Abstract
NKT cells are known to play a role against certain microbial infections, including malaria and HIV, two major global infectious diseases. NKT cells exhibit either protective or pathogenic role against malaria. They are depleted by HIV infection and have a direct pathogenic role against many opportunistic infections common in end-stage AIDS. This review discusses the various features of the interaction between NKT cells and malaria parasites and HIV, and the potential to harness this interaction for therapeutic and vaccine strategies.
Keywords: NKT cell, glycolipid, CD1d, HIV, malaria, vaccine, adjuvant
1. Introduction
Natural killer T (NKT) cells are a relatively recently described subset of innate immune cells that have features of both T cells and natural killer (NK) cells. Their ability to rapidly secrete large amounts of pre-formed cytokines upon activation allows them to bridge the innate and adaptive immune responses, as this NKT cell activation leads to downstream recruitment and activation of dendritic cells (DCs), NK cells and CD4+ and CD8+ T cells. A significant proportion of NKT cells, called type I NKT cells, express an invariant T cell receptor (invTCR), characterized in mice by Vα14-Jα18 and Vβ8.2, Vβ7, or Vβ2, and in humans by Vα24-Jα18 and Vβ11, although the β chain can be somewhat variable. This invTCR recognizes lipid-based antigens in the context of CD1d molecules, an MHC Class I-like molecule expressed on antigen presenting cells (APCs) and other cell types. A second subgroup of CD1d-restricted NKT cells, also called type II NKT cells, utilizes other, more diversified TCRs to recognize CD1d molecules. NKT cells develop in the cortical thymus and undergo selection by CD1d molecules on cortical thymocytes [Reviewed in 1].
NKT cells are known to play a role in cancer surveillance, largely through their ability to activate natural killer cells upon binding lipid-based tumor antigens [2, 3]. NKT cells were initially discovered based on the finding that a novel glycolipid compound, α-galactosyl ceramide (α-GalCer), had anti-tumor activity [4]. Mechanistic studies elucidated that α-GalCer binds CD1d and then α-GalCer-CD1d complex activates NKT cells through their invTCR, leading to secondary activation of NK cells, DCs, and other leukocytes. α-GalCer has been used in humans as a potential therapy for cancer [5-10]. NKT cells also play a role in suppressing autoimmune disease. Circulating numbers of NKT cells are decreased in patients with certain autoimmune diseases, such as diabetes, lupus, and multiple sclerosis [reviewed in 11, 12]. Understanding the role of NKT cells in cancer and autoimmunity may yield promising new therapies.
NKT cells bridge the adaptive and innate immune responses to foreign lipid antigens. Known exogenous NKT cells ligands, which bind CD1d molecules and trigger activation of NKT cells through their invTCR, include α-linked glycosphingolipids from Sphingomonas [13-16], galactosyl diacylglycerols from Borrelia burgdorferi (b. burgdorferi) [13-16], lipophosphoglycan (LGP) on Leishmania donovani (l. donovani) [17] and phosphatidylinositol tetramannoside (PIM4) from Mycobacterium leprae (m. leprae) [18]. Upon binding CD1d molecules, these ligands are recognized by invTCR expressed by NKT cells and rapidly activate both NKT cells and APCs. Given the broad range of known NKT cell function, this article is focused on the role of NKT cells in infection and immunity against two major global pathogens, human immunodeficiency virus (HIV) and malaria.
2. NKT Cells in Malaria
2.1 Role of NKT cells in protective immunity against malaria
The role of NKT cells in protective immunity against malaria was first implicated in a study by Schofield et al [19]. In this study, CD1d-deficient mice that lack NKT cells, as well as wild-type control mice, were immunized twice with sporozoites of a rodent malaria parasite, Plasmodium berghei, and it was found that the level of humoral response against the circumsporozoite (CS) protein was strongly diminished in CD1d-deficient mice compared to wild-type mice. The study further demonstrated that glycosylphosphatidylinositol (GPI) purified from blood stages of a human malaria parasite, Plasmodium falciparum, was able to stimulate murine CD4+ NKT cells in vitro, suggesting that CD4+ NKT cells act as a helper T cell to facilitate the production of anti-CS antibody by B cells in vivo [19]. The role of NKT cells as a helper T cell against anti-malarial humoral response in vivo, however, was later questioned by two independent studies [20, 21]. Using CD1d-deficient mice and wild-type mice as a control, two research groups, compared the levels of humoral response against the CS protein upon immunization with radiation-attenuated sporozoites of P. yoelii or P. berghei, respectively. In the first study, performed by our group [20], it was shown that the level of anti-CS antibody response was not affected by the absence of CD1d molecules regardless of the genetic background of mice, i.e. BALB/c and C57BL/6, but was almost completely abolished in MHC-II-deficient mice. The second study by Romero et al [21] further demonstrated that both the level of humoral response against malaria and the level of protective immunity against malaria were comparable between CD1d-deficient and wild-type mice after P. berghei sporozoite immunization. Taken together, these latter two studies indicate the dispensable role of NKT cells in protective immunity against pre-erythrocytic stages of malaria.
Overall, NKT cells do not seem to have a clear physiological role against pre-erythrocytic stages of malaria. However, once NKT cells get activated by the artificial ligand α-GalCer, they are shown to display an inhibitory effect against the development of liver stages of malaria in vivo [22]. The fact that up to half of the T cells present in the liver consist of NKT cells favors the inhibitory activity of NKT cells against the hepatic stages of rodent malaria parasites. In this study, α-GalCer was able to display anti-parasite activity regardless of the strain of malaria parasite, i.e. P. yoelii or P. berghei, or mouse strain, i.e. BALB/c or C57BL/6 mice [22]. Using mice lacking CD1d and V 14 NKT cells, the activity of α-GalCer was confirmed to be CD1d- and iNKT cell-dependent. Furthermore, it was found that IFN-mediates the anti-plasmodial activity of α-GalCer-activated NKT cells (Fig. 1A), since the activity was completely abolished in mice lacking IFN-γ or IFN-γ receptors, but not in mice lacking TNF-α, perforin, or Fas-ligand. The study did not address whether the action of α-GalCer-activated NKT cells was due to bystander mechanism or through direct recognition of malaria-infected hepatocytes. It is interesting to note that a C-glycoside analogue of α-GalCer, called α-C-GalCer, which preferentially stimulates a Th1-type response in mice, was shown to display superior anti-plasmodial activity against the liver stages than the parental α-GalCer upon in vivo administration [23].
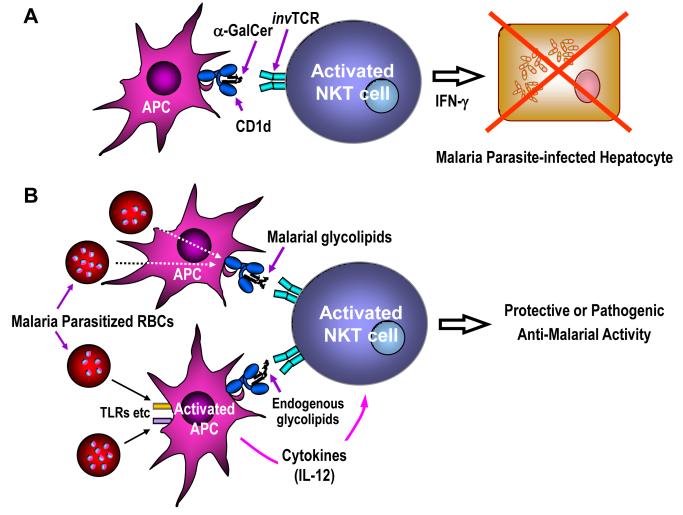
Figure 1.
