Abstract
C-glycoside analogues of α-galactosylceramide were shown to activate both human and mouse invariant NKT (iNKT) cells. Among these analogues, GCK152, which has an aromatic ring in the acyl chain, exhibited a stronger stimulatory activity against human iNKT cells and a much weaker activity against murine iNKT cells than GCK127 that has an almost identical fatty acyl chain as α-galactosylceramide. In this study, we have found that invariant TCR (invTCR) expressed by iNKT cells, but not CD1d expressed by APCs, command the species-specific preferential activity of C-glycosides, and that their preferential activity against human vs murine iNKT cells correlate with the binding affinity of glycolipid-CD1d complex to invTCR of respective iNKT cells rather than that of glycolipid to human or murine CD1d molecules. Overall, the structural difference of invTCR appears to supersede those of CD1d molecule in shaping the strength of the biological activity of C-glycoside analogues.
Murine invariant NKT (iNKT)3 cells express an invariant TCR (invTCR), which consists of α-chain encoded by a Vα14-Jα18 rearranged gene that is preferentially associated with β-chain encoded by Vβ8.2, Vβ2, or Vβ7. In human, iNKT cells express an invTCR, consisting of α-chain encoded by Vα24-Jα18 gene segment mainly associated with Vβ11 β-chain (1). A marine sponge-derived glycolipid, α-galactosylceramide (α-GalCer), is the first identified ligand being recognized by invTCR of both human and murine iNKT cells in the context of a CD1d molecule, a nonclassical MHC class I-like molecule. When activated by α-GalCer, iNKT cells rapidly produce both Th1 (IFN-γ) and Th2 (IL-4) cytokines (2, 3). More recently, various analogues of α-GalCer have been synthesized and shown to induce altered ratios of Th1/Th2 production by iNKT cells (4 –7).
In our previous study, we found that several C-glycoside analogues, which have an E-alkene linker between sugar and lipid moieties, were able to activate both human and murine iNKT cells in vitro (8). Interestingly, among these analogues, GCK152, which has an aromatic ring in the tail of the acyl chain, displayed a potent stimulation of human iNKT cells to secrete both Th1 and Th2 cytokines, but failed to significantly stimulate murine iNKT cells both in vivo and in vitro (8). In contrast, GCK127, an analog with a fatty acyl chain very similar to α-GalCer, exhibited a potent stimulatory activity against murine iNKT cells but a weaker activity than GCK152 against human iNKT cells (8). We hypothesized that the distinct activities of GCK127 and GCK152 against human vs murine iNKT cells could be due to the structural difference between human and mouse CD1d molecules, or due to those between the invTCRs of mouse vs human iNKT cells. In the current study, using human/mouse CD1d:Ig dimeric proteins, we have determined the relative binding affinity of GCK127 and GCK152 against human vs mouse CD1d molecules, as well as against human vs mouse iNKT cells. By coculturing cells that express human or mouse invTCR in combination with APCs that express either mouse or human CD1d, we identified the cells that are accountable for species-specific preferential activity of the two C-glycoside analogues.
Materials and Methods
Glycolipids and mice
GCK127 and GCK152 were synthesized following the procedures described (9). Six- to 8-wk old female C57BL/6 mice were purchased from Taconic Farms and maintained under standard conditions in The Laboratory Animal Research Center of The Rockefeller University.
Determination of serum cytokine concentration
C57BL/6 mice were administrated i.v. with 1 μg of each glycolipid. The sera were collected from mice 2, 6, 12, 24, and 48 h after the glycolipid treatment, and the serum concentration of IFN-γ and IL-4 were measured by way of a sandwich ELISA (eBioscience).
Isolation and generation of immature human dendritic cells (DCs) and human iNKT cell lines
Immature DCs and human iNKT cell lines, expressing Vα24 TCR, were generated, as previously reported (8). In brief, Vα24+ cells isolated from peripheral blood mononuclear cells were cocultured with mitomycin-C (Sigma-Aldrich) treated autologous immature DCs for 24 h in the presence of 100 ng/ml α-GalCer and 10 IU/ml a recombinant human IL-2 (R&D Systems), and further cultured for 7–10 days in the presence of 10 IU/ml human IL-2 alone. After two cycles of stimulations, >95% of NKT cells were shown to be Vα24+ cells by FACS.
