adenoviruses and protection against sporozoite challenge
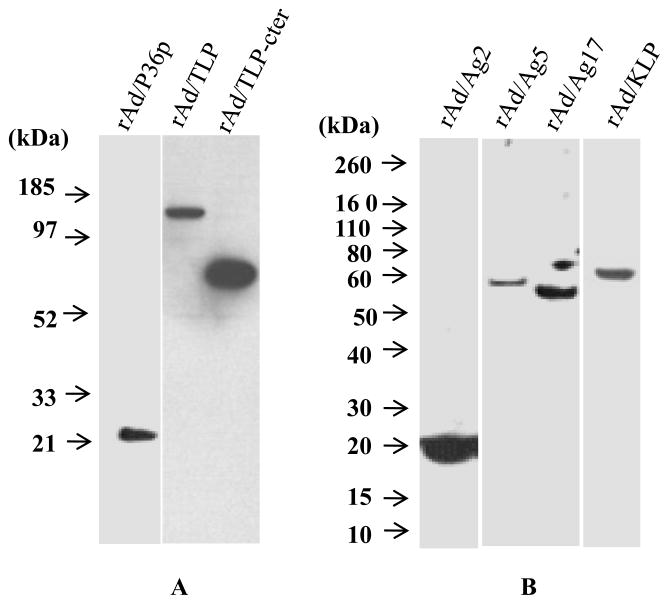
Of the antigens listed in Figs 1 and 2, we selected 6 for expression by a recombinant adenoviral vector. Our aim was to obtain preliminary information about their immunogenicity, in particular their ability to elicit CD8+ T cells and protection against challenge with sporozoites. Three of the chosen antigens P36p (Py52) - PY01340, Krueppel-like protein - PY00839, and TRAP-like protein - PY01499 are highly transcribed during the sporozoite stage [27,28]. The other three antigens namely Ag2 (PyCelTOS), Ag 5 (Hypothetical Protein), and Ag 17 (Hypothetical Protein) were immunogenic in human volunteers vaccinated with falciparum IrSp. [31]. Immunization of BALB/c mice and outbred mice with adjuvanted recombinant CelTOS led to sterile protection in a sizable proportion of the animals [21].]. We performed Western blot assays using the extracts of cells infected with each of recombinant adenoviruses and confirmed the expression of c-Myc tagged antigens, as a transgene (Fig. 4).
Fig 4.
Western blot analysis of the expression of recombinant adenovirus c-Myc tagged antigen in transfected AD-293 cells. Total cell lysates were prepared and the presence of recombinant protein was detected using the c-Myc monoclonal antibody.
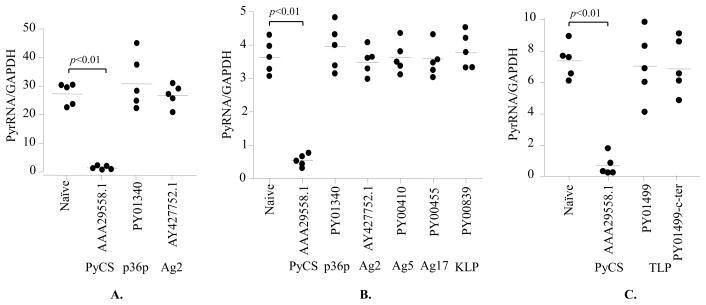
After constructing recombinant adenoviruses expressing those six antigens, we compared their immunogenicity and protective efficacy with that of a recombinant adenovirus bearing CSP. The immunogenicity was only evaluated by an IFN-γ ELISPOT assay using selected CD8+ T cell epitopes in two separate experiments (Fig. 5). Notably, the splenocytes of mice immunized with adenovirus expressing P36p, Ag2 (CelTOS) and Ag5 produced similar or even higher numbers of IFN-γ ELISPOT, when compared to splenocytes of mice immunized with adenovirus-expressing CSP. To test the protective capacity of each antigen, mice immunized with recombinant adenoviruses were challenged with 104 live sporozoites of P. yoelii, 2 weeks after the immunization. Two days after the challenge, the livers were collected from the challenged mice and the amount of parasite loads was determined by a real-time RT-PCR [37,38]. We performed three independent experiments shown in Fig. 6. Because the infectivity of sporozoites varies from week to week, the amounts of the parasite load in the liver also varies. Nevertheless, it is apparent that the parasite load in the livers of the group of mice immunized with adenovirus-expressing CSP was significantly (p<0.01) lower compared to that of mice immunized with adenovirus-expressing non-CSP antigens. Thus only mice immunized with adenovirus-expressing CSP were protected (Fig. 6).