Interaction between NKT cells and malaria parasites. A: NKT cells activated by a CD1d-binding glycolipid, α-GalCer, can exhibit inhibitory activity against the development of the liver stages of malaria, which is mediated by IFN-γ. B: NKT cells may get activated directly by plasmodial glycolipids/phospholipids that bind CD1d. Alternatively, plasmodial lipids may cause up-regulation of CD1d-binding endogenous glycolipids by stimulating antigen presenting cells (APCs) through toll-like receptors (TLRs) and other receptors. The endogenous glycolipids together with IL-12 secreted by stimulated APCs may, in turn, induce activation of NKT cells. Upon activation, NKT cells may exhibit protective or pathogenic anti-malarial activity.
Although α-GalCer and its analogue can display strong anti-plasmodial activity, it is not adequate to use these compounds as therapy against malaria infection, for two reasons. First, the malaria parasite resides within hepatocytes for only a brief period of time [24]. Second, once activated by a strong ligand such as α-GalCer, NKT cells rapidly become into an anergic state that lasts a 4-6 weeks [25, 26]. The study showed, in fact, that α-GalCer had to be administered 2 days before sporozoite-induced infection in order for the glycolipid to be able to eliminate malaria parasites from hepatocytes. Thus, there would be a very narrow time window for α-GalCer to be fully effective against the liver stage infection, and, hence, it is not practical to use α-GalCer as a preventive and/or therapeutic measure against malaria infection, unless it is used against a uniquely long-lasting liver stage infection, such as hypnozoite, caused by P. vivax infection.
With regard to the role of plasmodial GPI as a ligand to stimulate NKT cells, phosphoglycolipids have been isolated from protozoan parasites and have been shown to bind to CD1d molecules (Fig. 1B). In one study, both GPI mucins and glycoinositolphospholipids (GIPLs) were isolated and purified from Trypanosoma cruzi (T. cruzi) and have been shown to bind to plate-bound CD1d molecules in vitro [27]. However, these phosphoglycolipids were unable to stimulate NKT cells in vitro or in vivo, and humoral responses to these phosphoglycolipids in vivo were shown to be elicited in an MHC class II-restricted fashion, independent of CD1d [27]. This indicates that despite binding to CD1d, GPI mucins and GIPLs expressed by T. cruzi do not appear to evoke significant CD1d-restricted immune responses in vivo.
In another study, lipophosphoglycan (LPG) and GIPLs purified from L. donovani were shown not only to bind CD1d, but also to trigger a CD1d-dependent IFN-γ response mediated by a fraction of naive intrahepatic lymphocytes [17]. Since the binding of the CD1d/LPG or CD1d/GIPLs complex to the invTCR of NKT cells was not determined in this study, it remains possible that TCR binding to CD1d or other receptors, such as TLRs, LPG and GIPLs may up-regulate the expression of CD1d that bears endogenous glycolipids, which may, in turn, stimulate CD1d-restricted NKT cells with help from cytokines, such as IL-12, as demonstrated by several studies [28-31] (Fig. 1B). In other words, there has been no compelling evidence to date showing protozoan GPI to be a NKT cell ligand. In spite of the lack of evidence for malaria-derived antigens as a ligand for NKT cells, a number of studies have indicated that NKT cells get expanded in vivo during the course of blood stage infection by rodent malaria parasites, and that the NKT cells can exhibit anti-plasmodial activity.
An initial study by Pied et al. [32] showed that upon acute blood stage infection by P. yoelii, there was a preferential expansion of CD4−CD8− NKT cells in the liver. The study further showed that these NKT cells exhibit inhibitory activity against the liver stages in vitro, which was in part mediated by IFN-γ. This was somewhat in agreement with the study in which α-GalCer-activated NKT cells inhibit the liver stages [22]. However, more recently, this group has found that, when CD1d-deficient mice with C57BL/6 background were infected with malaria, there was no significant difference between wild-type and CD1d-deficient mice with regards to either the parasite burden in the liver or the parasitemia in the blood, thus indicating that CD1d and CD1d-dependent NKT cells are not required for the control of a primary P. yoelii infection in vivo [33]. Therefore, although NKT cells seem to display anti-plasmodial activity in vitro, CD1d-dependent NKT cells expanded in the liver do not seem to play a significant role in controlling malaria infection in vivo [33].
The protective role of CD1d-dependent NKT cells in controlling blood stage infection was underscored in a study by Abo et al. [34], which showed that parasitemia was prolonged in the blood of CD1d-deficient mice compared to wild-type control mice upon P. yoelii blood stage infection. However, the same group has later shown that the course of blood stage infection with P. yoelii was not significantly different between β2-microglobulin-deficient mice that also lack CD1d and wild-type control mice [35], suggesting the dispensable role of NKT cells. Although it is unclear why the two studies done by the same group resulted in different outcomes, in view of the great variability of the course of blood stage infection amongst individual mice, as determined by parasitemia, the mere prolongation, i.e. 2 days, of parasitemia in CD1d-deficient mice shown in their earlier study might not have led to a valid conclusion to support the definitive protective role of NKT cells. In addition, in view of the recently published studies demonstrating the biological activity of CD1d-dependent, type II NKT cells [36-38], the involvement of type II NKT cells in influencing the course of malaria infection cannot be ruled out.
An interesting study by Hansen et al. [39] has shown that when CD1d-dependent NKT cells get activated during the blood stage infection of P. berghei, they promote a Th2 type response that correlates with the enhancement of P. berghei-specific antibody responses, in particular those against GPI-anchored MSP-1 protein. In this study, it is noteworthy that antibody production against the GPI-anchored MSP-1 was significantly reduced in CD1d-deficient mice only during early time points after parasite challenge, whereas no differences between CD1d-deficient and wild-type mice were observed when anti-MSP-1 antibody response was evaluated after a longer time period of infection [39]. Although the precise mechanism is unknown, this study suggests the role of NKT cells as helper T cells to assist anti-malarial antibody production by B cells.
2.2 Pathogenic role of NKT cells against malaria
Infection by blood stages of P. berghei induces liver injury in mice, although these stages of parasites do not actively invade hepatocytes. Nakanishi et al. [40] have shown that DX5+ NKT cells obtained from the liver of CD1d-deficient mice infected with P. berghei blood stages could cause liver injury by killing normal hepatocytes. The hepatotoxicity of these hepatic DX5+ NKT cells occurs through an MHC-unrestricted fashion and does not require TCR engagement [40]. It would be interesting to know whether the liver injury caused by DX5+ NKT cells is mediated by bystander killing or other undefined cell-mediated killing mechanisms.
Hansen et al. [39] have shown that CD1d-deficient mice displayed considerably reduced splenomegaly associated with a reduced expansion of the splenic B cell population in response to infection by P. berghei. This indicates that CD1d-dependent NKT cells could contribute to the development of splenomegaly induced by P. berghei blood stage infection. In view of the high levels of CD1d expression by marginal zone B cells, a unique B cell subset that trap blood-born antigens such as malarial blood stages parasite products and present T-independent antigens, the study also indicates the role of CD1d-dependent NKT cells in promoting the development of B cell-mediated response to malaria infection.