A mouse iNKT hybridoma and mouse iNKT cell lines
A mouse iNKT hybridoma, 1.2, which coexpresses mouse invariant Vα14-Jα18 and Vβ8.2 chains, was provided by Dr. Mitchell Kronenberg (La Jolla Institute of Allergy and Immunology, San Diego, CA). Generation of mouse iNKT hybridoma, 58αβ, which coexpresses human invariant Vα24-Jα18 and Vβ11 chains has previously been described (10, 11). Mouse iNKT cell lines were generated as described with some modifications (12, 13). Bone marrow progenitor cells from C57BL/6 mice were cultured with 10 ng/ml mouse GM-CSF for 7 days to generate immature DCs. Thymocytes were then cocultured with autologous immature DCs in the presence of 100 ng/ml α-GalCer for 10 days. After being purified with lympholyte-M (Accurate Chemical), cells were restimulated with immature DCs in the presence of 100 ng/ml α-GalCer and 10 μg/ml mouse IL-2 (Cell Sciences) for 1 wk. After three cycles of stimulations, >95% of NKT cells were shown to react with mouse CD1d-dimer loaded with α-GalCer by FACS.
Generation of Hela cells expressing mouse CD1d molecules
A plasmid, pCDNA3-CD1.1, which encodes mouse CD1d gene was provided by Dr. Chyung-Ru Wang (Northwestern University, Chicago, IL) (14). Five × 105 wild-type Hela cells were cultured overnight and then transfected with 4 μg of pCDNA3-CD1.1 plasmid, using 10 μl of Lipofectamine 2000 (Invitrogen). The expression of mouse CD1d molecules, consisting of mouse CD1d H chain associated with human β2 microglobulin, were confirmed, 24 h later, by staining transfected Hela cells with PE labeled-anti-mouse CD1d Ab, followed by FACS analysis. More than 60% of the transfected Hela cells were found to express mouse CD1d molecules (data not shown).
Quantification of the level of cytokines produced by human iNKT cell lines, mouse iNKT cell lines and mouse iNKT hybridoma cells by ELISA
Twenty thousand human iNKT cells, or the same number of mouse iNKT cells or mouse iNKT hybridoma cells, were cocultured with 2 × 104 Hela cells transfected with a human CD1d gene (provided by Dr. Steven Porcelli, Albert Einstein College of Medicine, Bronx, NY), Hela cells transfected with a mouse CD1d gene, or A20 cells transfected with a mouse CD1d gene (provided by Dr. Mitchell Kronenberg), in the presence of an indicated concentration of each glycolipid. After 24-h incubation, the culture supernatants were collected and the concentrations of human IFN-γ and IL-4 and mouse IFN-γ, IL-2, and IL-4 in the supernatants were determined by ELISA (eBioscience).
A competitive ELISA, using 18:1 PE lipid as an indicator
Affinities between glycolipid analogues and human or mouse CD1d molecules were measured by a competitive ELISA, using 18:1 Biotinyl PE (1,2-Dioleoyl-sn-Glycero-3- Phosphoethanolamine-N-(Biotinyl)) (Avanti Polar Lipid) as a competitor, as described (15). In brief, flat-bottom 96-well plates (Thermo Fisher Scientific) coated with 3 μg/ml goat anti-mouse IgG1 Ab (Thermo Fisher Scientific) were washed and incubated with 1 μg/ml hCD1d: mIgG dimer (BD Biosciences), or mCD1d: mIgG dimer (BD Biosciences) for 2 h. After washing, glycolipid analogues were serially diluted with PBS in the presence of 1 μg/ml 18:1 Biotinyl PE lipid and then added into wells immediately, followed by overnight incubation at 37°C. In brief, 18:1 Caproylamine PE lipid (1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine- N-(hexanoylamine)) (Avanti Polar Lipid) was used as a positive control. After 1 h of incubation, the plates were washed and the amount of Biotinyl PE-CD1d complex was determined by adding HRP-labeled Avidin (eBioscience). Inhibition curve was made with GraphPad Prism software (Ver 4.0 GraphPad Software).
hCD1d: Ig dimer or mCD1d: Ig dimer loaded with glycolipids
hCD1d: mIgG dimer or mCD1d: mIgG dimer molecules were conjugated with PE or allophycocyanin, using Lynx Rapid RPE Ab Conjugation Kit (ABD Serotec) or Lynx Rapid APC Ab Conjugation Kit (ABD serotec), respectively. Each PE or allophycocyanin-labeled CD1d: mIgG dimer molecule was loaded with 1000 folder molar ratio excess of GCK127, GCK152, or α-GalCer for overnight at 37°C in dark and unloaded glycolipids were washed off by Microcon Centrifugal Filter Devices (Millipore). These glycolipid-loaded, PE/allophycocyanin-labeled hCD1d dimers or mCD1d dimers were used to stain human or mouse iNKT cells.