Fig 5.
CD8+ T- cell responses induced by recombinant adenoviruses expressing non-CSP antigens. Groups of naive BALB/c mice (4 mice per group in A and 5 mice per group in B except for Ag2, which consists of 3 mice per group) were immunized i.m. with 1010 virus particle of the recombinant adenovirus expressing the Plasmodial sequence. Two weeks later, splenocytes were isolated and the number of IFN-γ secreting, epitope specific CD8+ T- cells was determined by an ELISPOT assay. The horizontal bars represent median values. In all experiments, the results represent one of two experiments that showed similar results.
Fig 6.
The parasite burdens in the livers of immunized and naive mice, as determined by real-time PCR. Groups of BALB/c mice (5 mice per group) were immunized i.m. with 1010 virus particle of the recombinant adenovirus expressing the Plasmodial sequence and challenged two weeks after the immunizations by i.v. injection of 104 P. yoelii live sporozoites. The protection against a sporozoite challenge is shown as the inhibition of liver infection. The horizontal bars represent median values. In all experiments, the results represent one of two similar experiments.
4. Discussion
More than forty years have passed since the demonstration that immunization of mice with irradiated sporozoites (IrSP) induces sterile protection against a sporozoite challenge [2]. This was followed by the discovery of the circumsporozoite protein (CSP), its role in the parasite infectivity and efforts to develop CSP-based vaccines [39]. CSP makes up the bulk of the proteins at the sporozoite surface [40], is the main target of antibody responses [8] and displays two non-restricted CD4+ T-cell epitopes. Although CSP is an immunodominant protective antigen [1], two studies using distinct approaches showed that sterile immunity against malaria infection can be obtained independently of CSP. One study used CSP-transgenic mice that cannot make antibodies and are T-cell tolerant to CSP. After triple immunization with a high dose of IrSp the transgenic mice were fully protected, and the protection was mediated by CD8+ T cells. [1]. In the other approach, mice were immunized with P. berghei IrSp, and were fully protected from challenge with recombinant P. berghei parasites bearing the CSP of P. falciparum. Since there was no cross-reactivity between the two CSPs, the authors concluded that sterile immunity to the recombinant parasite was not CSP-dependent [41].
The main objective of our studies was to characterize the non-CSP antigens that can elicit powerful protective CD8+ T cell responses. When approaching this question it is important to consider the multiple complexities that have to be overcome for the T-cell mediated elimination of the infected liver cells (exoerythrocytic forms or EEFs). One of them is the scarcity of EEFs among non-infected liver cells. If the challenge after immunization is with 104 sporozoites, the ratio of EEFs to normal liver cells will be only about 1/10,000 even if the efficiency of infection is 100%. Therefore very large numbers of CD8+ T cells are needed to find and destroy all of the EEFs [42,43]. To complicate the task of the T cells, the main lymphokine that inhibits the liver stages, interferon γ, is only effective during the very early development of the parasite [44]. Thus not only the T cells targets are scarce but the time window for the EEF destruction is limited.
None of the selected 34 P. yoelii non-CSP antigens (Table 1) came even close to the immunogenicity of CSP in BALB/c mice. The explanation is that CSP is very abundant in salivary gland sporozoites [39], has a very potent Kd-restricted epitope, and is released while the parasites traverse cells while traveling from the site of mosquito bite to the liver [45,46]. The released CSP can be picked up by dendritic cells where it remains for weeks for antigen presentation [47]. Even after sporozoites invade hepatocytes and the parasite is enclosed in a vacuole, CSP traverses the vacuole membrane and enters the hepatocyte cytoplasm where it enters the antigen processing pathway. The traversal of the membrane of the vacuole is facilitated by the presence in CSP of two functional Pexel motifs [48]. Thus CSP is not only poised to generate powerful CD8 responses, but is an excellent target for CD8+ T cells. As shown in Figures 1 and 2 the number of interferon-γ ELISPOTs generated by the non-CSP antigens was 20 to 100 times lower than those obtained with CSP. Thus we failed to discover any single antigen that accounts for the sterile immunity observed in the CSP-Tg/JhT(−/−)] BALB/c mice hyper-immunized with IrSp. A tentative conclusion is that the observed protection reflects the sum of activities of many minorprotective antigens within the IrSp[49].