The most important pathology of malaria is cerebral malaria, which is in fact the main cause of the mortality in this disease. P. berghei ANKA is known to cause cerebral malaria in mice, and the incidence of cerebral malaria is known to vary among mice having different genetic backgrounds. The study by Hansen et al. [41] found that during the course of P. berghei ANKA infection, CD1d-restricted NKT cells in BALB/c mice appear to polarize towards a Th2 response, which is associated with resistance to cerebral malaria, whereas these NKT cells induce early IFN-γ production and promote pathology in susceptible C57BL/6 mice. It seems that the differential expression of molecules encoded by the natural killer complex (NKC) located on chromosome 6 accounts in part for the opposing roles of NKT cells in C57BL/6 and BALB/c mice in response to P. berghei infection [41]. The study further indicates that the NKC is a significant genetic determinant of murine cerebral malaria, imparting partial protection or susceptibility depending on its genotype. Overall, this study has discovered that the expression of NKC is not only associated with the functional properties of CD1d-dependent NKT cells, but appears to be a significant genetic determinant of murine malarial fatalities due to cerebral malaria [41]. It would be important to determine the parasite-derived antigen(s), such as malarial GPI, which can stimulate CD1d-restricted NKT cells upon malaria infection.
More recently, Mitchell et al. [42] induced cerebral malaria in susceptible C57BL/6 mice by P. berghei ANKA infection, which was associated with an absence of a range of cytokine production at 24 h p.i. but a surge of IFN-γ production at 3 to 4 days p.i. In contrast, when they infected susceptible mice with a closely related strain, P. berghei K173 that does not cause cerebral malaria, infected mice developed transient production of a range of cytokines, most notably IFN-γ, in the spleen and liver. Surprisingly, when mice were co-infected with both ANKA and K173, a similar pattern of cytokine production was seen with respect to K173 alone, specifically, a burst of IFN-gamma at 24h post infection. This pattern correlated with the failure to develop cerebral malaria [42]. Early IFN-γ production was present in NK-depleted, γδ-depleted, and Jα281−/− (NKT-deficient) mice, but absent from β2-microglubulin-deficient mice that had been infected with P. berghei K173. These results suggest that the absence of an active suppression of immunopathological process involving IFN-γ and CD8+ T cells, but not other cells including NKT cells, in P. berghei ANKA infection allows the development of cerebral malaria [42].
2.3 Role of NKT cells in eliciting acquired immunity against malaria
Although NKT cells do not seem to have a direct “physiological” role in protection against pre-erythrocytic stages, as described earlier, once NKT cells are activated by the artificial ligand, α-GalCer, they are able to rapidly secrete cytokines, including IFN-γ, and display biological activity against the liver stages of malaria. This unique property of the swift activation of NKT cells by α-GalCer had led us to investigate whether activated iNKT cells could enhance acquired immunity elicited by various malaria vaccines. Our group found that when α-GalCer was co-administered to mice with irradiated P. yoelii sporozoites or a recombinant adenovirus expressing the CS protein of P. yoelii, the level of CS-specific T cell responses were greatly enhanced compared to those elicited by malaria immunogens alone [43] (Fig. 2). Furthermore, co-injection of α-GalCer with these malaria vaccines resulted in the enhancement of the level of protective anti-malaria immunity upon challenge with live P. yoelii sporozoites [43]. Interestingly, though, co-injection of α-GalCer failed to significantly enhance antigen-specific T cell responses induced by a poxvirus-based vaccine (data not shown). Using CD1d-deficient, as well as V 14NKT cell-deficient mice, we confirmed that the “adjuvant” effect of α-GalCer was dependent on CD1d/NKT pathway. Because α-GalCer was able to exhibit similar adjuvant effect both in BALB/c and B10.D2 mice, non-MHC background does not seem to have a significant effect on α-GalCer induced NKT cell activation. Finally, using mice lacking various effector molecules, we found that IFN-γ presumably produced by activated NKT cells, is the key mediator for the “adjuvant effect” [43] (Fig. 2). Two independent studies have shown that CD40-CD40 ligand interaction is also indispensable for α-GalCer to induce maturation/activation of dendritic cells, thereby displaying its adjuvant effect [44, 45] (Fig. 2).
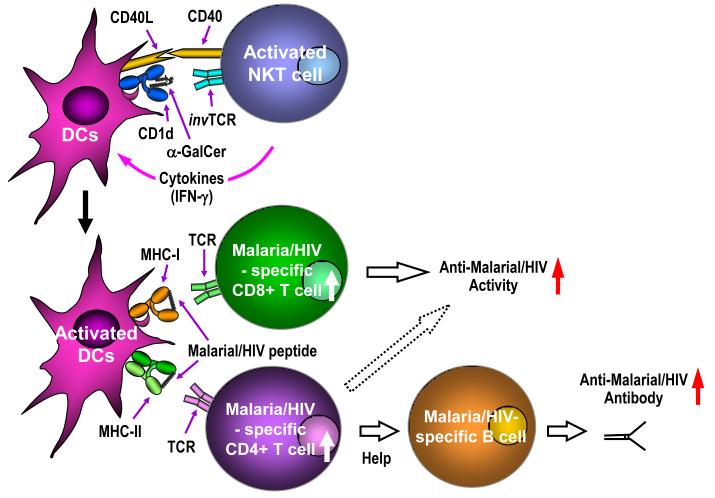
Figure 2.
Role of NKT cells in adaptive immunity against malaria and HIV. In the context of CD1d molecules, α-galactosyl ceramide (α-GalCer) stimulates NKT cells through their invariant T cell receptor (invTCR). Upon activation, NKT cells rapidly secrete cytokines such as IFN-γ, and together with CD40-CD40L interaction, induce activation and maturation of dendritic cells (DCs). Thus, co-administration of α-GalCer with vaccines expressing malaria or HIV antigens are able to enhance the efficacy of the vaccines by augmenting the levels of antigen-specific T cell and humoral responses.
More recently, Korten et al. [46] addressed an “intrinsic” adjuvant effect caused by CD1d-dependent NKT cells. This study showed that vaccination of mice with recombinant poxviruses, fowl pox and modified vaccinia Ankara (MVA) expressing a P. berghei CS protein, induced activation of both iNKT cells and NK cells in the liver of BALB/c mice, while inducing CS-specific CD8+ T cells secreting both IFN-γ and TNF-α. In this study, it was observed that when iNKT-deficient mice were vaccinated with the poxviruses, the number of double cytokine producing, CS-specific CD8+ T cells slightly decreased, with similar levels of anti-malarial protection at relatively early time points after vaccination [35]. However, at later time points after vaccination, a slightly better rate of protection was observed in V 14NKT-deficient mice compared to wild-type mice. This study altogether indicates that NKT cells activated by poxvirus vaccines help to generate malaria-specific T cells, but are not required for anti-malarial protection induced by the vaccines [46]. The finding of poxvirus-induced activation of NKT cells may explain why we failed to observe the adjuvant effect of α-GalCer only against poxvirus-based vaccines.
3. NKT Cells in HIV
3.1 Depletion of NKT cells in early HIV/SHIV infection
HIV causes acquired immune deficiency syndrome (AIDS) primarily via infection of CD4+ T cells, by simultaneous attachment to the CD4 receptor and the CCR5 or CXCR4 co-receptor, leading to immune dysfunction and CD4+ T cell depletion. Because a subset of NKT cells express the CD4 receptor on their cell surface, they are vulnerable to direct infection by HIV.