Staining of iNKT cells with a serially diluted PE-labeled CD1d-glycolipid complex
Human and mouse iNKT cells were stained with PE-conjugated CD1d: mIgG dimer loaded with glycolipids according to the published protocols (12, 16). In brief, varying doses, from 10 −.5 to 102.5 nM, of PE-labeled CD1d dimer-loaded with glycolipids and 10 μg/ml FITC-labeled anti-CD3ε mAb were incubated with 2 × 105 human iNKT cells or mouse iNKT hybridoma cells on ice for 30 min. After washing the cells, the stained cells were gated with CD3 and analyzed with FACS LSRII instrument (BD Biosciences). Flow cytometric analysis was done using FlowJo v8.8 software (Tree Star). The level of dimer fluorescence was normalized to the level of the surface staining with FITC-labeled anti-CD3 Ab and then plotted against dimer concentration. Binding curves were fitted by Graph-Pad Prism software.
A competitive staining of iNKT cells using α-GalCer-CD1d complex as an indicator
First, a suboptimal dose of α-GalCer-CD1d complex that binds human or mouse iNKT cells was determined by staining them with serially diluted allophycocyanin-labeled CD1d: Ig dimer fully loaded with α-GalCer. After identifying the suboptimal dose for α-GalCer-CD1d complex staining to be 4 nM, human iNKT cells or mouse iNKT hybridoma cells were then stained on ice for 30 min with 10 μg/ml FITC-labeled anti-CD3ε mAb and 4 nM of allophycocyanin-labeled CD1d dimer loaded with α-GalCer, in the presence of a varying doses, 10−3 to 104.5 nM of PE-labeled CD1d dimer-loaded with glycolipid analogues, as a competitor. After washing the cells, iNKT cells were gated with CD3 and analyzed for allophycocyanin color staining with FACS LSRII instrument (BD Biosciences). Flow cytometric analysis was performed, using FlowJo v8.8 software.
Data analysis
All data were expressed as the mean ± SD of triplicate wells from each sample. Statistical analysis of experimental and control data was evaluated by a Student t test. In all studies, a value of p < 0.05 was considered statistically significant.
Human subjects
Human peripheral blood mononuclear cells from anonymous blood donors was obtained from leukopacks provided by the New York Blood Center. NYBC does not select donors on the basis of gender or race but ensures that all donors are above 18 years of age. The work we performed, therefore, did not require an approval from the Institutional Review Board.
Results
Two C-glycoside analogues of α-GalCer, namely GCK127 and GCK152, have an E-alkene linker between sugar and lipid moieties. GCK127 has an almost identical, 2 carbons longer, fatty acyl chain as α-GalCer, whereas GCK152 has an aromatic ring in the tail of the acyl chain (Fig. 1A). We have previously shown that GCK152 displayed a stronger stimulatory activity against human iNKT cells than GCK127 in vitro (8). In sharp contrast, GCK152, unlike GCK127, failed to induce a significant level of both IFN-γ and IL-4 in the sera of mice at any time points after in vivo administration (Fig. 1B).
FIGURE 1.

Structure of two C-glycoside analogues, GCK127 and GCK152, and their ability to induce cytokine production in vivo. A, Chemical structures of GCK152 and GCK127. B, The levels of cytokines in the sera of mice administered with glycolipids. One μg of each glycolipd, GCK127 or GCK152, was administered into wild-type C57BL/6 mice i.v., and the sera were collected at the indicated time points after the administration. The levels of cytokines, including IFN-γ and IL-4 in the sera were determined by ELISA. The data were expressed as the mean ± SD of sera from five mice. Results represent one of two similar experiments.