We also generated recombinant adenovirus expressing a few non-CSP antigens. Among them are antigen 2 (CelTOS, [23]) and 5 [31] that were specifically recognized by CD4+ and/or CD8 + T cells of at least 50% of humans volunteers immunized with IrSp of P. falciparum. The number of IFN-γ ELISPOTs generated by peptides from CelTOS, antigens 5 and P36p (pointed out by arrows in figures 1 and 2) was similar or even greater that those obtained by the dominant Kd epitope of CSP (Figure 5). Unexpectedly, after challenge of the immunized mice with live P. yoelii sporozoites, only CSP was protective. A plausible explanation for this paradoxical result is that the non-CSP antigens did not enter the class I pathway of antigen presentation in the EEFs or, more likely, because they are much less abundant than CSP. In other words, the non-CSP antigens were very immunogenic but were poor targets to the CD8+ T cells. Of course, other methods of immunization that elicit good antibody responses, or effector CD4+ T cells may lead to protective immunity. For example, the injectionin mice of recombinant CelTOS P. falciparum or P. berghei protein in Montanide adjuvant, or a plasmid encoding P. berghei CelTOS, induced sterile immunity after challenge with P. berghei sporozoites [21,23]. The serum of the immune mice immobilized P. berghei sporozoites, suggesting that the protection was at least in part antibody mediated.
In summary, we failed in our attempt to discover powerful protective non-CS antigens in our P. yoelii model system. Nevertheless, as recently reported [50], it might be worthwhile to investigate whether a mixture of antigens would lead to protection. In fact, one of the protective antigens in reference 50 (PY03424) was among those that elicited a higher numbers of ELISPOTS in our studies (Figures1 and 2).
Supplementary Material
Acknowledgments
V.N. and R.S.N are supported by the Bill and Melinda Gates Foundation, NIH R56 AI073658 and GlaxoSmithKline (GSK). M.T. is supported by NIH R56 AI073658 and GSK. E.A.W. was supported by grants from the W. M. Keck Foundation and by National Institutes of Health Grant R01AI059472.
Footnotes
Author contributions: V.N., M.T., R.S.N., E.A.W. and S.M. designed research; M.T., U.R., T.S., X.L. and S.M. performed research; V.N., M.T., R.S.N., J.C., Y.V. and S.M. analyzed data; and V.N., M.T., R.S.N. and S.M. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–40. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 4.Doolan DL, Martinez-Alier N. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med. 2006;6(2):169–85. doi: 10.2174/156652406776055249. [DOI] [PubMed] [Google Scholar]
- 5.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–67. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan AM, Wang R, Kappe SH. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum Vaccin. 2010;6:107–13. doi: 10.4161/hv.6.1.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(-) and uis4(-) Plasmodium sporozoites. PLoS One. 2009;4:e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985;228(4706):1436–40. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 9.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, et al. Cloned cytotoxic T cells recognize an epitope on the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–25. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–85. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 11.Renia L, Grillot DA, Marussig M, Corradin G, Miltgen F, et al. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol. 1993;150:1471–78. [PubMed] [Google Scholar]
- 12.Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis. 1998;178:1139–44. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 13.Ballou WR. Malaria vaccines in development. Expert Opin Emerg Drugs. 2005;10(3):489–03. doi: 10.1517/14728214.10.3.489. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6(1):90–6. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 15.Sacarlal J, Aide P, Aponte JJ, Renom M, Leach A, et al. Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. J Infect Dis. 2009;200:329–36. doi: 10.1086/600119. [DOI] [PubMed] [Google Scholar]
- 16.Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, Pinder M, Gilbert SC, Walraven G, Greenwood BM, Hill AS. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1(2):e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. 2010;78(1):145–53. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtis JD, Lanar DE, Opollo M, Duffy PE. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect Immun. 1999;67(7):3424–29. doi: 10.1128/iai.67.7.3424-3429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2010;28(31):5135–44. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 20.Daubersies P, Ollomo B, Sauzet JP, Brahimi K, et al. Genetic immunisation by liver stage antigen 3 protects chimpanzees against malaria despite low immune responses. PLoS One. 2008;3(7):e2659. doi: 10.1371/journal.pone.0002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse CF, Angov E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine. 2011 Jun 28; doi: 10.1016/j.vaccine.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 22.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann-Leitner ES, Mease RM, De La Vega P, Savranskaya T, Polhemus M, Ockenhouse C, Angov E. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS One. 2010;5(8):e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–74. [PubMed] [Google Scholar]