Van der Vliet et al. showed that circulating numbers of Vα24+ Vβ11+ NKT cells were reduced in a cross-sectional study of chronically infected HIV+ patients, independent of CD4+ T cell counts, CD4:CD8 ratios, and HIV plasma viral load, and irrespective of treatment with highly active antiretroviral therapy (HAART) in 37/50 patients. A separate longitudinal cohort study pre and post HIV seroconversion demonstrated that a substantial proportion of this NKT cell depletion occurs within the first year of infection. Because the authors had found that only a small percentage of NKT cells expressed the CD4+ receptor and the CCR5 co-receptor, while the majority expressed the Fas molecules, they surmised that this depletion was largely due to a continuous process of Fas-mediated activation induced cell death, rather than direct infection by HIV [47].
NKT cell depletion in HIV infected individuals has since been confirmed by several other groups [48-50], but the mechanism of depletion was contradicted by Sandberg et al., who described two distinct sets of CD4+ and CD4− NKT cells, defined by co-expression of Vα24 and CD161, a natural killer cell surface marker. The authors found that 93.4±2.5% of CD4− NKT cells and 33.3±14% of CD4+ NKT cells expressed CCR5, and almost all NKT cells expressed CXCR4. Furthermore, these distinct NKT cell subsets expressed differential homing receptors, with CD4+ NKT cells expressing the CD62L receptor for homing to lymph nodes, and CD4− NKT cells expressing the CD11a receptor for infiltration into tissues. The authors found that NKT cell depletion in HIV infected children was limited to the CD4+ NKT cells [49].
More recently, Fernandez et al. described a similar decline of NKT cells (defined as CD3+ cells binding α-GalCer-loaded CD1d: Ig dimer) in pigtail macaques following infection with CXCR4-tropic Simian Human Immunodeficiency Virus (SHIV)mn229. NKT cell depletion in pigtail macaques following CCR5-tropic SIVmac251 infection was slower and more variable, which mimics the relative patterns of CCR5-tropic and CXCR4-tropic HIV infection in humans [51]. These observations are consistent with the prior findings that CCR5 expression on human NKT cells is variable, whereas CXCR4 expression is nearly ubiquitous [49].
3.2 Direct infection of CD4+ NKT cells by HIV
The ability of HIV to directly infect NKT cells was confirmed by two separate groups. Motsinger et al. demonstrated that both the CXCRX4-tropic HIV NL4-3 and CCR5-tropic HIV BAL were able to infect three separate clonal NKT cell lines, all derived from primary human NKT cells, in both resting and active states. In contrast to the in vivo findings above, investigators observed that these clonal cell lines were more susceptible to the CCR5-tropic strain, corresponding with higher levels of CCR5 expression relative to CXCR4 expression [48]. α-GalCer activated NKT cells were more susceptible to HIV infection than resting NKT cells, possibly due to upregulation of CCR5 expression after activation. This same group later demonstrated that although macaque NKT cells are more highly skewed towards the CD8+, CD4− phenotype, the CD4+ NKT cell subset is highly susceptible to SIV infection, is depleted via cytolysis in vitro, and is capable of supportive productive virion replication [52].
In parallel, Fleuridor et al. also demonstrated the ability of R5 tropic HIV to productively infect three clonal NKT cell lines, which expressed higher levels of CCR5 than CXCR4. Interestingly, the kinetics of viral production lagged by about seven days with respect to HIV production from primary CD4+ T lymphocytes [53]. Taken together, these studies convincingly demonstrate that CD4+ NKT cells are preferentially infected by R5-tropic HIV, which is the predominant form of transmitted HIV. This likely accounts for the rapid depletion of NKT cells observed early in the course of HIV infection (Figure 3A).
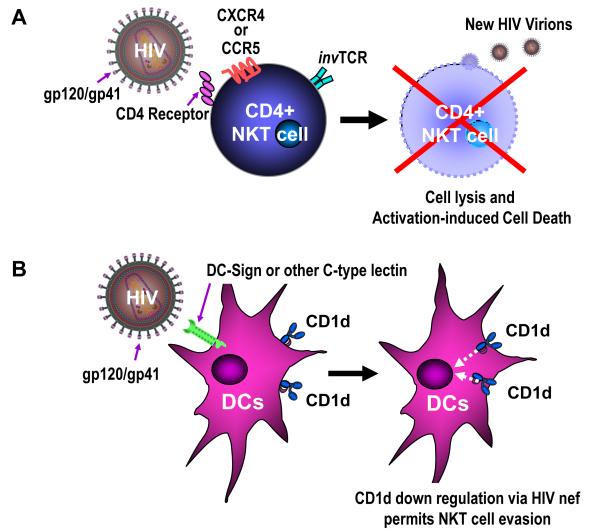
Figure 3.
Two primary mechanisms of HIV evasion against NKT cells. A: HIV directly infects CD4+ NKT cells in the same manner as conventional CD4+ T cells, via attachment of the gp120/gp41 envelope complex to the CD4 receptor, causing direct NKT cell lysis and/or activation-induced cell death. B: HIV can directly infect dendritic cells (DCs) through binding of DC-Sign or other C-type lectins on the DC surface. The HIV nef gene downregulates CD1d surface expression on the DC surface, impairing the ability for these antigen presenting cells (APCs) to bind glycolipids and activate NKT cells through binding of their invariant T cell receptor (invTCR) by the CD1d/glycolipid complex.
3.3 Partial reconstitution of NKT cell levels with HAART
There is some debate in the literature on whether initiation of highly active antiretroviral therapy (HAART) can prevent NKT cell depletion in HIV infection. Van der Vliet et al. initially reported that circulating NKT cell numbers remained low despite HAART in a chronically HIV-infected cohort [47]. A later more detailed study by the same group revealed that circulating NKT cell percentages in the peripheral blood increased significantly within three months of initiation of HAART, in a very similar pattern to the reconstitution of conventional CD4+ T cells [54]. Because it is well established that the initial rise in circulating CD4+ T cells reflects a redistribution of previously sequestered memory lymphocytes from the lymphoid tissue to the circulation, whereas the later more chronic rise in CD4 T+ cell count is a result of de novo proliferation and expansion of these cells [55,56], the authors hypothesized that a similar phenomenon was occurring within the NKT cell compartment. In support of this hypothesis, they observed that the early rise of circulating NKT cells in HIV+ patients initiating HAART consisted of predominantly CD4− NKT cells, suggesting that these cells were merely redistributed from NKT cells sequestered in the periphery.
Our group studied NKT cell levels in a cohort of 75 individuals who initiated HAART during acute infection (within 3-4 months of seroconversion). Although these individuals did not experience a rise in percentage of circulating NKT cells after one year of HAART compared to pre-treatment levels, they also did not experience any decline in NKT cell levels, suggesting a role of HAART in stabilizing the rate of NKT cell depletion by HIV [50]. A concurrent study by Yang et al. found that the increase in CD4+ NKT cells after initiation of HAART is detectable but gradual in the first year, and more pronounced after two years on therapy. Thus, reconstitution of NKT cells may parallel the biphasic reconstitution of conventionally CD4+ T cells [55,56], but in a more delayed manner. Simultaneous administration of interleukin 2 (IL-2) with HAART leads to a significant expansion of both the CD4+ and CD4− NKT cell compartments, again paralleling the CD4+ T cell reconstitution of both naïve and memory cells [57].