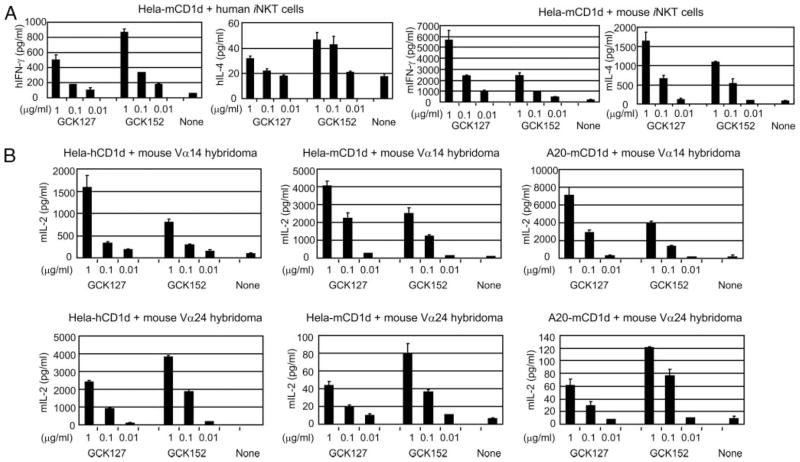
Firstly, we determined the preferential biological activity of these two glycolipids in vitro, by using Hela cells expressing human CD1d, or A20 cells expressing mouse CD1d as APCs to stimulate human vs mouse iNKT cells (Fig. 2). We found that GCK152 presented by Hela-hCD1d induced nearly 2-fold higher levels (p < 0.05) of IFN-γ and IL-4 secreted by human iNKT cells than GCK127 (Fig. 2A), whereas GCK152 presented by A20-mCD1d induced almost a half of the level (p < 0.05) of the cytokines secreted by murine iNKT cells than GCK127 (Fig. 2B). We hypothesized that the preferential stimulatory activities of GCK152 and GCK127 against human vs mouse iNKT cells are due to the difference between human vs mouse CD1d molecules in view of a previous study showing that the affinity of glycolipid-CD1d interaction correlated with the level of cell-based cytokine production (17). To test this hypothesis, we used A20-mCD1d cells to present GCK152 and GCK 127 to human iNKT cells, and conversely, used Hela-hCD1d cells to present the glycolipids to mouse iNKT cells. To our surprise, when presented by mouse CD1d molecules expressed by A20 cells, GCK152 induced almost 2- to 3-fold stronger stimulatory activity (p < 0.05) against human iNKT cells than GCK127 (Fig. 2C). In contrast, GCK127 presented by human CD1d molecules expressed by Hela cells displayed a significantly stronger stimulatory activity (p < 0.05) against murine iNKT cells than GCK152 (Fig. 2D).
FIGURE 2.
Differing stimulatory activities of GCK127 and GCK152 in the context of human vs mouse CD1d molecules against human vs murine iNKT cells in vitro. In A and B, 2 × 104 human iNKT cells or mouse iNKT cells were cocultured with 2 × 104 Hela-hCD1d or A20-mCD1d, respectively, in the presence of various concentrations of each glycolipid, ranging from 0.01 to 1 μg/ml in a 96-well culture plate. In C and D, 1 × 105 human iNKT cells or mouse iNKT cells were cocultured with 1 × 105 of A20-mCD1d or Hela-hCD1d, respectively, in the presence of various concentrations of each glycolipid, ranging from 0.01 to 1 μg/ml in a 96-well culture plate. After 24 h of incubation, supernatants were collected, and the concentration of hIFN-γ and hIL-4, or mIFN-γ and mIL-4, was determined by ELISA. Results represent one of three similar experiments.
It still remains possible that the preferential activity of the two glycolipids may be somewhat affected by the origin of the species of costimulatory molecules and/or cytokines/chemokines expressed by APCs. To eliminate such possibilities, we have generated Hela cells expressing mouse CD1d molecules, Hela-mCD1d, and used this cell line as APCs. As shown in Fig. 3A, the preferential stimulatory activity of GCK152 against human iNKT cells was clearly unaltered even with Hela-mCD1d, as APCs. In contrast, GCK127 was able to maintain a far stronger stimulatory activity (p < 0.05) against mouse iNKT cells than GCK152 when presented by Hela-mCD1d.
FIGURE 3.
Differing stimulatory activities of GCK127 and GCK152 presented by human or mouse CD1d molecules against human vs mouse invTCR expressing iNKT cells in vitro. In A, 1 × 105 human iNKT cells were cocultured with 1 × 105 Hela-mCD1d cells, and 3 × 104 mouse iNKT cells were cocultured with 3 × 104 of Hela-mCD1d cells, respectively, in the presence of indicated concentrations of each glycolipid in a 96-well culture plate. After 24 h of incubation, supernatants were collected, and the concentration of hIFN-γ and hIL-4, or mIFN-γ and mIL-4, was determined by ELISA. In B, 5 × 104 cells of mouse Vα14 hybridoma that expresses mouse invTCR, or mouse Vα24 hybridoma that expresses human invTCR, were cocultured with 5 × 104 cells of either Hela-hCD1d, Hela-mCD1d, or A20-mCD1d in the presence of indicated concentrations of each glycolipid in a 96-well culture plate. After 24 h of incubation, supernatants were collected, and the concentration of mIL-2 was determined by ELISA. In all experiments, results represent one of three similar experiments.
To further confirm that invTCR alone could command the preferential stimulatory activity of GCK127 and GCK152 against mouse and human iNKT cells, respectively, we used a mouse hybridoma that coexpress human invariant Vα24-Jα18/Vβ11 chains (11), designated mouse Vα24 hybridoma, and used this hybridoma as responder cells. As shown in Fig. 3B, the superior stimulatory activity of GCK127 on mouse Vα14 hybridoma was totally diminished when mouse Vα24 hybridoma was used as responding cells. In sharp contrast, GCK152 exhibited two-fold stronger stimulatory activity (p < 0.05) against mouse Vα24 hybridoma, as determined by the level of IL-2 secretion.