- 25.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17(8):1333–9. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, Hill AV. CD8+ T Effector Memory Cells Protect against Liver-Stage Malaria. J Immunol. 2011 Jun 29; doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De la Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite lifecycle. Science. 2003;301 (5639):1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, Henson K, Nussenzweig V, Carlton J, Vinetz JM, Duraisingh MT, Winzeler EA. Evidence-based annotation of the malaria parasite’s genome using comparative expression profiling. PLoS ONE. 2008;3(2):e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–33. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 30.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–37. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 31.Doolan DL, Southwood S, Freilich DA, et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci USA. 2003;100:9952–57. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 33.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–68. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douradinha B, van Dijk MR, Ataide R, van Gemert GJ, Thompson J, Franetich JF, Mazier D, Luty AJ, Sauerwein R, Janse CJ, Waters AP, Mota MM. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int J Parasitol. 2007;37(13):1511–19. doi: 10.1016/j.ijpara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Heiss K, Nie H, Kumar S, Daly TM, Bergman LW, Matuschewski K. Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test. Eukaryot Cell. 2008;7:1062–70. doi: 10.1128/EC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreira CK, Templeton TJ, Lavazec C, Hayward RE, Hobbs CV, Kroeze H, et al. The Plasmodium TRAP/MIC2 family member, TRAP-Like Protein (TLP), is involved in tissue traversal by sporozoites. Cell Microbiol. 2008;10:1505–16. doi: 10.1111/j.1462-5822.2008.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruña-Romero O, Hafalla JC, González-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 200;31(13):1499–502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 38.Ophorst OJ, et al. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect Immun. 2006;74:313–20. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol. 2004;20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida N, Potocnjak P, Nussenzweig V, Nussenzweig RS. Biosynthesis of Pb44, the protective antigen of sporozoites of Plasmodium berghei. J Exp Med. 1981;154(4):1225–36. doi: 10.1084/jem.154.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruner AC, Mauduit M, Tewari R, Romero JF, Depinay N, et al. Sterile Protection against Malaria Is Independent of Immune Responses to the Circumsporozoite Protein. PLoS ONE. 2007;2:e1371. doi: 10.1371/journal.pone.0001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105(37):14017–22. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6(7):e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, Nussenzweig RS, Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232(4752):881–84. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 45.Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol. 2004;20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Pahtak S. Malaria vaccine: a current perspective. J Vector Borne Dis. 2008;45:1–20. [PubMed] [Google Scholar]
- 47.Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, et al. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 2010;6(5):e1000877. doi: 10.1371/journal.ppat.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AP, Buscaglia CA, Wang Q, Levay A, Nussenzweig DR, Walker JR, et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131(3):492–504. doi: 10.1016/j.cell.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman SL, Doolan DL. Malaria vaccines-targeting infected hepatocytes. Nat Med. 2000;6(11):1218–9. doi: 10.1038/81315. [DOI] [PubMed] [Google Scholar]
- 50.Limbach K, Aguiar J, Gowda K, Patterson N, Abot E, et al. Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malaria Journal. 2011;10:65. doi: 10.1186/1475-2875-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.