3.4 Restoration of NKT cell function by HAART during acute HIV infection
HAART can restore the circulating levels of innate immune cells, such as NK cells, but despite the restoration in total numbers of circulating cells, certain key effector functions, such as IFN-γ secretion, remain significantly impaired [58]. In contrast to NK cells, van der Vliet et al reported that although the reconstitution of NKT cells immediately following HAART was limited to the CD4− NKT cell subset, these cells remained functional in their ability to secrete both IFN-γ and IL4 in response to stimulation with α-GalCer in co-culture with CD1d transfected HeLa cells [54].
We conducted a study in HIV-infected subjects who initiated HAART during acute infection to test whether HIV-1 infection impairs NKT cell function with respect to HIV-uninfected controls, and whether function improves with antiretroviral therapy. NKT cell-enriched PBMCs from HIV-uninfected controls or from HIV-infected subjects were stimulated with increasing concentrations of CD1d:Ig dimeric protein loaded with α-GalCer. IFN-γ and IL-4 secretion from HIV-1 infected donors at pre-treatment baseline was also significantly lower than uninfected controls, indicating that NKT cell function is impaired in early HIV infection. In all subjects, both IFN-γ and IL-4 cytokine secretion was significantly greater one year after initiation of HAART, and comparable to levels in uninfected donors. This finding occurred irrespective of the change in total NKT cell percentage, indicating this was not merely due to reconstitution of NKT cells [50]. The mechanism for this augmentation remains to be determined, but may be multifactorial. CD1d is downregulated on CD14+ monocytes in HIV infection, and restored by HAART [59]. Differences in responsiveness to α-GalCer may therefore be due to altered presentation by antigen presenting cells. It is also plausible to speculate that, like CD4+ T cells, NKT cell function is compromised in early HIV infection due to increased general immune activation and downstream apoptosis.
In contrast, Moll et al. recently found that NKT cells were unable to proliferate or produce IFN-γ in response to stimulation with α-GalCer in a cohort of chronically HIV infected individuals. Furthermore, HAART had little impact on restoring NKT cell function [60]. This difference may be due in part to the fact that Moll et al used autologous antigen presenting cells expressing CD1d to measure function, whereas our study stimulated NKT cells with CD1d:ig dimmer loaded with αGalCer. Thus, the impaired function in the latter study may have been due in part to direct impairment of the antigen presenting cells by HIV infection. However, Synder-Cappione et al, found that impairment of IFN-γ and TNF-α secretion in response to α-GalCer occurred regardless of treatment with ART in chronically infected individuals, and correlated inversely with the number of years of HIV infection [61]. While there is still debate among clinicians on when to initiate antiretroviral therapy in HIV-infected patients, the contrast between these studies [48,60,61] may lend evidence to the importance of beginning early HAART in order to preserve NKT cell function.
3.5 NKT cell interactions with HIV
While NK cells have been shown to form a strong early cytotoxic response against HIV (62-64), differing mechanisms have been postulated on the role of NKT cells in HIV infection. NKT cells might either activate T cells to make them more prone to HIV infection, or secrete soluble factors to fight HIV infection [65]. Our studies indicate that when stimulated by glycolipid, the latter is likely the case, as supernatants from a stimulated population of PBMCs enriched for NKT cells caused potent suppression of HIV-1 replication [50]. The NKT cell-enriched population was stimulated in a ligand-specific, CD1d-restricted fashion, indicating that initial responding cells were strictly NKT cells. However, the effector mechanism for this anti-HIV suppression may be multifactorial. It has been shown in other diseases that NKT cells facilitate anti-tumor activity by secondary activation of NK cells and other lymphocytes via cytokine activation [66-68]. We therefore used supernatants from a purified NKT cell line to demonstrate that this anti-HIV effect is primarily caused by NKT cells. The addition of soluble anti-IFN-γ antibody abrogated the effect, indicating that the anti-HIV effect displayed by NKT cells is mediated by IFN-γ [50]. While secretion of IFN-γ is the primary mechanism for the anti-HIV effect of NKT cells in this in vitro system, it is plausible that NKT cells exert this effect in vivo by a combination of cytokine secretion, direct cytolysis, and secondary activation of other immune cells. The exact mechanism by which NKT cells suppress HIV replication remains to be elucidated.
At the same time, HIV has evolved mechanisms to evade the innate immune response from the host. In addition to depletion of NKT cells by direct infection and cytolysis, HIV also evades detection of NKT cells by directly downregulating CD1d cell surface expression on antigen presenting cells (Figure 3B). It has been well established that the nef gene of HIV induces downregulation of the MHC-I expression, thereby reducing presentation of HIV antigens to circulating T cells [69]. Hage et al first demonstrated that CD1d expression is downregulated on the surface of CD14+ monocytes derived from patients infected with HIV [70]. Subsequent reports confirmed that this down regulation is due to the HIV nef gene, as infection of CD1d-expressing cells with mutant HIV lacking nef did not lead to a similar downregulation of CD1d cell surface expression [71,72].
3.6 Consequences of NKT cell depletion and CD1d downregulation during HIV
The primary mechanism by which HIV causes morbidity and mortality is through depletion and functional impairment of CD4+ T cells, leading to acquired immunodeficiency syndrome (AIDS), where the host is rendered susceptible to a broad array of otherwise less harmful pathogens, known as “opportunistic infections”. While CD4+ T cell depletion may be the primary mechanism for immunosupression of the host, HIV also impairs the function of other immune cells, such as NK cells and dendritic cells [58,73,74]. It is therefore possible that NKT cell depletion and CD1d downregulation may also contribute to the development of opportunistic infections in end stage AIDS.
Two separate groups have reported an inverse correlation between both total NKT cell number and CD4+ NKT cell number with HIV plasma viral load [48,49], although CD4− NKT cells numbers are preserved. NKT cells have been documented to play protective role in host defense in a variety of bacterial, viral, and fungal pathogens that are common in AIDS patients, including mycobacterium tuberculosis (MTB), a bacterial pathogen causing chronic respitatory and extrapulmonary disease, which occurs with greater frequency and severity in persons co-infected with HIV. In the murine model, NKT cells contribute to the classic granulomatous reaction caused by MTB, likely through direct activation of NKT cells triggered by phosphatidylinositolmannosides (PIMs) from mycobacterial cell walls [75]. Individuals with active MTB infection have lower peripheral circulating NKT cell levels than both uninfected individuals and those with MTB exposure, and these levels do not increase within six months of MTB therapy [76]. NKT cells restrict MTB replication in vitro, and protect from aerosolized MTB challenge in mice [77]. Some controversy remains regarding the relevance of NKT cells to the mycobacterial response, as Vα24 deficient mice mounted similar pulmonary and systemic immune responses to mycobacterium bovis BCG [78]. Taken together, evidence points towards a role of NKT cells in contributing to the immune response to this important global pathogen.
Cryptococcus neoformans (c. neoformans) is a fungal pathogen which causes fatal meningoencephalitis in end stage AIDS patients. NKT cells are found to accumulate rapidly in the lungs of patients infected with c. neoformans [79]. Streptococcus pneumoniae (S. pneumoniae) is a ubiquitous gram positive bacteria that is a common cause of otitis media, sinusitis, and pneumonia in healthy individuals. It can cause recurrent, invasive, pneumococcal pneumonia (PCP) in HIV infected patients who are not on antiretroviral therapy. Jα281 knock out mice that lack Vα14+ NKT cell subset had marked exacerbation in disease course after exposure to S. pnuemoniae, as evidenced by shorter survival time and increased bacteria in the lung with respect to wild type mice [80]. Cytomegalovirus (CMV) is a viral pathogen that can cause chorioretinitis, enterocolitis, pneumonitis, and radiculopathy (central nervous system disease) in HIV infected patients with low CD4+ T cell counts. In the murine cytomegalovirus (MCMV) model, NKT cells become activated and produce IFN-γ in response to infection. CD1d-deficient mice display higher mortality to high dose MCMV infection [81]. Thus, the list of infectious diseases in which NKT cells play a role is long, diverse, and ever expanding. While further studies are needed, it is plausible to speculate that the NKT cell depletion caused by HIV contributes to the general state of immunocompromise and eventual susceptibility to opportunistic infections in end stage AIDS.