Altogether, the results shown in Figs. 2 and 3 indicated that the preferential stimulatory activity of the glycolipids toward human vs murine iNKT cells appears to be governed by the origin of the species of invTCR rather than that of CD1d molecules. These findings have led us to further investigate the binding affinity of the two glycolipids to CD1d, as well as iNKT cells of human vs mouse.
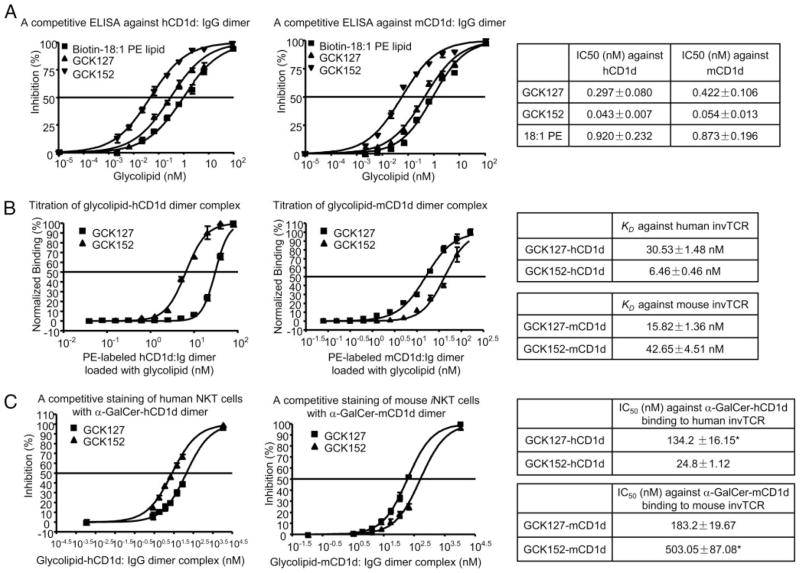
To assess the strength of glycolipid-CD1d interaction, biotinylated 18:1 PE lipid was used as an indicator in our assay, because 18:1 PE lipid, which has diacylglycerol that is similar to the lipid portion of α-GalCer, but lacks a sugar moiety, was shown to bind CD1d molecules (15) without being recognized by iNKT cells. We indeed confirmed that 18:1 PE lipid loaded onto CD1d: IgG dimer failed to stain iNKT cells, albeit it retained the ability to competitively inhibit the staining of iNKT cells with CD1d: IgG dimer loaded with α-GalCer (data not shown). Thus, we have developed a novel competitive binding ELISA to investigate the molecular interaction between CD1d and glycolipids, i.e., GCK152 and GCK127, independent of invTCR recognition. Through this assay, we were able to determine the binding of GCK152 and GCK127 to both human and mouse CD1d molecules (Fig. 4A). Interestingly, GCK152 consistently displayed 6- to 8-fold lower IC50 (p < 0.05) than GCK127 to either human and mouse CD1d molecules in the competitive binding ELISA, thus indicating that GCK152 had a stronger affinity to CD1d molecules, regardless of their origin of the species. In view of the differing biological activity of the two C-glycoside analogues against human vs murine iNKT cells (Figs. 2 and 3), it appears that the binding affinity of the glycolipids to CD1d molecules does not translate into the strength of their activity against iNKT cells.
FIGURE 4.
Relative binding affinity of GCK127 and GCK152 against human vs mouse CD1d molecules, as well as against human vs mouse iNKT cells. A, A competitive ELISA against CD1d dimer, using 18:1 PE lipid. After being coated with anti-mouse IgG1 Ab overnight, Maxisorp ELISA plate was incubated with hCD1d: mIgG or mCD1d: mIgG dimer for 2 h. Serially diluted glycolpids were then added onto the plate in the presence of 1 μg/ml 18:1 Biotinyl PE. After 24 h of incubation at 37°C, bound 18:1 Biotinyl PE lipid was determined with HRP-labeled Avidin and TMB substrate. Inhibition curves were fitted with Sigmoidal dose-response formula using Prism 4.0 software. B, Staining of iNKT cells with a serially diluted PE-labeled CD1d-glycolipid complex. PE-labeled hCD1d: mIgG dimer or PE-labeled mCD1d: mIgG dimer was loaded with 1000 molar ratio excess of GCK127 or GCK152. Glycolipid-hCD1d dimer complex or glycolipid-mCD1d dimer complex was then serially diluted and incubated with human iNKT cells or mouse 1.2 iNKT hybridoma cells, respectively, together with FITC-labeled anti-CD3ε mAb for 30 min. After washing twice, the cells were gated with CD3 and analyzed with FACS LSRII instrument. Binding curves were fitted by GraphPad Prism software. The binding isotherms were then subjected to a Scatchard transformation to access the apparent equilibrium dissociation constant characteristic of the avidity, and generated KD value. C, A competitive staining of iNKT cells with glycolipid-CD1d dimer complex. Allophycocyanin-labeled CD1d dimer was loaded with 1000 molar ratio excess of α-GalCer to form α-GalCer-CD1d-allophycocyanin complex. PE-labeled CD1d dimer was loaded with 1000 molar ratio excess of GCK127 or GCK152 to form GCK127-CD1d-PE or GCK152-CD1d-PE complex, respectively. Human iNKT cells or murine iNKT hybridoma cells were stained for 30 min with 10 μg/ml FITC-labeled anti-CD3ε mAb together with 4 nM of α-GalCer-hCD1d-allophyco-cyanin complex or 3 nM of α-GalCer-mCD1d-allophycocyanin complex, respectively, in the presence of serially diluted GCK127-hCD1d-PE or GCK152-hCD1d-PE complex for human iNKT cells, or serially diluted GCK127-mCD1d-PE or GCK152-mCD1d-PE complex for mouse iNKT hybridoma cells. After washing twice, the cells were gated with CD3 and analyzed with FACS LSRII instrument. The degree of percentage inhibition of α-GalCer-hCD1d-allophycocyanin fluorescence or α-GalCer-mCD1d-allophycocyanin fluorescence was plotted against the concentration of GCK127/GCK152-hCD1d-PE or GCK127/GCK152-mCD1d-PE complex, respectively. Inhibition curves were fitted with Sigmoidal dose-response formula using Prism 4.0 software. Asterisk indicated a predicted IC50 according to calculation of the binding curve. In all experiments, the results represented the mean of triplicate wells from each sample, and expressed as mean ± SD. Compared two curves by t test statistics analysis, p < 0.05 was considered as significant difference.
To determine the binding affinity of glycolipid-CD1d complex to invTCR of iNKT cells, we have used a FACS-based method to evaluate the avidity between glycolipid-CD1d complex and in-vTCR, as previously described (16). In this assay, we loaded PE-labeled human or mouse CD1d: Ig dimer with GCK152 or GCK127. We then stained human iNKT cells or murine iNKT hybridoma cells with a serially diluted amount of each CD1d dimer-glycolipid complex and peformed FACS analysis.
Because glycolipid-loaded CD1d dimer stains iNKT cells through interacting with their invTCR, it is reasonable to say that this method can assess the binding strength of glycolipid-CD1d complex to the TCR. We found that human CD1d dimer loaded with GCK152 exhibited a 5-fold stronger avidity (p < 0.05) to human iNKT cells than that loaded with GCK127, and in sharp contrast, mouse CD1d dimer loaded with GCK152 showed a 2–3-fold weaker avidity (p < 0.05) than that loaded with GCK127 (Fig. 4B). These results indicate that GCK152 and GCK127, as a complex with CD1d molecules, have a different avidity to the invTCR of human vs mouse iNKT cells, with GCK152-hCD1d complex having a stronger avidity to human in-vTCR than GCK127-hCD1d complex, and vice versa, GCK127-mCD1d complex having a stronger avidity to mouse invTCR than GCK152-mCD1d.
To further confirm the affinity of the interaction between glycolipid-CD1d complex and invTCR of iNKT cells, we used allophycocyanin-labeled CD1d: Ig dimer loaded with α-GalCer (α-GalCer-CD1d-allophycocyanin), as an indicator, and serially diluted PE-labeled CD1d: Ig loaded with GCK127 or GCK152, as a competitor. In brief, human or mouse iNKT cells were stained with 4 nM of α-GalCer-CD1d-allophycocyanin complex in the presence of serially diluted GCK127-CD1d-PE or GCK152-CD1d-PE complex. Then, the mean fluorescence intensity was determined by FACS analysis, and their IC50 was calculated (Fig. 4C).
We found that IC50 of GCK127-hCD1d complex against α-GalCer-hCD1d complex binding to human iNKT cells is more than 5-fold (p < 0.05) greater than that of GCK152-hCD1d, whereas GCK127-mCD1d complex required 3-fold less amount (p < 0.05) than GCK152-mCD1d to inhibit 50% of α-GalCer-mCD1d binding to murine iNKT cells (Fig. 4C). These results indicate that GCK152-hCD1d complex has a stronger affinity to human invTCR than GCK127-hCD1d, and vice versa, GCK127-mCD1d complex had a stronger affinity to mouse invTCR than GCK152-mCD1d.