4. NKT-Cell Based Therapies for Infectious Diseases
4.1 Glycolipids as direct therapy
Because the earliest known NKT cell agonist, α-GalCer, was discovered based on its anti-tumor activity in vitro, clinical trials of glycolipid compounds to date have focused treating or controlling cancer. α-GalCer has been administered as a stand alone compound or through pulsing autologous dendritic cells ex vivo. Overall, the trials indicate that glycolipid-based therapies are safe, well-tolerated, and capable of augmenting both the innate natural killer response and the adaptive immune response [5-10].
There is evidence for the benefit of glycolipid therapy in treating infectious disease in the mouse model. As described earlier, our group has shown that inperitoneal administration of α-GalCer to mice infected with the rodent malaria parasites decreased the parasite burden [22]. Kawakami et al showed that mice treated with α-GalCer 3 days after infection with S. pneumoniae had a significantly reduced number of live bacteria in the lung compared to wild type mice. This effect was not seen in Vα14 deficient mice, indicating the specific role of NKT cells in the observed protective effect [80]. Chakerian et al found that treatment of mice with αGalCer reduced the bacterial burden in the lungs, diminished tissue injury, and prolonged survival of mice following inoculation with virulent MTB [82]. Finally, our demonstration of the role of NKT cells in suppressing HIV infection raises the question of the potential therapeutic effect of αGalCer or other NKT-activating glycolipids on HIV, either through innate immune activation or through reactivation of the latent reservoir. Exploration of the clinical utility of glycolipid therapy in the treatment of infectious diseases is warranted.
4.2 NKT cells in vaccine development
The development of effective vaccines against malaria and HIV has been difficult for a number of reasons. Because both are intracellular pathogens, an effective vaccine will likely have to induce both humoral and cell mediated immunity. To date, the majority of effective, licensed vaccines rely on inducing a pathogen-specific antibody response as the primary means of protection. Thus, our knowledge on how best to induce a protective adaptive T cell response is relatively limited. The ability of NKT cells to augment the adaptive immune response allows us to harness these cells to improve the magnitude and the quality of the cell mediated immune response to vaccines.
As described earlier, our group first demonstrated that co-administration of αGalCer with malaria vaccines enhanced not only the magnitude of malaria-specific CD8+ T cell reponse, but also the level of protective anti-malaria immunity [43]. We have subsequently shown that intramuscular coadministration of α-GalCer with DNA-based vaccines encoding HIV antigens increases both antigen-specific IFN-γ secreting splenocytes as well as humoral responses, although protection against HIV can not be established due to lack of a mouse challenge model [83]. This is not route-specific, as intradermal administration of α-GalCer with a DNA vaccine encoding a Leishmania antigen improves boosting with a vaccinia-based vaccine encoding the same antigen in mice [84]. Incorporating small amounts of αGalCer and a synthetic analogue, α-C-GalCer, into live Bacillus Calmette-Guérin (BCG) vaccine augments anitgen-specific CD8+ T cell responses and improves protection against challenge by virulent MTB in mice [85].
Glycolipids have the potential to boost not only the T cell response, but also antibody responses to vaccine antigens. Galli et al demonstrated that α-GalCer can boost antibody responses to protein vaccines by 1-2 logs, and that this effect is seen in mice lacking MHC classII molecules, indicating that NKT cells have the potential to substitute for CD4+ T cell help to B cells [86]. By coupling B cell antigens directly to α-GalCer or to beads coated with α-GalCer, two independent recent studies have extended these results by demonstrating that the cross-talk between antigen specific B cells and iNKT cells results in B cell differentiation into plasma cells and higher antibody titers [87, 88].
Because many infectious diseases are transmitted via respiratory or genital mucosal surfaces, recent attention has turned to eliciting mucosal immunity to contain the pathogen at the initial point of entry. Ko et al demonstrated that intranasal adminsitration of αGalCer with PR8 hemagglutinin antigen to mice led to subsequent protection from influenza challenge. Intranasal administration of αGalCer with a replication-deficient live adenoviral vaccine expressing LacZ elicited humoral responses in nasal washes, lung and sera, and LacZ-specific IFNγ producing CD8+ splenocytes at higher levels than vaccine alone [89]. Although NKT cell frequency at the nasal mucosa is low, nasal administration of αGalCer induces a localized increase in the NKT cell population, which is partly dependent on CXCL16/CXCR6. Antigen-specific IgA production is dependent upon IL-4 production from NKT cells [90]. Despite previously described phenomenon of systemic NKT cell anergy, intranasal administration of α-GalCer is effective in eliciting antigen-specific responses to HIV peptides when delivered repeatedly to mice at intervals of 5 days for up to three vaccinations, suggesting that mucosal delivery may be more effective in overcoming NKT cell anergy [91]. Lindqvist et al showed that both intranasal and intravaginal administration of aGalCer with herpes simplex virus-2 (HSV-2) glycoprotein D (gD) elicited gD-specific lymphoproliferative and IFNγ responses in the genital lymph nodes and spleen, and that vaginally immunized mice were protected against HSV2 challenge [92].
Because NKT cells secrete both Th1 and Th2 cytokines, the balance between these two cytokines can drive the adaptive immune response to immune activation or immune suppression. A study by Miyahira et al showed that coadministration of α-GalCer with a DNA vaccine encoding an antigen for T. cruzi actually impaired induction of epitope-specific CD8+ T cells and resulted in increased parasitemia in mice challenged with T. cruzi compared to vaccine alone [93]. It is possible that the adjuvant effect of αGalCer is disease specific, and may be heavily influenced by the balance of Th1 versus Th2 cytokine secretion. Because of this, efforts are underway to identify novel synthetic glycolipid adjuvants with differing Th1:Th2 activation profiles. It is thought that production of Th1 cytokines correlates with antitumor, antibacterial, antiviral, and vaccine adjuvant effects, while Th2 cytokine production leads to immunosuppression, which may be advantageous in the control of autoimmune disease [94, 95]. Several studies have elucidated the structure activity relationship (SAR) between glycolipid structure and Th1 versus Th2 cytokine production from NKT cells. Variables influencing this ratio include length of the lipid tail of the glycolipid compound, alpha versus beta-anomeric compounds, binding affinity to TCR, binding affinity to CD1d, stability of the CD1d/glycolipid complex [96-100]. Interestingly, Liang et al. found that the binding affinity of the glycolipid with the CD1d complex correlates strongly with IFN-γ secretion from NKT cells, but not IL-4 secretion [100]. Most recently, our group has demonstrated that the binding affinity to the invariant TCR of NKT cells supercedes the binding affinity to CD1d as a predictor of the biological potency of glycolipids [101]. Taken together, these studies allow for rational design of novel glycolipid compounds skewed towards eliciting a stronger Th1 or Th2 response. Several analogues of α-GalCer have been synthesized to date [96,97,100,102-105], some of which have shown equivalent or superior adjuvant activity in mice [85,106].
5. Conclusion
While the mechanism of the anti-HIV effect of NKT cells may be non-specific, the rapidity and magnitude of the cytokine response ensures that NKT cells are key players in the initial host immune response. Their role in preventing autoimmunity, protection against neoplasia, and in fighting other viral, fungal and parasitic pathogens has been well established. Strategies to preserve NKT cell number and function during HIV and other infections may therefore become important in improved long-term prognosis. Glycolipids that activate NKT cells have therapeutic potential in the treatment of infectious diseases, as well as adjuvants for vaccines against these pathogens.
Acknowledgements
This work was supported in part by an NIH Grant AI070258, and by support from Cytheris, Otsuka Pharmaceutical Co., the Irene Diamond Foundation, and Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- [2].Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–71. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- [3].Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- [4].Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–34. [PubMed] [Google Scholar]
- [5].Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- [6].Nieda M, Okai M, Tazbirkova A, Lin H, Yamamura A, Ide K, et al. Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- [7].Ishikawa A, Shinichiro M, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A Phase I Study of α-Galactosylceramide (KRN7000)-Pulsed Dendritic Cells in Patients with Advanced and Recurrent Non-Small Cell Lung Cancer. Clin Cancer Res. 2005;11:1910–17. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- [8].Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–45. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- [11].Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- [12].Ronchi F, Falcone M. Immune regulation by invariant NKT cells in autoimmunity. Front Biosci. 2008 May 1;13:4827–37. doi: 10.2741/3042. [DOI] [PubMed] [Google Scholar]
- [13].Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- [14].Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- [15].Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- [16].Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–15. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci USA. 2004;101:10685–90. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–9. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- [20].Molano A, Chiu Y-H, Nosseir S, Bendelac A, Tsuji M. Cutting Edge: The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: Exploring the role of GPIs in NKT cell activation and antimalarial responses. J. Immunol. 2000;164:5005–9. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- [21].Romero JF, Eberl G, MacDonald HR, Corradin G. CD1drestricted NK T cells are dispensable for specific antibody responses and protective immunity against liver stage malaria infection in mice. Parasite Immunol. 2001;23:267–9. doi: 10.1046/j.1365-3024.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- [22].Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. α-GalCer-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, et al. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232:881–4. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- [25].Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor downmodulation and expansion. Proc. Natl. Acad. Sci. USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Procopio DO, Almeida IC, Torrecilhas AC, Cardoso JE, Teyton L, Travassos LR, et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J Immunol. 2002;169:3926–3933. doi: 10.4049/jimmunol.169.7.3926. [DOI] [PubMed] [Google Scholar]
- [28].Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 29].Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–6. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- [31].Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, et al. Liver CD4−CD8− NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- [33].Soulard V, Roland J, Sellier C, Gruner AC, Leite-de-Moraes M, Franetich JF, et al. Primary infection of C57BL/6 mice with Plasmodium yoelii induces a heterogeneous response of NKT cells. Infect Immun. 2007;75:2511–22. doi: 10.1128/IAI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mannoor MK, Weerasinghe A, Halder RC, Reza S, Morshed M, Ariyasinghe A, et al. Resistance to malarial infection is achieved by the cooperation of NK1.1(+) and NK1.1(−) subsets of intermediate TCR cells which are constituents of innate immunity. Cell Immunol. 2001;211:96–104. doi: 10.1006/cimm.2001.1833. [DOI] [PubMed] [Google Scholar]
- [35].Taniguchi T, Tachikawa S, Kanda Y, Kawamura T, Tomiyama-Miyaji C, Li C, et al. Malaria protection in beta 2-microglobulin-deficient mice lacking major histocompatibility complex class I antigens: essential role of innate immunity, including gammadelta T cells. Immunology. 2007;122:514–21. doi: 10.1111/j.1365-2567.2007.02661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Teng MW, Yue S, Sharkey J, Exley MA, Smyth MJ. CD1d activation and blockade: a new antitumor strategy. J Immunol. 2009;182:3366–71. doi: 10.4049/jimmunol.0802964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–36. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- [38].Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–12. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hansen DS, Siomos MA, De Koning-Ward T, Buckingham L, Crabb BS, Schofield L. CD1d-restricted NKT cells contribute to malarial splenomegaly and enhance parasite-specific antibody responses. Eur J Immunol. 2003;33:2588–98. doi: 10.1002/eji.200323666. [DOI] [PubMed] [Google Scholar]
- [40].Adachi K, Tsutsui H, Seki E, Nakano H, Takeda K, Okumura K, et al. Contribution of CD1d-unrestricted hepatic DX5+ NKT cells to liver injury in Plasmodium berghei-parasitized erythrocyte-injected mice. Int Immunol. 2004;16:787–98. doi: 10.1093/intimm/dxh080. [DOI] [PubMed] [Google Scholar]
- [41].Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity. 2003;18:391–402. doi: 10.1016/s1074-7613(03)00052-9. [DOI] [PubMed] [Google Scholar]
- [42].Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun. 2005;73:5645–53. doi: 10.1128/IAI.73.9.5645-5653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gonzalez-Aseguinolaza G, Van Kear L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, et al. The natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- [45].Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Korten S, Anderson RJ, Hannan CM, Sheu EG, Sinden R, Gadola S, et al. Invariant Valpha14 chain NKT cells promote Plasmodium berghei circumsporozoite protein-specific gamma interferon- and tumor necrosis factor alpha-producing CD8+ T cells in the liver after poxvirus vaccination of mice. Infect Immun. 2005;73:849–58. doi: 10.1128/IAI.73.2.849-858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van der Vliet HJ, von Blomberg BM, Hazenberg MD, Nishi N, Otto SA, van Benthem BH, et al. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168:1490–5. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- [48].Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869–79. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, Palumbo P, et al. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–34. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vasan S, Poles MA, Horowitz A, Siladji EE, Markowitz M, Tsuji M. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int Immunol. 2007;19:943–51. doi: 10.1093/intimm/dxm055. [DOI] [PubMed] [Google Scholar]
- [51].Fernandez CS, Chan AC, Kyparissoudis K, De Rose R, Godfrey DI, Kent SJ. Peripheral NKT cells in simian immunodeficiency virus-infected macaques. J Virol. 2009;83:1617–24. doi: 10.1128/JVI.02138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Motsinger A, Azimzadeh A, Stanic AK, Johnson RP, Van Kaer L, Joyce S, et al. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J Virol. 2003;77:8153–8. doi: 10.1128/JVI.77.14.8153-8158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fleuridor R, Wilson B, Hou R, Landay A, Kessler H, Al-Harthi L. CD1d-restricted natural killer T cells are potent targets for human immunodeficiency virus infection. Immunology. 2003;108:3–9. doi: 10.1046/j.1365-2567.2003.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54].van der Vliet HJ, van Vonderen MG, Molling JW, Bontkes HJ, Reijm M, Reiss P, et al. Cutting edge: Rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J Immunol. 2006;177:5775–8. doi: 10.4049/jimmunol.177.9.5775. [DOI] [PubMed] [Google Scholar]
- [55].Pakker NG, Notermans DW, de Boer RJ, Roos MT, de Wolf F, Hill A, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- [56].Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–8. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, Nixon DF. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006;107:3081–3. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–70. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- [59].Hage CA, Kohli LL, Cho S, Brutkiewicz RR, Twigg HL, 3rd, Knox KS. Human immunodeficiency virus gp120 downregulates CD1d cell surface expression. Immunol Lett. 2005;98:131–5. doi: 10.1016/j.imlet.2004.10.025. [DOI] [PubMed] [Google Scholar]