Collectively, all the binding assays have led to the conclusion that although GCK152 has a stronger affinity to both human and mouse CD1d molecules than GCK127, GCK152-hCD1d complex has a stronger affinity to human invTCR than GCK127-hCD1d, whereas GCK127-mCD1d complex has a stronger affinity to mouse invTCR than GCK152-mCD1d.
Discussion
In our previous studies, among C-glycoside analogues of α-Gal-Cer, having an E-alkene linker between sugar and lipid moieties, GCK152, which has an aromatic ring in the tail of the acyl chain, was shown to exhibit a stronger stimulatory activity against human iNKT cells than GCK127, which has an almost identical fatty acyl chain as α-GalCer (8). We have also shown that, on the contrary to human iNKT cells, GCK152 displayed a much weaker activity against murine iNKT cells than GCK127 both in vivo and in vitro (8). This striking difference in the level of stimulatory activity against human vs murine iNKT cells by these two C-glycosides had prompted us to investigate in our current studies the main reason why the two structurally similar glycolipids cause such opposing activities.
Recent crystal structure studies have shown that the lipid portion of α-GalCer fits into the binding groove of human CD1d and mouse CD1d molecules in an almost identical fashion (18, 19). More recently, it has been demonstrated that invTCR recognizes the glycoside head by a unique “lock-and-key” mode (20). A similar structural study has shown that the CDR2β loop of invTCR mainly interacts with CD1d backbone, and germline-encoded residues of both CDR1α and CDR3α loops contribute to the recognition of α-GalCer by interacting with CD1d and the bound α-GalCer molecules (21). Thus, by this predominantly rigid lock-and-key interaction, the CDR loops that contact with the α-Gal-Cer-CD1d complex minimally change conformation on ligation, indicating that the recognition by invTCR of iNKT cell was highly conserved. The early study showed the α-anomeric conformation of sugar moiety was essential for the iNKT cells activation and β-galactosylceramide or mannosylceramide did not result in proliferative responses of iNKT cells (22). A few recent studies, however, have shown that recognition of invTCR had diversity, albeit a limited degree, because some nonglycoside α-GalCer and β-linked glycoshingolipid were able to activate iNKT cells (23, 24). A hypothesis was that the high degree of variability in the CDR3β sequences in invTCR of iNKT cell, coupled with the location of this loop within the complex, was likely to provide plasticity of the invTCR recognition for different CD1d-restricted Ags (20). Although galactose head positioning had up to a 3Å shift between CD1d-α-GalCer complex structure of mice and man (25), some studies have demonstrated that there is interspecies cross-reactivity between human and mouse iNKT cells with α-GalCer-CD1d complex from both species (13, 21, 26), because of the plasticity of invTCR recognition.
In our present study, although GCK152 and GCK127 had the same α-E-alkene-linkage glycoside, they displayed different affinities to human and mouse invTCR when presented as a complex with human and mouse CD1d molecules. Because the only difference between GCK152 and GCK127 is their lipid portion, the lipid chain binding to CD1d molecules should result in a minor conformation shift of the sugar head of the glycolipids. This shift is likely beyond the plasticity of the invTCR recognition, having preferential recognition to this shift by human vs mouse invTCR. Our study implies that there should be a significant, if not a major, difference between recognition by invTCR of human and mouse iNKT cells. Moreover, this difference might partially account for different biological activities of some of α-GalCer analogues against mouse vs human iNKT cells. Although invTCR recognition has been considered highly conserved, our study presents functional evidence that there is still some restriction specificity in iNKT activation through invTCR. In this regard, the effect of invTCR restriction should not be neglected when designing the next generation of CD1d-binding iNKT-stimulating glycolipids. Finally, in view of a most recent x-ray crystallography study on the structure of mouse invTCR-glycolipid-mCD1d complex in which a differential recognition of CD1d-α-GalCer by the Vβ8.2 and Vβ7 semi-invTCR of mouse iNKT cells has been revealed (27), our findings further imply that the affinity of the mouse or the human invTCR for conserved endogenous lipids might shape, upon positive selection, the different Vβ usage in the two species.
In summary, two C-glycoside analogues, GCK152 and GCK127, having identical structure except their acyl chain, are shown to display differing biological activities against human vs murine iNKT cells. Through a series of binding assays, it was found that this different activity correlates with the binding affinity of glycolipid-CD1d complex to invTCR of human vs murine iNKT cells rather than that of glycolipid to human or murine CD1d molecules. By experiments, in which interspecies cross-reactivity between human and mouse iNKT cells with glycolipid-presenting APCs from both species was examined, we have found that iNKT cells, but not APCs that express CD1d, influence the species-dependent, preferential activity of C-glycosides. Furthermore, using Hela cells expressing mouse CD1d, as APCs, and mouse hybridoma expressing human invTCR, as responding cells, we were able to determine that the origin of the species of invTCR, but not of CD1d molecule, commands the biological activity of C-glycoside. These studies indicate that the affinity to invTCR rather than to CD1d molecule appears to govern in shaping the strength of the biological activity of C-glycoside analogues against iNKT cells of different species.
Acknowledgments
We thank Drs. Vincent Sahi and Raquel Garcia-Navarro for help with FACS analysis. We also thank Drs. Mitch Kronenberg, Chyung-Ru Wang, Steven Porcelli, and Kirin Brewery for providing a mouse iNKT hybridoma 1.2 and A20-mCD1d, a pCDNA3-CD1.1, Hela-hCD1d, or α-GalCer, respectively.
Footnotes
This work was funded by National Institutes of Health Grants R21 AI062842 and R56 AI070258 (to M.T.), Cytheris, Otsuka Pharmaceutical Company, the Irene Diamond Foundation, Bill and Melinda Gates Foundation, a subcontract from N01-AI-25456-MODno.5 (to R.W.F.), and Italian Association for Cancer Research and Italian Ministry of Health (to G.C. and P.D.).
Abbreviations used in this paper: iNKT, invariant NKT cell; invTCR, invariant T cell receptor; α-GalCer, α-galactosylceramide; DC, dendritic cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuji M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63:1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Wang X, Besra GS, Gumperz JE. Modulation of CD1d-restricted NKT cell responses by CD4. J Leukocyte Biol. 2007;82:1455–1465. doi: 10.1189/jlb.0307163. [DOI] [PubMed] [Google Scholar]
- 5.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 6.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 8.Li X, GC, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Chien M, Tsuji M, Franck RW. E and Z α-C-galactosylceramides by Julia-Lythgoe-Kocienski chemistry: a test of the receptor-binding model for glycolipid immunostimulants. Chembiochemistry. 2006;7:1017–1022. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 10.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 11.Thedrez A, de Lalla C, Allain S, Zaccagnino L, Sidobre S, Garavaglia C, Borsellino G, Dellabona P, Bonneville M, Scotet E, Casorati G. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood. 2007;110:251–258. doi: 10.1182/blood-2007-01-066217. [DOI] [PubMed] [Google Scholar]
- 12.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protocol. 2008;3:70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 13.Molling JW, Moreno M, van der Vliet HJ, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, Bontkes HJ. Generation and sustained expansion of mouse spleen invariant NKT cell lines with preserved cytokine releasing capacity. J Immunol Methods. 2007;322:70– 81. doi: 10.1016/j.jim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Mandal M, Chen XR, Alegre ML, Chiu NM, Chen YH, Castano AR, Wang CR. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 15.Rauch J, Gumperz J, Robinson C, Skold M, Roy C, Young DC, Lafleur M, Moody DB, Brenner MB, Costello CE, Behar SM. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508– 47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidobre S, Hammond KJ, Benazet-Sidobre L, Maltsev SD, Richardson SK, Ndonye RM, Howell AR, Sakai T, Besra GS, Porcelli SA, Kronenberg M. The T cell antigen receptor expressed by Vα14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc Natl Acad Sci USA. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang PH, Imamura M, Li X, Wu D, Fujio M, Guy RT, Wu BC, Tsuji M, Wong CH. Quantitative microarray analysis of intact glycolipid-CD1d interaction and correlation with cell-based cytokine production. J Am Chem Soc. 2008;130:12348–12354. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch M, V, Stronge S, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6:819– 826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 19.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810– 818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44– 49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 21.Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, Richardson SK, Thakur M, Howell AR, Scott-Browne JP, Gapin L, Godfrey DI, McCluskey J, Rossjohn J. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med. 2008;205:939–949. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 23.Silk JD, Salio M, Reddy BG, Shepherd D, Gileadi U, Brown J, Masri SH, Polzella P, Ritter G, Besra GS, et al. Cutting edge: non-glycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180:6452– 6456. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 24.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey DI, McCluskey J, Rossjohn J. CD1d antigen presentation: treats for NKT cells. Nat Immunol. 2005;6:754–756. doi: 10.1038/ni0805-754. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, et al. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, et al. Differential recognition of CD1d-α-galactosyl ceramide by the V β 8.2 and V β 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]