- [60].Moll M, Kuylenstierna C, Gonzalez VD, Andersson SK, Bosnjak L, Sönnerborg A, et al. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol. 2009;39:902–11. doi: 10.1002/eji.200838780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Snyder-Cappione JE, Loo CP, Carvalho KI, Kuylenstierna C, Deeks SG, Hecht FM, et al. Lower cytokine secretion ex vivo by natural killer T cells in HIV-infected individuals is associated with higher CD161 expression. AIDS. 2009;23:1965–70. doi: 10.1097/QAD.0b013e32832b5134. [DOI] [PubMed] [Google Scholar]
- [62].Bernstein HB, Kinter AL, Jackson R, Fauci AS. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res Hum Retroviruses. 2004;20:1189–95. doi: 10.1089/aid.2004.20.1189. [DOI] [PubMed] [Google Scholar]
- [63].Alter G, Teigen N, Ahern R, Streeck H, Meier A, Rosenberg ES, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–60. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- [64].Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179:2097–104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- [65].Unutmaz D. NKT cells and HIV infection. Microbes Infect. 2003;5:1041–7. doi: 10.1016/s1286-4579(03)00185-0. [DOI] [PubMed] [Google Scholar]
- [66].Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl Acad Sci USA. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nakui M, Iwakabe K, Ohta A, Sekimoto M, Sato M, Makuuchi H, et al. Natural killer T cell ligand alpha-galactosylceramide inhibited lymph node metastasis of highly metastatic melanoma cells. Jpn J Cancer Res. 1999;90:801–4. doi: 10.1111/j.1349-7006.1999.tb00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, et al. Human invariant Valpha24+ natural killer Tcells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumor activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99:229–34. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- [70].Hage CA, Kohli LL, Cho S, Brutkiewicz RR, Twigg HL, 3rd, Knox KS. Human immunodeficiency virus gp120 downregulates CD1d cell surface expression. Immunol Lett. 2005;98:131–5. doi: 10.1016/j.imlet.2004.10.025. [DOI] [PubMed] [Google Scholar]
- [71].Cho S, Knox KS, Kohli LM, He JJ, Exley MA, Wilson SB, Brutkiewicz RR. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology. 2005;337:242–52. doi: 10.1016/j.virol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- [72].Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu XN. HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol. 2006;36:278–86. doi: 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- [73].Andrieu M, Chassin D, Desoutter JF, Bouchaert I, Baillet M, Hanau D, et al. Downregulation of major histocompatibility class I on human dendritic cells by HIV Nef impairs antigen presentation to HIV-specific CD8+ T lymphocytes. AIDS Res Hum Retroviruses. 2001;17:1365–70. doi: 10.1089/08892220152596623. [DOI] [PubMed] [Google Scholar]
- [74].Kawamura T, Gatanaga H, Borris DL, Connors M, Mitsuya H, Blauvelt A. Decreased stimulation of CD4+ T cell proliferation and IL-2 production by highly enriched populations of HIV-infected dendritic cells. J Immunol. 2003;170:4260–6. doi: 10.4049/jimmunol.170.8.4260. [DOI] [PubMed] [Google Scholar]
- [75].Apostolou I, Takahama Y, Belmant C, Kawano T, Huerre M, Marchal G, et al. Murine natural killer T(NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc Natl Acad Sci U S A. 1999;96:5141–6. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76].Snyder-Cappione JE, Nixon DF, Loo CP, Chapman JM, Meiklejohn DA, Melo FF, et al. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J Infect Dis. 2007;195:1361–4. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- [77].Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4(12):e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Minimal contribution of Valpha14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol Immunol. 2002;46:207–10. doi: 10.1111/j.1348-0421.2002.tb02687.x. [DOI] [PubMed] [Google Scholar]
- [79].Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol. 2001;167:6525–32. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- [80].Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–30. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- [81].Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4(7):e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70:6302–9. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–16. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [84].Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, et al. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol. 2008;38:706–19. doi: 10.1002/eji.200737660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Venkataswamy MM, Baena A, Goldberg MF, Bricard G, IM JS, Chan J, et al. Incorporation of NKT Cell-Activating Glycolipids Enhances Immunogenicity and Vaccine Efficacy of Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol. 2009;183:1644–56. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–9. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–44. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105:8345–50. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89].Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–17. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- [90].Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, Mori K, et al. Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1:208–18. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- [91].Courtney AN, Nehete PN, Nehete BP, Thapa P, Zhou D, Sastry KJ. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine. 2009;27:3335–41. doi: 10.1016/j.vaccine.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lindqvist M, Persson J, Thörn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182:6435–43. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- [93].Miyahira Y, Katae M, Takeda K, Yagita H, Okumura K, Kobayashi S, et al. Activation of natural killer T cells by alpha-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect Immun. 2003;71:1234–41. doi: 10.1128/IAI.71.3.1234-1241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- [95].Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- [96].Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–3. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- [97].Shimamura M. Glycolipid stimulators for NKT cells bearing invariant V alpha 19-J alpha 33 TCR alpha chains. Mini Rev Med Chem. 2008;8:285–9. doi: 10.2174/138955708783744119. [DOI] [PubMed] [Google Scholar]
- [98].McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–44. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Parekh VV, Singh AK, Wilson MT, Olivares-Villagómez D, Bezbradica JS, Inazawa H, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173:3693–706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- [100].Liang PH, Imamura M, Li X, Wu D, Fujio M, Guy RT, et al. Quantitative microarray analysis of intact glycolipid-CD1d interaction and correlation with cell-based cytokine production. J Am Chem Soc. 2008;130:12348–54. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Li X, Shiratsuchi T, Chen G, Dellabona P, Casorati G, Franck RW, et al. Invariant TCR rather than CD1d shapes the preferential activities of C-glycoside analogues against human versus murine iNKT cells. J Immunol. 2009;183:4415–21. doi: 10.4049/jimmunol.0901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Reddy BG, Silk JD, Salio M, Balamurugan R, Shepherd D, Ritter G, et al. Nonglycosidic agonists of invariant NKT cells for use as vaccine adjuvants. ChemMedChem. 2009;4:171–5. doi: 10.1002/cmdc.200800354. [DOI] [PubMed] [Google Scholar]
- [103].Fuhshuku K, Hongo N, Tashiro T, Masuda Y, Nakagawa R, Seino K, et al. RCAI-8, 9, 18, 19, and 49-52, conformationally restricted analogues of KRN7000 with an azetidine or a pyrrolidine ring: Their synthesis and bioactivity for mouse natural killer T cells to produce cytokines. Bioorg Med Chem. 2008;16:950–64. doi: 10.1016/j.bmc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- [104].Shimamura M, Huang YY, Okamoto N, Suzuki N, Yasuoka J, Morita K, et al. Modulation of Valpha19 NKT cell immune responses by alpha-mannosyl ceramide derivatives consisting of a series of modified sphingosines. Eur J Immunol. 2007;37:1836–44. doi: 10.1002/eji.200636689. [DOI] [PubMed] [Google Scholar]
- [105].Ndonye RM, Izmirian DP, Dunn MF, Yu KO, Porcelli SA, Khurana A, et al. Synthesis and evaluation of sphinganine analogues of KRN7000 and OCH. J Org Chem. 2005;70:10260–70. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]
- [106].Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, Franck RW, et al. Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine. 2009;27:3766–74. doi: 10.1016/j.vaccine.2009.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]